Abstract
Delirium is a common complication in acute stroke yet there is uncertainty regarding how best to screen for and diagnose delirium after stroke. We sought to establish how delirium after stroke is identified, its incidence rates and factors predicting its development. We conducted a systematic review of studies investigating delirium in acute stroke. We searched The Cochrane Collaboration, MEDLINE, EMBASE, CINHAL, PsychINFO, Web of Science, British Nursing Index, PEDro and OT Seeker in October 2010. A total of 3,127 citations were screened, full text of 60 titles and abstracts were read, of which 20 studies published between 1984 and 2010 were included in this review. The methods most commonly used to identify delirium were generic assessment tools such as the Delirium Rating Scale (n = 5) or the Confusion Assessment Method (n = 2) or both (n = 2). The incidence of delirium in acute stroke ranged from 2.3–66%, with our meta-analysis random effects approach placing the rate at 26% (95% CI 19–33%). Of the 11 studies reporting risk factors for delirium, increased age, aphasia, neglect or dysphagia, visual disturbance and elevated cortisol levels were associated with the development of delirium in at least one study. The outcomes associated with the condition are increased morbidity and mortality. Delirium is found in around 26% of stroke patients. Difference in diagnostic and screening procedures could explain the wide variation in frequency of delirium. There are a number of factors that may predict the development of the condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Delirium (or acute confusional state) is a severe but potentially preventable disorder which is common among elderly hospital patients [1, 2], with reported prevalence of 20–30% across a variety of settings [3]. Delirium is associated with increased mortality, morbidity and length of hospital stay [4, 5]. Delirium may be hyperactive (accompanied by overt psychotic symptoms and agitation); hypoactive (characterised by sedation); or mixed (i.e. both hypoactive and hyperactive). The hypoactive type can often be undetected or misdiagnosed as depression [6].
Although stroke is a recognised predisposing factor for the development of delirium, there is currently no clear guidance on whether stroke patients should be routinely screened for delirium, no guidelines on the best way to screen for delirium and no multidisciplinary treatment recommendations for the condition [7, 8]. This is despite recent national guidance on the importance of early identification of delirium in hospital patients over the age of 65 presenting with significant illness [9]. Potentially, this means that delirium in acute stroke may be missed, particularly the hypoactive type [10].
There is, to our knowledge, no published systematic review on delirium after stroke. As a systematic review is the least biased way of collating and examining evidence from the literature [11], we undertook a systematic review to determine the following in acute stroke:
-
1.
The incidence of delirium, the patient-related factors associated with its development, and the association between developing delirium and outcome.
-
2.
How best to screen for delirium, specifically, the feasibility of the screening tools, and their sensitivity and specificity.
Materials and methods
In October 2010 we searched Cochrane Stroke Group Trials Register and the Cochrane Dementia and Cognitive Improvement Group Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, latest issue), MEDLINE (1950–), EMBASE (1980–), CINAHL (1981–), PsycINFO (1840–), Web of Science (1970–); British Nursing Index (1985–), Physiotherapy Evidence Database (PEDro) and OT Seeker for the systematic evaluation of evidence in occupational therapy practice. See appendix for keyword combinations used. Reference lists of identified articles were scrutinised to identify studies that were not identified by the electronic searches. Authors of published studies were contacted on two occasions for clarification and seeking out of further details.
Inclusion criteria
We included cross-sectional studies, longitudinal studies, cohort studies case control studies and case series. All adult participants (≥18 years) presenting as hospital inpatients with a clear diagnosis of stroke [12] or subarachnoid haemorrhage (SAH) were included. Full publications in English, Hebrew, French, German, Dutch or Spanish were considered for this review.
Exclusion criteria
We excluded conference proceedings, editorials, opinion pieces, review papers, letters, single case studies, case series of three patients or fewer, studies presenting patients admitted due to delirium (rather than stroke or SAH), studies reporting on acquired brain injury or progressive neurological brain damage (e.g. multiple sclerosis, dementia) or delirium tremens.
Study selection
Titles and abstracts identified from database searches were reviewed by one author (GCL) and obviously irrelevant work was eliminated. This author categorised all citations as either ‘include’, ‘exclude’ or ‘possible’ using an agreed paper form, the reasons for exclusion were also logged on this form. All abstracts of both included, possible and excluded studies were reviewed by the first author plus a second review author (FvW, GEM or KN) who independently screened for relevance and fulfilment of inclusion criteria. Disagreements were resolved by discussion with a third reviewer.
Data extraction and quality assessment
Paper data extraction forms were designed, piloted on three studies, revised and subsequently used to extract data from the studies which met the inclusion criteria. We extracted data on (1) year of publication, study design, and characteristics of study participants, (2) sample size, inclusion and exclusion criteria, (3) tools used to diagnose and or screen for delirium including any data provided regarding psychometric properties and the suitability of tool use with stroke patients. This was judged based on the necessity of the patient to be able to understand and use language in order to participate in the assessment, (4) number of patients who experienced delirium, predictors of developing delirium and outcomes associated with delirium in acute stroke. Our data extraction forms also incorporated the 14 item tool for the Quality Assessment of studies of Diagnostic Accuracy included in systematic reviews known as the QUADAS tool [13]. Each item in this checklist had been designed to assess the reliability of specific aspects of a study’s methodology (see Table 1 for full details). Individual items are scored as ‘yes’, ‘no’ or ‘unclear’. ‘Yes’ scores indicate that the methodology has minimised bias and increased reliability of the study outcomes while a high number of ‘no’ or ‘unclear’ scores question the reliability of the diagnostic procedure [13]. In some cases, we had to score ‘non-applicable’ due to the nature of some of the papers. When completing the QUADAS checklist, the Reference Standard was regarded as a clinical assessment of delirium using established diagnostic criteria [14] such as DSM-III [15], DSM-III-R [16], DSM-IV [17] or DSM-IV-R [18]. The index test was regarded as any delirium diagnostic or screening tool such as the Confusion Assessment Method (CAM) [19], the Delirium Rating Scale (DRS) [20], Organic Brain Syndrome (OBS) Scale [21] or the Mini Mental State Examination (MMSE) [22].
One review author (GCL) extracted all data and assessed quality and three other authors (GEM, FvW and KN) independently extracted the data from a third of the papers each. In instances where there were discrepancies in scoring QUADAS items, raters discussed the specific items and reached agreement as to the definitive scores. Full scores for each paper are presented in Table 1.
Statistical analysis
Data on incidence were extracted from each study and a 95% confidence interval (CI) produced. These were combined in a meta-analysis to synthesise single descriptive statistics across the studies. To determine the pooled estimate, the DerSimonian and Laird random effects [14] meta-analytic approach was undertaken. Statistical heterogeneity was assessed using the Q statistic, with p < 0.05. The metan procedure in Stata version 9.2 was employed in the analysis and production of the associated Forest plot.
Results
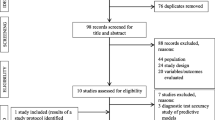
A total of 3,127 citations were identified by one author (GCL), of which, 198 were retrieved for abstract and/or full text scrutiny. A total of 138 studies were rejected as per our inclusion/exclusion criteria, leaving 60 titles, the full texts of which were further scrutinised. Of these articles, a total of 40 were excluded due to not meeting our inclusion criteria leaving a total of 20 studies which met the inclusion criteria for this review. No new titles were identified from reference lists of the available studies.
Description of studies included in this review
All included studies originated from hospital-based cohorts, most of which stated that delirium assessments were conducted within 1 week of hospital admission (Table 2). The designs employed in the studies included in this review were prospective studies (n = 11), retrospective studies (n = 3) case controls (n = 3), one cross-sectional study, one pilot study of treatment intervention and one study which was described as “observational” (see Table 2).
Diagnostic and screening tools used
A total of 12 studies reported applying established diagnostic criteria when assessing patients for delirium: Six (30%) studies applied DSM IV [2, 23–27], three (15%) studies applied DSM III-R [28–30], two studies applied DSM IV-R [31, 32], and one (5%) study applied DSM III [33]. Three studies referred to “clinical assessment” but did not detail any diagnostic guidelines [34–36], and one study referred to the diagnosis of “disorientation” using a simple three-point scale [37]. As for tools used to screen for delirium, of the 14 studies utilising such tools, five used the DRS or DRS R-98 [2, 23, 24, 31, 32]; two studies used the CAM [38]; two studies used both the DRS and the CAM [39, 40]; three studies used the OBS Scale [25, 29, 30] and two studies used the MMSE [35, 36]. See Table 2 for full details.
The DRS [20] and the CAM [19] are frequently used tools both of which are based on DSM criteria and have been designed to identify delirium in a variety of settings. The DRS is a tool comprising ten items, designed for use by medical staff with specific training [20]. The highest possible score is 32, with a cut-off score of ten indicating the presence of delirium, thus the DRS may be used to rate the severity of delirium [10]. Comprising of four features (acute onset and fluctuating course, inattention with either: disorganised thinking or altered level of consciousness) the CAM was developed for use by any health professional, it has high sensitivity, specificity and reliability and is easy to administer [19].
The Organic Brain Syndrome (OBS) Scale was developed for the evaluation of disturbance of awareness and orientation and other signs of confusion in the elderly [41]. Reportedly taking up to 1 h to complete [42], the OBS Scale consists of two subscales: OBS1 (16 items) for disorientation and OBS2 (39 items) for confusion. For both subscales, the severity of symptoms are ranked in a four-point ordinal scale from zero to three, where zero denotes a correct response and three denotes incorrect response. The Mini Mental State Examination (MMSE) [22] is a screening test of cognitive impairment.
One of the 20 studies included in this review reported data on sensitivity and specificity of the screening tools but this was not specific to stroke patients. However, all studies using either the DRS or the CAM referred to the original papers where data on sensitivity and specificity were available. There was no attempt in any of the studies to assess the suitability of using a generic delirium screening tool in acute stroke. A number of studies considered the challenge of using the above tools in acute stroke, as ten studies reported excluding patients with reduced consciousness [23, 27, 29, 30, 32, 33, 36–39] and four studies excluded patients with aphasia [24, 26, 29, 36]. Caeiro et al. [23, 31, 32] reported scoring zero in certain items of the DRS if patients had “language difficulties”; however, this term is somewhat vague. Henon et al. [2] considered the possibility of erroneously diagnosing demented or aphasic patients with delirium and report that patients had to score over 10 on the DRS. Gustafson et al. [30] referred to the use of clinical observation of rapid behavioural changes and disorientation on the ward as indicative of delirium in aphasic patients.
Evaluation of methodological quality
Studies which achieved the highest QUADAS scores tended to be those which utilised more than one method of identifying delirium in their cohort: a combination of a clinical assessment with the use of a screening tool [2, 23–25, 29–32] or two tools such as the CAM for detection and the DRS for severity of delirium [39, 40]. In studies which utilised only one method of identification of delirium items 7, 10 and 11 of QUADAS were removed and thus appear in Table 1 as “non-applicable” [13]. Item seven of the QUADAS was at times difficult to score as although the different tests utilised in practice are independent of each other, the DRS [20] and the CAM [19] are based on DSM diagnostic criteria and therefore one may argue that they are not entirely independent. Table 1 gives details of the scores given to each study as per QUADAS items.
Incidence of delirium in acute stroke and SAH
The overall incidence estimates of delirium in acute stroke and SAH is difficult to definitively establish due to the substantial heterogeneity observed (χ 2 = 587.49, degrees of freedom = 19, p = 0.000). This is often the case for single group studies, with 99% of the variation in the point estimate being attributable to heterogeneity [43]. Due to this, we report only the results of the random effects approach: incidence of delirium was 26% with a 95% CI of 19–33% (Fig. 1). The frequency of delirium assessment also varied: ten studies applied diagnostic procedures once within the first week of admission [23, 24, 26, 28, 31, 32, 36–39] and three studies applied these more than once daily [29, 30, 38] with the rest of the studies not reporting on the time points at which delirium assessments were carried out (Table 2).
Risk factors for developing delirium in acute stroke
Risk factors were examined in 17 of 20 studies (n = 478 with delirium). The most frequently cited risk factors for developing delirium in the acute stage of stroke were the following: older age [2, 23, 26, 27, 29, 30, 32]; specific symptoms resultant from the stroke (aphasia, neglect or dysphagia) [23, 26, 27, 31, 32, 38]; impaired vision [25–27, 38], either as a result of the stroke or pre-morbid visual disturbance, elevated cortisol levels [28, 29, 37] and drugs with anticholinergic effect [30, 31]. Eight studies (n = 209 with delirium) reported the association between lesion location and development of delirium: three studies found an association between right-sided lesions [33, 36, 44] and two for left-sided lesions [30, 34]. One study associated lesions of the posterior cerebral artery (PCA) with the development of delirium [35] while another reported a longer duration of delirium in patients with right hemisphere lesions, but the findings were not statistically significant [24]. Two studies [2, 27] found no significant association between lesion type or location with the development of delirium. Two studies [23, 26] found that delirium was most frequent and most severe following intracerebral haemorrhage, and two studies [26, 38] found an association between a total anterior circulation infarct (TACI) stroke and the development of delirium. The remaining studies did not investigate the association between lesion location or type and delirium.
Outcomes associated with delirium in acute stroke
Ten studies (n = 331 with delirium) related outcomes to the presence of delirium in acute stroke. All ten studies showed that those who experience delirium had unfavourable outcomes, with the other ten studies not reporting data on outcomes. It was consistently reported that patients who experienced delirium in the acute stage of stroke were more likely to have unfavourable outcomes such as increased hospital stay [23, 26, 29, 30, 38], increased mortality rates [23, 24, 26, 37, 38] and increased dependence: measured by rates of institutionalisation [26, 29, 38] or by means of standardised assessment of ability to perform activities of daily living [2, 23, 25–27].
Discussion
Incidence rates of delirium in acute stroke
Our meta-analysis finding of an incidence rate of 26% (95% CI 19–33%) of delirium in acute stroke is consistent with the rate of delirium found in other medical settings [3]. However, this result must be seen in the context of other findings of this review which relate to the variation in diagnostic and screening methods used to identify delirium after stroke. This variation and the varying methodological rigor, discussed below, are factors which may explain the wide range of incidence of delirium observed and the heterogeneity observed across the studies (Fig. 1).
Rigour of delirium diagnostic procedures
The rigor of diagnostic procedures across the studies included in this review must be seen in light of the decade the studies were conducted in. Three of the studies included in this review predate the development of validated delirium diagnostic or screening tools [33, 36, 44], one of which was a retrospective case note review and shall be discussed separately below [33]: Mori and Yamadori conducted a study investigating the presence of acute agitated delirium and ACS following right MCA infarcts. The authors state that mental state examinations were performed; however, there is no mention of diagnostic criteria used. Also, the MMSE was applied within 2 weeks; arguably, this time period is too long, as it is possible that cases of ACS were missed within that time period [36]. Schmidley and Messing do not explicitly refer to diagnostic criteria used; however, they do detail the definition of ACS which follows DSM III criteria [44].
Four of the studies included in this review conducted retrospective reviews of patient case notes to establish the incidence of delirium after stroke [33–35, 44]; while these methods are valid, the rigour of diagnostic procedures is impossible to critique as the reporting of these is lacking, perhaps understandably as the researchers did not conduct the delirium assessments. Other instances where we had difficulty commenting on the rigor of diagnostic procedures relate to lack of sufficient detail reported: Sandberg et al. conducted a study of sleep apnea and its relation to delirium, the scales used to diagnosed delirium are described; however, the frequency and timing of assessment are not detailed thus it is difficult to critique the methods beyond the choice of tools [25]. Dostovic et al. [24] and Fassbender et al. [28] also do not give sufficient details regarding the execution of the delirium assessments; thus it is difficult to judge the methods employed to diagnose delirium in their cohort. Marklund et al. [37] provided sufficient detail of their diagnostic protocol as they aimed to investigate the relationship of serum cortisol levels post stroke and relate these to the presence of disorientation. It is curious that they chose to investigate the presence of ‘disorientation’, which is a manifestation of delirium, but in itself, it does not constitute a medical or psychiatric condition and seen alone, it is not enough to determine a delirium diagnosis as per DSM criteria [15, 16, 18]. Marklund et al. [37] assessed ‘disorientation’ by means of a non-standardized three-point scale, the validity and sensitivity of which is unknown. Overall, it appears that in those studies where more rigorous assessment protocols were followed, there is greater confidence in the incidence rates found.
The use of general delirium tool in a stroke cohort
An important finding of this review is the application of diagnostic tools developed for use in a general medical environment, within a stroke cohort. Among the studies included in this review, none had addressed the fact that the tools used were not specifically designed to detect delirium in a stroke cohort. The question regarding the suitability of the tools used to screen for delirium in stroke patients was asked by McManus et al. [10] as they offered some drawbacks for the use of both the CAM and the DRS in a stroke population, based mainly on language difficulties and the fluctuating nature of cognitive function within the acute phase of stroke. Albeit, McManus et al. [39] compared the CAM and the DRS in the acute stroke population and found that there was high level of agreement between the two screening tools and that there is a strong correlation between a low MMSE score and delirium in this setting. They concluded that the CAM is favourable due to its ease of use but cautioned that appropriate training is essential for use of either tool. Oldenbeuving et al. [45] also favour the CAM for use in a stroke cohort, despite the fact that it was not tested for use in this setting. The tool less frequently used by studies in this review is the OBS Scale. According to Bjorklund et al. [41] various studies have assessed the OBS scale’s sensitivity to detecting a range of organic brain syndromes and found high inter-rater reliability. The OBS Scale has also been reported to show good responsiveness to cognitive symptoms in an elderly population [41] but there is no published reference to any psychometric properties of this tool [46]. A comparison between the OBS scale and the MMSE as applied to patients with dementia was carried out by Jensen et al. [47] and the two were found to have good agreement; however, the sample comprised of patients with dementia and the applicability of the OBS scale in a stroke setting is not described in the literature. The fourth tool reported in this review is the MMSE, a tool which has reported restrictions in the application in stroke due to its score being influenced by language, mood and sensory and motor function [10]. It seems clear that greater uniformity in the method and frequency of application of delirium assessment batteries would enable the establishment of greater clarity on the incidence of delirium after stroke.
Sources of bias
Some of the studies we reviewed were limited by selection bias. Mori and Yamadori and Schmidley and Messing investigated the presence of ACS in MCA infarcts, reportedly due to the fact that the relationship between right hemisphere infarctions and ACS had been previously described [36, 44]. Similarly, Nicolai and Lazarino restricted their cohort to PCA territory infarcts; however, unlike the aforementioned studies, the presence of ACS in this type of infarct is not well documented in the literature, and indeed, they report a small number of new cases of PCA infarcts with ACS [35]. Another factor relevant to selection bias is exclusion criteria. A total of four studies excluded patients with aphasia [24, 26, 29, 36]. Aphasia has been reported in up to 38% of patients with acute stroke [48]; therefore it is possible that a substantial proportion of patients have been excluded from the study of incidence rates of delirium. Another point to note is that the CAM was validated for use with non-verbal patients in the intensive care environment (CAM-ICU) [49], it is therefore surprising that researchers chose to exclude patients with aphasia when there is a validated tool available for use with patients who are unable to communicate. A total of seven studies excluded patients with a history of dementia [24, 26, 33–36, 44], presumably to enable more accurate distinction between delirium and dementia; however, other authors have reported an association between pre-existing cognitive impairment and developing delirium in acute stroke [27, 30, 38]. Thus, by excluding this group of patients, the incidence rates of delirium would be potentially affected.
Risk factors and outcomes in delirium after stroke
Some of the risk factors for the development of delirium identified in this review are consistent with the general medical and geriatric literature. Older age, severe illness and visual impairment are established risk factors for delirium [5, 9, 50]. The importance of anticholinergic medication as precipitating factor for the development of delirium is documented in the medical literature [5, 51]; however, only two of the studies included in this review examined this as a risk factor for delirium in acute stroke. More specifically to stroke, a number of studies included in this review have found that a stroke in the territory of the middle cerebral artery (MCA) is a precipitating factor for the development of delirium, which has been reiterated by Caplan, who reviewed studies that we have excluded on the grounds that the presenting feature of the patients’ admission was the delirium rather than stroke [52]. To the best of our knowledge, other stroke-specific factors highlighted in this review have not previously been discussed in the literature. As for outcomes associated with delirium after stroke, these are consistent with published literature, as it is well established that delirium is associated with an increased length of hospital stay and increased mortality and morbidity [4, 5, 9, 53].
Strengths, weaknesses and future research
To our knowledge, this is the first time the literature on delirium after stroke has been systematically reviewed. We are confident that our search strategy has identified all the available literature in the field, and we had followed a rigorous protocol when applying inclusion/exclusion criteria and during data extraction. We have applied a validated, rigorous checklist [13] for the quality assessment of included studies, which we believe has strengthened the review. However, the main restriction of this review stems from the substantial heterogeneity of the studies included. It was difficult to compare and group studies because of the wide variation in the way delirium was detected and the timing and frequency of delirium assessment. This has highlighted the importance of establishing delirium screening guidelines within stroke medicine, to enable an early identification, treatment and potential minimisation of the effects of the condition on patients and healthcare systems. We propose that an important direction for future research lies in either adapting an existing screening tool for the use within a stroke cohort, or the development of a new tool, specifically designed to be used with patients after stroke.
References
Inouye SK, Bogardus ST, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, Cooney LMJ (1999) A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 340:669–676
Henon H, Lebert F, Durieu I, Godefroy O, Lucas C, Pasquier F, Leys D (1999) Confusional state in stroke: relation to preexisting dementia, patient characteristics, and outcome. Stroke: J Cereb Circ 30:773–779
Siddiqi N, House AO, Holmes JD (2006) Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing 35:350–364
McCusker J, Cole MG, Dendukuri N, Belzile E (2003) Does delirium increase hospital stay? J Am Geriatr Soc 51:1539–1546
Young J, Inouye SK (2007) Delirium in older people. BMJ (Clinical Research Ed) 334:842–846
Carson A, Zeman A, Brown T, Sharpe M (2004) Organic disorders. In: Johnstone E, Cunningham Owens D, Lawrie S, Sharpe M, Freeman C (eds) Companion to psychiatric studies, 7 ed. edn. Churchill Livingstone, Edinburgh, pp 341–344
Scottish Intercollegiate Guidelines Network (2010) Management of Patients with Stroke: Rehabilitation, prevention and management of complications, and discharge planning. A National Clinical Guideline. NHS Quality Improvement Scotland
National Institute for Health and Clinical Excellence (2008) Stroke: National Clinical Guidelines for treatment and initial management of acute stroke and transient ischaemic attack (TIA). London, Royal College of Physicians
National Institute for Clinical Excellence (2010) Delirium: Diagnosis prevention and management. NICE
McManus J, Pathansali R, Stewart R, Macdonald A, Jackson S (2007) Delirium post-stroke. Age Ageing 36:613–618
Downs SH, Black N (1998) The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 52:377–384
Hatano S (1976) Experience from a Multicentre stroke register: a preliminary report. bulletin of the World Health Organisation; 54 Available at: URL: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2366492/. Accessed 15 Sept 2010
Whiting P, Rutjes AWS, Reitsma JB, Bossuyt PMM, Kleijnen J (2003) The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 3:25
Deeks J (2001) Systematic reviews of evaluations of diagnostic and screening tests. In: Egger M, Davey Smith G, Altman D (eds) Systematic reviews in health care: Meta-Analysis in Context. BMJ Publishing Group, London, pp 248–282
American Psychiatric Association (1980) Diagnostic and Statistical Manual of Mental Disorders DSM III, 3rd edn. American Psychiatric Association, Washington, DC
American Psychiatric Association (1987) Diagnostic and statistical manual of mental disorders: DSM-III-R. American Psychiatric Association, Washington, DC
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders: DSM IV. American Psychiatric Association, Washington, DC
American Psychiatric Association (2002) Diagnostic and statistical manual of mental disorders (DSM-IV-R), 4th edn. American Psychiatric Association Press, Washington DC
Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI (1990) Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 13:941–948
Trzepacz PT, Baker RW, Greenhouse J (1988) A symptom rating scale for delirium. Psychiatry Res 23:89–97
Gustafson L, Lindgren M, Westling B (1995) The OBS Scale—a factor analysis approach to evaluation of confusional states and other organic brain syndromes. Unpublished work available from: http://www.med.lu.se. Accessed 1 Jun 2011
Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Caeiro L, Ferro JM, Albuquerque R, Figueira ML (2004) Delirium in the first days of acute stroke. J Neurol 251:171–178
Dostovic Z, Smajlovic D, Sinanovic O, Vidovic M (2009) Duration of delirium in the acute stage of stroke. Acta Clin Croat 48:13–17
Sandberg O, Franklin KA, Bucht G, Gustafson Y (2001) Sleep apnea, delirium, depressed mood, cognition, and ADL ability after stroke. J Am Geriatr Soc 49:391–397
Sheng AZ, Shen Q, Cordato D, Zhang YY, Yin Chan DK (2006) Delirium within three days of stroke in a cohort of elderly patients. J Am Geriatr Soc 54:1192–1198
Dahl MH, Ronning OM, Thommessen B (2010) Delirium in acute stroke—prevalence and risk factors. Acta Neurol Scand 122(suppl 190):39–43
Fassbender K, Schmidt R, Mossner R, Daffertshofer M, Hennerici M (1994) Pattern of activation of the hypothalamic-pituitary-adrenal axis in acute stroke. Relation to acute confusional state, extent of brain damage, and clinical outcome. Stroke: J Cereb Circ 25:1105–1108
Gustafson Y, Olsson T, Asplund K, Hagg E (1993) Acute confusional state (Delirium) soon after stroke is associated with hypercortisolism. Cerebrovasc Dis 3:33–38
Gustafson Y, Olsson T, Eriksson S, Asplund K, Bucht G (1991) Acute confusional states (Delirium) in stroke patients. Cerebrovasc Dis 1:257–264
Caeiro L, Ferro M, Claro MI, Coelho J, Albuquerque R, Figueira ML (2004) Delirium in acute stroke: a preliminary study of the role of anticholinergic medications. Eur J Neurol 11:699–704
Caeiro L, Menger C, Ferro JM, Albuquerque R, Figueira ML (2005) Delirium in acute subarachnoid haemorrhage. Cerebrovasc Dis 19:31–38
Dunne JW, Leedman PJ, Edis RH (1986) Inobvious stroke: a cause of delirium and dementia. Aust N Z J Med 16:771–778
Shih HT, Huang WS, Liu CH, Tsai TC, Lu CT, Lu MK, Chen PK, Tseng CH, Jou SB, Tsai CH, Lee CC (2007) Confusion or delirium in patients with posterior cerebral arterial infarction. Acta Neurol Taiwanica 16:136–142
Nicolai A, Lazzarino LG (1994) Acute confusional states secondary to infarctions in the territory of the posterior cerebral artery in elderly patients. Ital J Neurol Sci 15:91–96
Mori E, Yamadori A (1987) Acute confusional state and acute agitated delirium: occurrence after infarction in the right middle cerebral artery territory. Arch Neurol 44:1139–1143
Marklund N, Peltonen M, Nilsson TK, Olsson T (2004) Low and high circulating cortisol levels predict mortality and cognitive dysfunction early after stroke. J Intern Med 256:15–21
McManus J, Pathansali R, Hassan H, Ouldred E, Cooper D, Stewart R, Macdonald A, Jackson S (2009) The course of delirium in acute stroke. Age Ageing 38:385–389
McManus J, Pathansali R, Hassan H, Ouldred E, Cooper D, Stewart R, Macdonald A, Jackson S (2009) The evaluation of delirium post-stroke. Int J Geriatr Psychiatry 24:1251–1256
Oldenbeuving AW, de Kort PLM, Jansen BPW, Kappelle LJ, Roks G (2008) A pilot study of rivastigmine in the treatment of delirium after stroke: a safe alternative. BMC Neurol 8:34
Bjorkelund KB, Larsson S, Gustafson L, Andersson E (2006) The organic brain syndrome (OBS) scale: a systematic review. Int J Geriatr Psychiatry 21:210–222
Sandberg O, Gustafson Y, Brannstrom B, Bucht G (1999) Clinical Profile of Delirium in Older Patients. J Am Geriatr Soc 47:1300–1306
Egger M, Davey Smith G, Schneider M (2001) Systematic reviews of observational studies. In: Egger M, Davey Smith G, Altman D (eds) Systematic reviews in health care meta analysis in context, 2nd edn. BMJ Publishing Group, London, pp 211–227
Schmidley JW, Messing RO (1984) Agitated confusional states in patients with right hemisphere infarctions. Stroke 15:883–885
Oldenbeuving AW, de Kort PLM, Jansen BPW, Roks G, Kappelle LJ (2007) Delirium in acute stroke: a review. Int J Stroke 2:270–275
White S, Bayer A (2007) Delirium: a clinical overview. Rev Clin Gerontol 17:45–62
Jensen E, Dehlin O, Gustafson L (1993) A comparison between three psychogeriatric rating scales. Int J Geriatr Psychiatry 8:215–229
Scottish Intercollegiate Guidelines Network (2002) SIGN 64: management of patients with stroke. Rehabilitation, prevention and management of complications, and discharge planning. A national clinical guideline. 1–51. Sign, Edinburgh
Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, Hart RP, Dittus R (2001) Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 286:2703–2710
Oldenbeuving AW, de Kort PLM, Jansen BPW, Algra A, Kappelle LJ, Roks G (2011) Delirium in the acute phase after stroke: incidence, risk factors, and outcome. Neurology 76:993–999
Tune LE, Egeli S (1999) Acetylcholine and delirium. Dement Geriatr Cogn Disord 10:342–344
Caplan LR (2010) Delirium: a neurologist’s view—the neurology of agitation and overactivity. Rev Neurol Dis 7:111–118
Burns A, Galllagley A, Byrne J (2004) Delirium. J Neurol Neurosurg Psychiatry 75:362–367
Acknowledgments
Ms Carin-Levy is a part time PhD student funded by Queen Margaret University, Edinburgh. No other funding has been received for this study. We are grateful to Prof. Marie Donaghy for her contribution during the development of this project and for her comments on manuscript drafts.
Conflicts of interest
There are no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Key words used in searches and their combinations:
Stroke: stroke; cerebrovascula/cereb.ral vascular + disorders/accident; cerebral/cerebellar/brain + infarct/ischemia/thrombo*/emoboli*; subarachnoid; brain attack
Delirium: delirium/deliri*; acute confusion/confusional state; acute + organic/psychoorganic + psycho/syndrome; acute brain syndrome; metabolic encephalopathy; clouded state; clouding of consciousness.
-
(1)
Stroke/post stroke/CVA
-
(2)
Cerebrovascula/cerebral vascular + disorders/accident/insult
-
(3)
Cerebral/cerebellar/brain + infarct/ischemia/thrombo*/emoboli*
-
(4)
Cerebral/brain/subarachnoid + haemorrhage/hemorrhage
-
(5)
Brain attack
-
(6)
Delirium/deliri*
-
(7)
Acute confusion/confusional state
-
(8)
Acute + organic/psychoorganic + psycho/syndrome
-
(9)
Acute brain syndrome
-
(10)
Metabolic encephalopathy
-
(11)
Clouded state
-
(12)
Clouding of consciousness
-
(13)
1 and 6–12
-
(14)
2 and 6–12
-
(15)
3 and 6–12
-
(16)
4 and 6–12
-
(17)
5 and 6–12
Rights and permissions
About this article
Cite this article
Carin-Levy, G., Mead, G.E., Nicol, K. et al. Delirium in acute stroke: screening tools, incidence rates and predictors: a systematic review. J Neurol 259, 1590–1599 (2012). https://doi.org/10.1007/s00415-011-6383-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-011-6383-4