Abstract
Standard neurology texts list a reduced blink rate as one of the clinical features of Parkinson’s disease. However, there are few clinical studies which have quantified this clinical sign. Here we present the results of a quantified study in a cohort of cases and controls using a standard protocol. Cases meeting standard criteria for a diagnosis of Parkinson’s disease were studied together with age- and sex-matched controls. Baseline data included age, sex, duration of disease, Hoehn and Yahr stage, mini-mental state examination and treatment. Subjects were videoed undertaking three different tasks: being interviewed, watching a video, and reading from a book. Blink rates were calculated as a mean ‘per minute’ figure for each of the three tasks. A meta-analysis of previous studies of blink rate was undertaken. A total of 20 cases and 41 controls were studied. A decline in blink rate with increasing age was seen for cases but not controls. A significant reduction in blink rate was seen in cases when compared with controls for each of the test conditions. Blink rates were highest in subjects when being interviewed and were lowest whilst reading a passage in both cases and controls. No effect of disease duration, severity or treatment was observed. We have quantified the reduction in blink rate which has long been recognised as a feature of Parkinson’s disease. We have identified factors which determine blink rate within individuals. We have also been able to define normal and abnormal levels for blink rate which may be of value clinically and for future research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease is a degenerative disorder primarily involving the substantia nigra which leads to progressive disability through a combination of tremor, bradykinesia, rigidity and postural instability [1]. It has been recognised for a long time that blink rate is reduced in Parkinson’s disease and it has been suggested that reduced blink rate can be used to aid diagnosis [2]. Blink rate has been incorporated into disease rating scales [3]. Whilst there are a number of studies which have looked at blink rate in diseased and healthy individuals [4–8] there is no consensus as to what represents a normal blink rate and what an appropriate ‘cut off’ to support a diagnosis of Parkinson’s disease might be.
Lesion studies and neuropharmacological experiments indicate a role for the paramedian pontine reticular formation, substantia nigra and superior colliculus with modulation from the cerebellum and occipital cortex through a combination of dopaminergic, cholinergic and GABAergic signals [9]. Dopamine acts to increase blink frequency whilst agonist effects of choline and GABA reduce blink rate.
Previous studies have indicated that blink rate can be affected by age [6], emotional state [10], dopaminergic treatment [11] and performance of mental tasks [12, 13]. Parkinson’s disease does not affect blink kinematics but does reduce blink amplitude [14].
The aim of this study was to examine factors influencing blink rate in Parkinson’s disease and determine threshold values for normal and abnormal blink rates. We have therefore undertaken a case–control study of blink rate under a variety of conditions in patients with Parkinson’s disease and age-matched controls. We have also undertaken a meta-analysis of previous studies of blink rate incorporating the current data.
Methods
Subjects
Cases were patients with Parkinson’s disease seen through neurology clinics at the Gold Coast Hospital. The diagnosis of Parkinson’s disease was confirmed using standard criteria [15]. Healthy controls matched for age (±5 years) and sex (two controls for each case) were selected at random from the patient register of a collocated general practice and were invited to participate by one of the investigators. Control subjects were excluded if they had any history of acute or chronic disabling neurological disease or other significant medical condition affecting their ability to comply with the study. The study was approved by the Griffith University Human Research Ethics Committee. All subjects gave written, informed consent.
Data collection
Basic demographic data (age and sex) were collected for all cases and controls. Cognitive assessment was undertaken for all cases and controls using the mini-mental state examination (MMSE) [16]. It was also noted if subjects were wearing glasses or not. Additional information collected in cases included duration of disease, drug treatment for Parkinson’s disease and dosages, and disease severity, measured using the Hoehn and Yahr staging of disease [17].
Test conditions and procedures
All subjects were filmed under three different test conditions for a minimum of 3 min and 20 s for each. The three test conditions were: (1) conversation with an interviewer, (2) watching a video, and (3) reading a book. The conversation involved questioning of the subjects hobbies and favourite activities or personal preferences, as well as general background. The video clip used was from “Travelling Birds—An Adventure in Flight”, directed by Jaques Perrin and the reading was from “White Oleander” by Janet Finch. Task order was assigned randomly for each subject. Digitally recorded video images focusing on the eyes were recorded for the duration of each task. Blinks were regarded as full blinks if at least 50% closure of the eye occurred, followed rapidly by reopening of the eye. Prolonged eye closure, often a voluntary action in response to deep concentration, was, therefore, excluded. Blinks resulting in less than 50% eye closure were counted separately but were not included in the overall blink rate. It was noted that in some subjects this occurs not infrequently upon re-fixation of the eye. There is conflicting evidence as to whether or not there is diurnal variation in blink rates with some studies suggesting an increase in the evening [18], whilst others have shown no such variation [7]. Therefore, all recordings were undertaken in the morning between 9 a.m. and 12 p.m. Patients were advised to take their usual medications. Previous studies have shown that dyskinesia associated with treatment for Parkinson’s disease can increase blink rate [2]. Patients were therefore not recorded if they displayed clinical evidence of dyskinesia.
Blink rates were later calculated by averaging blink frequencies over three separate 1 min time periods commencing no sooner than 20 s after the onset of the test condition and video recording. In most instances these time periods were sequential, but in some instances they were interrupted by external factors or technical difficulties with the video image. Blinks were counted later using a hand held click counter and timing was taken to the nearest 0.1 s using the digital video time signature. Counting blinks at a later time reduces the chances of a Hawthorne effect [19] which may have arisen from the noise of the click counter.
Statistical analysis
Blink rates are not normally distributed and, therefore, non-parametric tests have been used (Mann–Whitney U test and Wilcoxon matched pairs signed rank sum test). A Bonferroni correction for multiple testing was used for all principal p values quoted. P values < 0.05 were considered significant. Receiver operating characteristic (ROC) curves were utilised to determine appropriate cut offs for sensitivity and specificity estimation.
Meta-analysis
Studies of blink rate in Parkinson’s disease and control subjects were identified by searching PubMed and Medline databases using the terms blink rate and Parkinson’s disease. Cited articles of identified studies were also reviewed to identify additional potential studies. Articles were excluded if they were not case–control studies or did not include adequate data (mean and standard deviation for groups). Data have been combined in a forest plot using standard methodology for differences between means [20]. Heterogeneity was tested by calculating I2 [21].
Results
There were 20 subjects with Parkinson’s disease and 41 controls who met the inclusion and exclusion criteria and were enrolled in the study. Baseline demographic data for cases and controls is illustrated in Table 1. There were no statistically significant differences in baseline characteristics, although there were notably fewer males in the control group. The demographic and clinical characteristics of the cases are summarised in Table 2.
Comparisons of blink rate between cases and controls revealed a statistically significant difference for all three test conditions, with cases having lower blink rates than controls (Fig. 1). These differences were statistically significant for all test conditions (Table 3). There was considerable variation in blink rate between the three test conditions, with conversation having the highest blink rate and reading the lowest (p < 0.001 for all comparisons). The greatest difference between cases and controls was found for blink rates in conversation: the test condition most closely resembling a clinical consultation. The conversation condition has therefore been used for all further analyses. The mean blink rate (conversation) in cases was 18.0 per minute (range 0.7–52.3 per minute) compared with 34.4 per minute (range 12–70.3 per minute) for controls. This difference was statistically significant at a p value of < 0.0001.
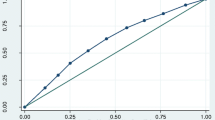
The initial meta-analysis search identified 29 publications of which five met the inclusion criteria, one further study was identified from cited publications. The calculated I2 (78%) suggested considerable heterogeneity and, therefore, a random effects model was used. It is likely that the heterogeneity reflects the differing test conditions applied in each of the studies with two using conversation [8, 22] and three using fixation on a point [4, 6, 7], whilst in one, no specific test conditions were stipulated [5]. Funnel plots of the conversation test condition studies (including the present data) and fixation studies showed no significant evidence of publication bias within each group (data not shown). Results of the meta-analysis (Fig. 2) show clear evidence of reduced blink rate in Parkinson’s disease compared with controls (p < 0.00001). Overall mean difference was −7.1 (95% CI: −9.9 to −4.3) showing reduced blink rates in subjects with Parkinson’s disease.
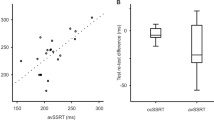
Amongst cases blink rate declined with age whilst in controls there was a slight upward trend (Fig. 3). Similarly there was a weak correlation between disease duration and reduced blink rate (data not shown). However, these correlations were not statistically significant. Overall, blink rate was not influenced by gender, disease severity (Hoehn and Yahr score), treatment, cognitive state or wearing of glasses.
ROC curve analysis suggested an optimal cut off score of 20 blinks per minute to distinguish cases from controls and yields a sensitivity of 65% and specificity of 83% which is good for a clinical diagnostic test [23, 24]. However, it should be noted that a significant number of apparently healthy controls had a blink rate which is within the ‘parkinsonian’ range and that a number of cases had scores which were well within the normal range for controls (Fig. 3). Among cases with blink rates above this threshold, no obvious trends in terms of disease duration, disease severity or treatment were evident. Reducing the cut off to 15 increased the specificity to 95% but reduced the sensitivity to 35%.
Discussion
We have conducted a case control study of blink rate in Parkinson’s disease finding reduced blink rate in cases under three test conditions. Our findings are statistically significant when adjusted for multiple comparisons and are consistent with previous studies. Meta-analysis of previous studies and our data confirms a significant difference in blink rate in a variety of testing conditions between subjects with Parkinson’s disease and healthy controls.
We propose a cut-off score for blink rate in conversation (20 blinks per minute) that has reasonable sensitivity (65%) and specificity (83%). Further validation of this finding is required in a larger cohort, preferably recently diagnosed or untreated cases. A lower cut off would be appropriate under different observation conditions or where higher specificity was required. It must be noted that there is considerable overlap in the range of blink rates for patients with Parkinson’s disease and controls. Thus, blink rate must be used in conjunction with other clinical features to improve the diagnostic accuracy of this clinical sign.
Previous studies have indicated that blink rate is reduced in other extra-pyramidal disorders [25] but not in other types of movement disorder [8]. Consequently blink rate is of little value in distinguishing between the various extra-pyramidal disorders.
The present data highlight the importance of the test conditions in determining blink rate, with a statistically significant and clinically meaningful hierarchy of states from conversation through watching a video to reading, which was associated with the lowest blink rate. This finding is consistent with previous studies in cases and controls [12, 26, 27]. It is, therefore, important that studies of blink rate take into account the testing conditions. Previous research has shown that spontaneous blinking is suppressed by tasks requiring visual attention such as reading and watching a video [13, 26]. Suppression of blinking occurs via the superior colliculus under the control of the blinking centre (globus pallidus) and the cortex [28]. The posterior parietal cortex appears to be particularly important for spontaneous blinking [29].
The mean blink rate in cases in conversation of 18.0 per minute is similar to values observed in previous studies [4, 5, 8]. We have found a small number of healthy controls who have blink rates in a similar range to patients with Parkinson’s disease and vice versa. Although numbers are small this overlap does not appear to be related to disease duration, treatment effects or severity of disease.
The finding of an inverse correlation between age and blink rate in cases was not surprising, but we did not expect to see a rise in blink rate with increasing age in controls. It is possible that this is a survival effect with only very healthy individuals surviving to ages >80 years or could reflect an age related degenerative change in the control of eye blinking. However, the number of controls over the age of 80 years in the present study was small and so no firm conclusions can be drawn. Previous studies have shown differing results with regards to the effect of age on blink rate in controls with one study showing a decline in blink rate with increasing age [6] and another suggesting an increase in blink rate with age, particularly for the conversation test condition [12].
We did not observe any effect of treatment for Parkinson’s disease but this is a complex area. Studies have shown an increase in the blink ‘habituation reflex’ (glabellar tap) with treatment [11] and an increased blink rate has been observed in advanced Parkinson’s disease as a form of ‘off’-period dystonia [30]. Reduction of blink rate has also been correlated with more severe disease [27] where treatment doses are likely to be higher.
In conclusion, we have confirmed the value of blink rate as part of the diagnostic algorithm in Parkinson’s disease and highlight the potential effects of differing test conditions. We have failed to find any clear correlation between blink rate and measures of disease severity, duration or treatment, suggesting that this may be a relatively early and fixed feature of the disease. However, the number of cases included in this study is relatively low and are insufficient to exclude subtle effects. From a clinical perspective, an observed blink rate of <20 per minute during an interview with a patient in someone presenting with appropriate symptoms and signs of Parkinsonism can be taken as an additional pointer towards a diagnosis of extra-pyramidal disease.
References
Parkinson J (1817) An essay on the shaking palsy. Sherwood, Neely and Jones, London
Karson CN, LeWitt PA, Calne DB, Wyatt RJ (1982) Blink rates in parkinsonism. Ann Neurol 12(6):580–583. doi:10.1002/ana.410120614
Goetz CG, Stebbins GT, Chmura TA, Fahn S, Poewe W, Tanner CM (2010) Teaching program for the Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale: (MDS-UPDRS). Mov Disord 25(9):1190–1194. doi:10.1002/mds.23096
Agostino R, Bologna M, Dinapoli L, Gregori B, Fabbrini G, Accornero N, Berardelli A (2008) Voluntary, spontaneous, and reflex blinking in Parkinson’s disease. Mov Disord 23(5):669–675. doi:10.1002/mds.21887
Biousse V, Skibell BC, Watts RL, Loupe DN, Drews-Botsch C, Newman NJ (2004) Ophthalmologic features of Parkinson’s disease. Neurology 62(2):177–180
Chen WH, Chiang TJ, Hsu MC, Liu JS (2003) The validity of eye blink rate in Chinese adults for the diagnosis of Parkinson’s disease. Clin Neurol Neurosurg 105(2):90–92. pii: S0303846702001075
Deuschl G, Goddemeier C (1998) Spontaneous and reflex activity of facial muscles in dystonia, Parkinson’s disease, and in normal subjects. J Neurol Neurosurg Psychiatry 64(3):320–324
Karson CN, Burns RS, LeWitt PA, Foster NL, Newman RP (1984) Blink rates and disorders of movement. Neurology 34(5):677–678
Karson CN (1989) Blinking. Bull Soc Belge Ophtalmol 237:443–457
Ponder E, Kennedy WP (1928) On the act of blinking. Quart J Exp Physiol 18:89–110
Penders CA, Delwaide PJ (1971) Blink reflex studies in patients with Parkinsonism before and during therapy. J Neurol Neurosurg Psychiatry 34(6):674–678
Bentivoglio AR, Bressman SB, Cassetta E, Carretta D, Tonali P, Albanese A (1997) Analysis of blink rate patterns in normal subjects. Mov Disord 12(6):1028–1034. doi:10.1002/mds.870120629
Fukuda K (1994) Analysis of eyeblink activity during discriminative tasks. Percept Mot Skills 79 (3 Pt 2):1599-1608
Korosec M, Zidar I, Reits D, Evinger C, Vanderwerf F (2006) Eyelid movements during blinking in patients with Parkinson’s disease. Mov Disord 21(8):1248–1251. doi:10.1002/mds.20930
de Rijk MC, Rocca WA, Anderson DW, Melcon MO, Breteler MM, Maraganore DM (1997) A population perspective on diagnostic criteria for Parkinson’s disease. Neurology 48(5):1277–1281
Folstein MF, Robins LN, Helzer JE (1983) The mini-mental state examination. Arch Gen Psychiatry 40(7):812
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17(5):427–442
Barbato G, Ficca G, Muscettola G, Fichele M, Beatrice M, Rinaldi F (2000) Diurnal variation in spontaneous eye-blink rate. Psychiatry Res 93(2):145–151. pii: S0165-1781(00)00108-6
Mayo E (1930) The human effect of mechanization. Am Econom Rev 20(Suppl 1):156–174
Hedges LV, Olkin I (1985) Statistical methods for meta-analysis. Academic Press, Orlando
Higgins JPT, Green S (eds) (2011) Cochrane handbook for systematic reviews of interventions Version 5.1.0 [updated March 2011]. Version 5.1.0 [Updated March 2011] edn. The Cochrane Collaboration. Available from www.cochrane-handbook.org
Karson CN, Bridge TP, Phelps BH, Wise CD, Potkin SG, Apostoles PS, Wyatt RJ (1982) The effect of oral glucose on platelet monoamine oxidase. Biol Psychiatry 17(9):1011–1015
Stephan F, Flahault A, Dieudonne N, Hollande J, Paillard F, Bonnet F (2001) Clinical evaluation of circulating blood volume in critically ill patients–contribution of a clinical scoring system. Br J Anaesth 86(6):754–762
Wallace DE, McGreal GT, O’Toole G, Holloway P, Wallace M, McDermott EW, Blake J (2000) The influence of experience and specialisation on the reliability of a common clinical sign. Ann R Coll Surg Engl 82(5):336–338
Pfaffenbach DD, Layton DD Jr, Kearns TP (1972) Ocular manifestations in progressive supranuclear palsy. Am J Ophthalmol 74(6):1179–1184
Hall A (1945) The origin and purposes of blinking. Br J Ophthalmol 29(9):445–467
Karson CN (1983) Spontaneous eye-blink rates and dopaminergic systems. Brain 106(Pt 3):643–653
Basso MA, Powers AS, Evinger C (1996) An explanation for reflex blink hyperexcitability in Parkinson’s disease. I. Superior colliculus. J Neurosci 16(22):7308–7317
Bodis-Wollner I, Bucher SF, Seelos KC (1999) Cortical activation patterns during voluntary blinks and voluntary saccades. Neurology 53(8):1800–1805
Kimber TE, Thompson PD (2000) Increased blink rate in advanced Parkinson’s disease: a form of ‘off’-period dystonia? Mov Disord 15(5):982–985
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fitzpatrick, E., Hohl, N., Silburn, P. et al. Case–control study of blink rate in Parkinson’s disease under different conditions. J Neurol 259, 739–744 (2012). https://doi.org/10.1007/s00415-011-6261-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-011-6261-0