Abstract
Fragile X Syndrome (FraX) is the most common inherited cause of learning disability worldwide. FraX is an X-linked neuro-developmental disorder involving an unstable trinucleotide repeat expansion of cytosine guanine guanine (CGG). Individuals with the full mutation of FraX have >200 CGG repeats with premutation carriers having 55–200 CGG repeats. A wide spectrum of physical, behavioural, cognitive, psychiatric and medical problems have been associated with both full mutation and premutation carriers of FraX. In this review, we detail the clinical profile and examine the aetiology, epidemiology, neuropathology, neuroimaging findings and possible management strategies for individuals with both the full mutation and premutation of FraX.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fragile X syndrome (FraX) is the most common heritable form of intellectual disability (learning disability) worldwide. It is an X-linked neurodevelopmental disorder with recent data suggesting that FraX affects approximately 1 in 2,500 individuals [1, 2] with approximately equal rates in males and females [3]. The prevalence of the premutation carrier state is significantly higher with estimates of up to 1 in 251 males [1] and 1 in 100 females noted [4].
Significant variation exists in relation to prevalence data, however, with figures from Israel finding the frequency of the premutation carrier state in females to be present in approximately 1 in 130, with the full mutation present in 1 in 2,500 females [3–5]; data from Canada, noting the prevalence of premutation carriers to be 1 in 800 males and 1 in 260 females [6, 7] and data from Taiwan finding that the frequency of premutation male carriers was much lower, at approximately 1 in 1,670 [8].
As FraX is an X-linked neurodevelopmental disorder, females, due to the presence of one normal allele, have a reduction, but not a complete absence of FMRP, resulting in a less severe physical, cognitive and behavioural phenotype. The levels of FMRP in females with FraX are related to lyonisation, or the X activation ratio (one of two X chromosomes is randomly inactivated with a consequent variation in the proportion of active X chromosomes that have an affected allele) [9–11]. Individuals with the full mutation of FraX have a characteristic physical, cognitive and psychological profile, whilst some individuals who are premutation carriers may exhibit some of these features, albeit to a lesser degree.
FraX involves an unstable trinucleotide repeat expansion of cytosine guanine guanine (CGG) in the 5′ promoter end (Xq27.3) of the fragile X mental retardation 1 gene (FMR1) [12]. Individuals with the full mutation of FraX have >200 CGG repeats with premutation carriers having 55–200 CGG repeats. In the vast majority of cases, the CGG expansion of the FMR1 gene is accompanied by methylation of the FMR1 gene and loss of FMR1 protein (FMRP) production [12, 13]. Absence of FMRP has primarily been associated with abnormal maturation of synaptic connectivity, which is argued to be the primary cause of the cognitive deficits frequently observed in FraX [14]. Approximately 15% of individuals with FraX display a mosaic pattern consisting of both premutation and full mutation alleles [15].
Clinical presentation
Full mutation FraX males (Table 1)
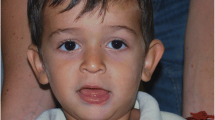
Individuals with FraX are classically characterized by cognitive and behavioural difficulties, facial dysmorphism, connective tissue anomalies and macro-orchidism. Macro-orchidism, although not specific for FraX, is the most consistent finding, present in 90% of boys by age 14 [16].
The physical phenotype of FraX males comprises a broad forehead, a long narrow face, large prominent ears, a high-arched palate, mitral valve prolapse, dermatoglyphic abnormalities including hyper-extendible finger joints, double-jointed thumbs, a single palmer crease and hand calluses and, as mentioned above, macro-orchidism [15–19]. The most common medical condition is epilepsy, which occurs in approximately 20% of individuals with FraX [20]; however several other medical conditions can also occur including recurrent otitis media and strabismus, which require treatment to prevent hearing impairments and amblyopia [21].
Frequent behavioural characteristics in FraX males include poor eye contact, hand flapping, tactile defensiveness, impulsivity and a resistance to environmental change [22]. Indeed, individuals with FraX frequently demonstrate a need for sameness and often over-react (including aggressively) to novelty [23]. Gaze aversion, anxiety, hyperactivity, and social-interaction deficits are other common behavioural characteristics found in individuals with FraX [24]. Autism spectrum disorders (ASD) are significantly over-represented in individuals with FraX with 25–47% of individuals fulfilling diagnostic criteria [25–28], with this rate further increased when a diagnosis of pervasive developmental disorder not otherwise specified (PDDNOS) is included [29]. In many cases individuals with FraX display qualitative differences in autistic symptoms and behaviour compared to people with ASD alone [30]. For example, individuals with both FraX and co-morbid ASD demonstrate social interaction patterns suggestive of social aversion rather than a lack of interest in the social environment more typical of ASD [31], however individuals with FraX and co-morbid ASD may be indistinguishable from idiopathic ASD and may show a similar social disinterest.
Other psychological difficulties noted in FraX males include mood instability and aggression [32, 33]. Furthermore, anxiety, shyness and even mutism have been described in children with FraX [34] with 86% fulfilling diagnostic criteria for an anxiety disorder, social phobia and specific phobia being the most commonly diagnosed [35]. Attention deficit hyperactivity disorder (ADHD) is also over-represented in individuals with FraX [36].
The cognitive phenotype of males with FraX includes a moderate to severe intellectual disability, although approximately 11% have an intelligence quotient (IQ) in the mild intellectual disability range [37]; deficits in executive function, abstract reasoning and short-term memory (particularly verbal short-term memory); difficulties with attentional control and arithmetic and poor visuo-spatial processing [14, 24, 38–40]. Tasks requiring short-term memory for complex sequential information are particularly problematic for FraX males [39]. However, individuals with FraX perform relatively well on measures of visuo-perceptual recognition, constructional ability and vocabulary [38, 41], and perform competently at tasks that require short-term memory for simple, meaningful information [42]. Furthermore, the patterns of cognitive deficits for individuals with FraX are different from those of other IQ matched intellectually disabled individuals. For example, an IQ matched Down Syndrome group of boys demonstrated greater attentional control during tasks involving selective attention, divided attention and executive functioning [43].
In summary, FraX males display a relative strength for learning simple verbal and non-verbal tasks and can recall simple meaningful information, but display significant impairments on tasks which require the manipulation of internal representations or the retention of abstract non-sequential information [44, 45].
Full mutation FraX females (Table 1)
In females, as discussed in the introduction, there is a reduction but not a complete absence of FMRP. These lower levels of FMRP typically result in a less severe physical, cognitive and behavioural phenotypes in girls and women. However, some females have severe impairments that are equivalent to those seen in males.
The physical phenotype in females is milder than that in males, however many of the same physical symptoms have been described, including a high arched palate, prominent ears, a long narrow face, genu valgum and flat feet [46]. These findings are more prevalent in those with an intellectual disability [47]. Several other more rare findings include hyper-extendible finger joints, double-jointed thumbs, cleft palate, precocious puberty and connective tissue dysplasia [15, 47–49]. Some females present with no physical abnormalities but can have significant psychiatric, cognitive and executive function deficits.
Cognitive deficits, whilst less common in females with FraX, have been noted, with executive function deficits most prominent [50]. Approximately 25% of females with FraX have an intellectual disability (an IQ < 70); however, most females have an IQ in the borderline to low-normal range (IQ 70–90) [48]. IQ and other cognitive deficits in females with FraX have been shown to correlate with levels of FMRP [51–54]. Deficits in attention and increased rates of ADHD have also been reported [55].
Females with FraX have several behavioural problems with depression, the most common of these, with up to 50% of individuals having been shown to suffer either from depression or dysthymia [56, 57]. Anxiety disorders are also common and have been reported to occur in up to 77% of females with FraX [32]. Other difficulties include shyness, social anxiety, specific phobias, impulsivity and schizotypal features, which present as a pattern of interpersonal socialization deficits such as excessive social anxiety, odd behaviour, odd speech and inappropriate affect [32, 58, 59].
Individuals with premutation FraX (males and females) (Table 1)
Some male premutation carriers of FraX exhibit subtle facial characteristics similar to individuals with full mutation FraX [60, 61] and display a wide range of subtle executive function, memory, and language deficits compared to healthy controls, although there are no significant differences in IQ compared to the general population [62]. Most premutation carriers however have no physical features of FraX and thus appear normal. Other difficulties noted include increased obsessionality and alcohol and drug misuse or dependence [63]. Male premutation carriers have been reported to have an increased prevalence of intellectual disability, ADHD, and ASD [64–68]. Schizotypal personality features and avoidant personality disorders have also been noted [69].
Female premutation carriers of FraX usually have no facial features of FraX though some demonstrate a mild form of the physical phenotype of FraX [50, 70]. Increased emotional problems [71], with high rates of major depressive disorder [57, 69], and some anxiety disorders, in particular panic disorder and agoraphobia without panic disorder are present in FMR1 premutation females [32, 57]. However, some anxiety disorders, including social phobia, specific phobias and post-traumatic disorders actually have been reported to not be increased in premutation female carriers of FraX in some but not all studies [57, 72]. Premutation carrier females of FraX have an increased risk of chronic muscle pain and thyroid disease—particularly hypothyroidism, which is associated with increased symptoms of depression and anxiety [73–75]. Elevated levels of follicle stimulating hormone are common [76], and ovarian insufficiency occurs in approximately 20% of individuals [77].
Two distinct adult onset medical disorders have been noted in premutation carriers of FraX: fragile X-associated tremor ataxia syndrome (FXTAS) and primary ovarian insufficiency (POI).
FXTAS is a progressive neurodegenerative disorder characterized by late-onset progressive cerebellar ataxia, intention tremor and cognitive decline in elderly male and female premutation carriers of FraX [78–80]. Other neurological findings that may occur with FXTAS include short-term memory loss, dementia, peripheral neuropathy, lower limb proximal muscle weakness, and autonomic dysfunction. FXTAS occurs in a subgroup of patients, with the prevalence of FXTAS estimated at 40–45% of males and 8–16% of females over 50 years of age with penetrance age-related [73, 78, 79, 81]. Whilst an increase in impulsivity and executive function deficits has been reported in male premutation carriers without FXTAS [82], no cognitive deficits have been observed in premutation carriers under 50 years of age [83].
Primary ovarian insufficiency (POI) is defined as the cessation of menses before the age of 40 and occurs in approximately 20% of premutation females with FraX [77, 84] compared to 1% of the general population [85]. POI is characterized by loss of oocytes, lack of folliculogenesis, reduced ovarian oestrogen production, elevated serum gonadotropin levels, amenorrhoea, and infertility in women before the age of 40.
In contrast to premutation carriers, women who carry full mutations do not have POI. The absence of ovarian dysfunction in full mutation females suggests that the lack of FMRP is not the cause of POI. Rather, it has been demonstrated that premutation carriers have an increased amount of mRNA in lymphocytes and neurons, but a normal quantity of FMRP. This combination of relatively high levels of an abnormal mRNA (which could trap some CGG binding proteins) and decreased levels of FMRP [4], may cause toxicity at blood lymphocytes, granulosa cells and the ovum [86], and consequently result in POI. Co-segregation of the premutation carriers of FraX and POI was first described in 1991.
Aetiology
Genetics
Several studies conducted since 1890 noted a 20–30% increased prevalence of males compared to females in institutions or schools for individuals with mental retardation (intellectual disability) [87]. This observation, and descriptions of families with an apparent X-linked inheritance of mental retardation, led to the suggestion in the late 1960s that mutations in genes on the X chromosome may be a significant factor accounting for this male predominance in intellectual disability. In 1969, Lubs [88] described a family with four “mentally retarded” males over three generations, all of whom showed a curious anomaly of the X chromosome upon cytogenetic examination, then termed marker X. After 1977, when the “fragile site” became more efficiently detectable under special conditions of karyotyping (culture of lymphocytes in low folate medium or in the presence of antifolates), FraX became increasingly recognized [89].
Fragile X mutations are unstable expansions of a CGG trinucleotide repeat, located in the first exon (non protein-coding) of the FMR1 gene. Several disabling neuro-psychiatric and neurological conditions result from similar expanded trinucleotide repeats including Huntington’s disease, myotonic dystrophy, Freidreich ataxia, spinal palsy and bulbar palsy. The full mutation inactivates the expression of the FMR1 gene, leading to the absence of FMRP [12, 13, 90]. Premutation carriers of FraX (expansions of 55–200 CGG repeats) have either normal levels or mild deficits in FMRP. Individuals with FraX may show a mosaic pattern: a mixture of premutation and full mutation alleles, most commonly caused by somatic instability of the full mutation in early embryogenesis leading to a retraction of the expanded CGG repeat [90]. This mosaic pattern occurs because the CGG repeat number expands to greater than 200 repeats in some cells (full mutation), whereas in other cells the repeat number fails to increase in size to 200 CGG repeats (premutation alleles). This mosaic pattern of FraX occurs in approximately 15% of cases [90], although figures as high as 40% have been noted [91]. A more rare type of mosaicism is “methylation mosaicism” and is associated with individuals having >200 CGG repeats (full mutation), but with incomplete methylation of the FMR1 gene and thus a reduced amount of FMRP. Therefore, mosaic mutations allow the expression of some FMRP (usually at low levels), and in some cases have been associated with lesser degrees of intellectual disability [90].
The transition from premutation carrier status of FraX to the full mutation occurs through maternal transmission of the abnormal allele, with a probability (up to 100%) depending on the size of the premutation, with the smallest premutation alleles observed to undergo transition to the full mutation in the next generation recorded at 56 CGGs repeats [92]. It has been shown that an adenosine guanine guanine (AGG) interspersion stabilizes the repeats as instability of the FMR1 gene is due to the length of uninterrupted CGGs. However, to our knowledge, technical difficulties have precluded the determination of this pattern of AGG interspersion in a diagnostic setting.
The FMR1 gene codes for the cytoplasmic protein FMRP, which has RNA-binding properties. The FMR1 gene spans approximately 40 kilobases (kb) of DNA and the protein encompasses 632 amino-acids, although several shorter forms have been observed in vivo as a result of alternative splicing of the 17 exons present [93, 94]. FMRP is abundant in neurons, particularly those in the cerebral cortex, cerebellum and hippocampus [94, 95], and is also present in other tissues, including spermatogonia and various epithelial tissues. FMRP has been detected in polyribosomes, particularly in the dendrites and contains functional domains allowing its transfer between the nucleus and the cytoplasm [96], and has been demonstrated to be involved in the shuttling of RNAs from the nucleus to the cytoplasm [97]. FMRP contains four RNA binding domains and binds to several mRNAs including its own mRNA [98]. FMRP associates with mRNAs and other proteins to form large messenger ribonucleoprotein (mRNP) complexes. These complexes are believed to participate in the transport, localization and translation of target mRNAs [99, 100]. The proteins comprising these mRNP complexes are largely unknown. However, certain candidate proteins exist, such as the autosomal homologs of FMRP, namely, the fragile X related proteins; fragile X mental retardation syndrome-related protein 1 (FXR1) and fragile X mental retardation syndrome-related protein 2 encoded by the FXR1 and FXR2 genes, respectively, and nucleolin, a protein very abundant in the nucleolus but also present in the cytoplasm [100]. All three proteins can shuttle RNAs between the nucleolus and cytoplasm. The FXR1 and FXR2 proteins have a high sequence similarity to FMRP, include similar functional domains identified in FMRP, such as RNA binding domains, and show a similar tissue distribution to that of FMRP [101]. Studies on FXR2 knockout mice demonstrate similar behavioural phenotypes to those in FMR1 knockout mice, implicating a similar role for FXR2 in central nervous system function [101].
FMRP has also been suggested to be involved in synaptic plasticity. Synaptic plasticity adjusts the strength of synapses during global changes in neural activity, thereby stabilizing the overall activity of neural networks. The mechanisms for FMRP’s putative involvement in synaptic plasticity include its regulation of mRNA translation and its effect on matrix metallo-proteinase-9 activity (MMP-9). The absence of FMRP in FraX is associated with a reduction in translation of mRNAs related to synaptic plasticity, in particular those specific dendritic mRNAs which encode cytoskeletal proteins and signal transduction molecules [102], and an increase in MMP-9 in the synapse [103]. MMP-9 is important for synaptic structure and plasticity. As will be described below, minocycline inhibits MMP-9 activity, and thus may potentially be a treatment for FraX.
Recent studies in Drosophila suggest that mRNA transport and translation are not only limited to dendrites but are also associated with axonal growth [93]. Alterations in the regulation of axonal growth and innervation in FMR1 neurons may contribute to the dendritic and spine pathology characteristic of FraX [104].
Neurotransmitters/neuropeptides
In a genome-wide expression profiling study in fragile X knockout mice, 3 complementary DNAs (cDNAs) were found to be differentially expressed: GABA-A receptor subunit δ, Rho guanine exchange factor 12 and expressed sequence tag BU563433. Of these, the δ subunit of the GABA-A receptor has been postulated to have a putative role in the cognitive and behavioural phenotype of FraX [105]. GABA-A receptors are the predominant inhibitory receptors in the brain and have been implicated in anxiety, depression, epilepsy, sleep patterns, learning and memory, all of which are affected to varying degrees in FraX [106]. Mouse and fly models of FraX have demonstrated a reduced expression of GABA-A receptors [107, 108], however no human studies (including neuropathology or PET imaging studies) have corroborated these findings to our knowledge to date.
Other amino acids, including glutamate (Glu) and N-acetyl aspartate (NAA) have also been implicated in the aetiology of FraX [109–112], with recent animal research particularly focusing on metabotrophic glutamate receptors (mGluR). We also look at evidence implicating matrix metallopeptidase 9 (MMP-9) with FraX.
FMRP is an RNA binding protein which modulates dendritic maturation and synaptic plasticity. One of the mechanisms postulated for this effect is its inhibition of mGluR1 and mGluR5 mediated mRNA translation in dendrites [113, 114]. Loss of FMRP may have several effects including long-term depression (LTD) of transmission at hippocampal synapses [115], which is associated with activity-guided synaptic elimination [116]. It has been suggested that neurological and psychiatric symptoms associated with FraX may be a consequence of an exaggerated responses to mGluR activation due to an absence of FMRP [115].
Mouse models have examined the mGluR5 antagonist 2-methyl-6-phenylethynyl-pyridine (MPEP). MPEP has been shown to block aberrant phenotypes in the FMR1 mouse model of fragile X and has effectively reversed several phenotypes, including hyperactivity, seizures and pre-pulse inhibition deficits, and have shown remarkable improvements in synaptic plasticity and spine morphology [117]. Repetitive behaviours common in ASD, but also present in FraX, may also be ameliorated with MPEP [117]. A recent human study investigated AFQ056, a receptor subtype-selective inhibitor of mGluR5, in 30 male individuals with FraX and noted an improvement in behavioural symptoms of FraX as measured by the Aberrant Behaviour Checklist-Community Edition (ABC-C) [118]. Another putative treatment for FraX via this mechanism is Fenobam, a high potency selective mGluR5 antagonist. Fenobam was previously investigated as an anxiolytic in a number of phase II studies in the early 1980s. However, a number of subjects suffered neurological and psychiatric symptoms including vertigo, paraethesias, hallucinations and insomnia [119, 120]. A more recent open label, single dose study demonstrated no such adverse effects [121].
As described above, the absence of FMRP has been associated with higher levels of matrix MMP-9 in the brain [122]. Minocycline, a broad spectrum tetracycline antibiotic, inhibits MMP-9 activity, and in FMR1 knockout mice has been shown to alleviate both synaptic and behavioural abnormalities [122]. An open-label add-on pilot trial evaluating the safety and efficacy of minocycline in treating behavioural abnormalities in humans with FraX demonstrated functional benefits to individuals with FraX, including an improvement in language and behaviours, and was also well-tolerated, with loss of appetite the only significant adverse effect noted [123]. These initial findings are consistent with the FMR1 knockout mouse model findings, suggesting that minocycline may modify underlying neural defects, which could account for some of the behavioural abnormalities found in individuals with FraX [122].
Neuropathology findings
Post-mortem studies in people with FraX have reported dendritic spines abnormalities; characterized by spines that are longer, thinner and more tortuous in shape and lacking the typical “mushroom shape” associated with mature dendritic spines [124–127]. While dendritic spine anomalies are present in several neuropsychiatric conditions associated with intellectual disability, they are increased in density in FraX, which appears to be unique to this condition [128]. FMRP has been suggested to be involved in dendritic maturation and this is supported by reports that FMR1 knockout mice have a significant decrease in the number and function of hippocampal neuronal synapses [127, 129, 130]. As described above, the dendritic spine dysgenesis found in FraX is typical of the morphology of the “immature brain” prior to synaptic elimination.
Eosinophilic, ubiquitin-positive inclusion bodies are the principal neuropathological finding in FXTAS and are located in the nuclei of neurons and astrocytes throughout the brain and the spinal column [131]. These inclusions are tau-negative and alpha-synuclein negative and contain FMR1 mRNA [132].
Further neuropathology findings in individuals with FXTAS include a patchy loss of axons throughout the brain, spongiosis of the middle cerebellar peduncles and loss of purkinje cells [131, 133].
Neuroimaging findings
Full mutation FraX
Structural magnetic resonance imaging (MRI) studies have noted several morphological differences in brain structure in individuals with FraX compared to healthy controls. The most replicated findings to date include increased volume of the caudate nucleus [134–140] and reduced volume of the cerebellum [136, 141, 142] compared to both healthy comparison groups and those with ASD. Other findings noted in some studies include increased volume of the lateral ventricles [135, 143, 144]; hippocampus [145–147]; parietal lobes [136, 139], and brainstem [139] and reduced volume of the cerebellar vermis [141].
The caudate nuclei are involved with regulation of impulse control and attention, and these neuroimaging findings may help explain the impulse control and attentional deficits frequently reported in individuals with FraX [33, 148]. This proposal has been supported by a recent report of altered fMRI activation in the right caudate of individuals with FraX engaging in an attention (go–no go) task [149]. The findings of cerebellar abnormalities may also explain some of the cognitive phenotype found in individuals with FraX.
The cerebellum is important in many higher order functions commonly impaired in individuals with FraX—e.g. attention [150], social interaction [151] and executive functioning [152] and has been found to be reduced in volume in ASD [153], which as mentioned above is increased in prevalence in individuals with FraX [24].
Premutations carriers of FraX
Most neuroimaging studies in premutation carriers of FraX are in individuals who have also been diagnosed with FXTAS. Studies in FXTAS have demonstrated reduced cerebral and cerebellar volume (males > females) compared to controls, with particular volume reductions in the middle cerebellar peduncles, the caudate nucleus and the parietal lobes [62, 153–156]. These regions have been implicated in motor and cognitive functions including co-ordination and attention [78], frequent difficulties in individuals with FXTAS. In premutation carriers of FraX without FXTAS, reduced volume in a number of regions including the amygdala–hippocampal complex bilaterally [62], the left hippocampus [156] and the left thalamus has been documented [62, 157], with a negative correlation between total hippocampal volume and anxiety in female carriers also reported [158, 159]. No difference in hippocampal or amygdala volumes between premutation carriers and normal controls has however also been observed [157, 158]. Although some studies have noted increased volume of the hippocampus in FraX individuals [145–147] and reduced hippocampal volume in premutation carriers of FraX [62, 157]; one study noted no difference between premutation and full mutation FraX individuals in relation to hippocampal volume [147].
Abnormalities in the hippocampus and amygdala have been suggested by findings in a number of fMRI studies in premutation carriers of FraX without FXTAS. Reduced hippocampal activation during a memory recall task [157] and reduced amygdala activation during a perceptual task when viewing fearful faces [158] have been observed.
Management
Screening
Screening individuals for any neuro-psychiatric disorder is a sensitive issue; however, given the recent evidence of the high frequency of FraX (both full mutation and pre-mutation carriers) in the population, it perhaps should be considered [14]. For example, an anonymous population screening, performed in Canada in 1995 on 10,624 females led to the detection of 41 previously undiagnosed premutation carriers of FraX (an incidence of 1 in 250) [6]. FraX is detected using DNA analysis: standardized Southern blot and polymerase chain reaction (PCR) analyses are performed followed by FMR1 specific probe hybridization [160]. The CGG repeat number is calculated from the Southern blot autoradiogram images. FMRP levels can be ascertained by calculating the percentage of peripheral lymphocytes containing FMRP using immuno-staining techniques [161]. More recently, a highly sensitive and specific enzyme-linked immunosorbent assay (ELISA) has also been developed to measuring FMRP levels in peripheral blood lymphocytes [162].
Prenatal diagnosis can be performed to determine if a foetus has inherited the full mutation; once a premutation or full mutation carrier of FraX has been identified in the mother, although such testing should only be performed in conjunction with appropriate counselling for the family concerned. The sensitivity of prenatal testing is approximately 99%, although in very rare cases, FraX may result from point mutations, deletions in the FMR1 FRAX-A gene or go undetected due to mosaicism.
Screening for FMR1 mutations has been a topic of consideration since the FMR1 gene was first identified. Advances in our understanding of the molecular basis of FraX and advances in genetic testing methods have elicited new prospects for identifying a greater number of individuals at risk for the disorder or at risk of transmitting the disorder [163]. McConkie-Rosell et al. [163] have suggested that individuals suitable for screening include children, grandchildren, or siblings with intellectual disability, autism, and social/behavioral, or learning disorders; daughters or female relatives with infertility, premature menopause, or both; and family members with tremor, ataxia or other neurological (neuropathy, multiple sclerosis), and/or psychiatric problems (anxiety disorders, depression, dementia, cognitive decline). This, however, is a very wide grouping of individuals, although there does appear to be merit in screening some of these individuals at least. We believe that there are two general types of circumstance in which fragile X testing should be considered: a clinical presentation suggestive of fragile X syndrome, including FXTAS or POI, or where there is a risk of inheritance of FraX due to a family history of FraX or intellectual disability of unknown cause.
In relation to clinical presentation; genetic testing for fragile X should be considered in children with developmental delay including specific speech, language or motor delay, children with a diagnosis of intellectual disability of unknown aetiology and ASD. Testing children with borderline cognitive deficits leading to a diagnosis of FraX can be used to improve educational strategies for the child and help their families better understand their child’s’ difficulties [15]. Individuals over 50 years old with a recent onset of tremors, balance disorders, or Parkinsonian-like findings without a diagnosis would also appear to be a group that should be tested for FXTAS. Similarly, we believe that women with unexplained infertility or POI should also be considered for testing [164].
Screening should also be considered in individuals with a family history of fragile X to determine if they may be carriers and at risk of transmitting it to future generations, and in individuals with a family history of mental retardation or autism of unknown cause.
The recent findings of significant phenotypes in premutation carriers of FraX have significant consequences for genetic counselling. Furthermore, in females, unlike males who are premutation carriers of FraX, the number of CGG repeats can increase to a full mutation when passed on to offspring.
Further epidemiological studies are required to better estimate fragile X allele frequencies for all racial and ethnic groups, and greater knowledge is needed regarding the penetrance of FMR1 associated disorders, FXTAS and fragile X-associated POI, in order to provide anticipatory guidance and to assist with the development of genetic counseling protocols [163].
Pharmacotherapy (see Table 2)
There are no pharmacological treatments presently available that ameliorate the cognitive deficits in FraX. However, a variety of agents have been utilized for the behavioural and psychological difficulties, although a paucity of controlled studies exist that formally measure their effectiveness [165]. Of those that are present, methylphenidate, dexamphetamine and l-acetylcarnitine have demonstrated some benefit for attention and behavioural difficulties [166, 167]. Anticonvulsants used to treat seizures may also improve autistic features, mood instability and tantrums [79], whilst antidepressant agents such as selective serotonin re-uptake inhibitors (SSRIs) may improve depression and anxiety disorders.
Future pharmaco-therapeutic strategies for FraX may focus on GABA and Glu, with evidence that the mGluR5 antagonist, 2-methyl-6-phenylethynyl-pyridine (MPEP), abolishes the audiogenic seizure phenotype in FMR1 knock-out mice [168], and decreases the mushroom body defects (fused β-lobes) [169]. These findings have been replicated in multiple animal models and with many phenotypes and have led to several human phase II trials that are on-going. As discussed above, a recent study investigated AFQ056, a receptor subtype-selective inhibitor of mGluR5, in 30 male individuals with FraX noted an improvement in behavioural symptoms of FraX [118]. Fenobam, a high potency selective mGluR5 antagonist is a further putative treatment option given recent evidence of a good safety profile [121]. Furthermore, minocycline, the broad spectrum tetracycline antibiotic, has also shown some promise in an initial open-label study for improving behavioural difficulties in individuals with FraX [123].
Psychological/environmental approaches
Individuals with FraX have shown benefits from non-pharmacological interventions such as speech, occupational and sensory integration therapies. Research in FMR1 knock-out mice has shown that an enriched environment can rescue many behavioural and neuronal abnormalities [170]. This suggests that early and intensive psychological and environmental interventions may substantially benefit the development of an individual with FraX.
Conclusions
FraX is a common genetic disorder resulting from a single-gene mutation on the X chromosome and is associated with a wide spectrum of physical, behavioural, cognitive, psychiatric and medical problems, with males more severely affected than females. Over the last decade our understanding of FraX has considerably increased, with conditions such as FXTAS and POI now known to affect premutation carriers of the condition. Early diagnosis of FraX is important to allow the introduction of appropriate educational and clinical interventions. Whilst there are few controlled trials to guide management to date, several medications can ameliorate medical, psychiatric and behavioural difficulties associated with FraX and can improve an individual’s quality of life. Future treatments should possibly be aimed at targeting specific synaptic mechanisms affected in FraX.
References
Fernandez-Carvajal I, Walichiewicz P, Xiaosen X et al (2009) Screening for expanded alleles of the FMR1 gene in blood spots from newborn males in a Spanish population. J Mol Diagn 11:324–328
Crawford DC, Meadows KL, Newman JL et al (2002) Prevalence of the fragile X syndrome in African Americans. Am J Med Genet 110:226–233
Pesso R, Berkenstadt M, Cuckle H et al (2000) Screening for fragile X syndrome in women of reproductive age. Prenat Diagn 20:611–614
Hagerman R, Hagerman P (2002) The fragile X premutation: into the phenotypic fold. Curr Opin Genet Dev 12:278–283
Toledano-Alhadef H, Basel-Vanagaite L, Magal N et al (2001) Fragile-X carrier screening and the prevalence of premutation and full-mutation carriers in Israel. Am J Hum Genet 69:351–360
Rousseau F, Rouillard P, Morel ML, Khandjian EW, Morgan K (1995) Prevalence of carriers of premutation-size alleles of the FMR1 gene—and implications for the population genetics of the fragile X syndrome. Am J Hum Genet 57:1006–1018
Dombrowski C, Lévesque S, Morel ML, Rouillard P, Morgan K, Rousseau F (2002) Premutation and intermediate-size FMR1 alleles in 10572 males from the general population: loss of an AGG interruption is a late event in the generation of fragile X syndrome alleles. Hum Mol Genet 11:371–378
Tzeng C, Tsai L, Hwu W et al (2005) Prevalence of the FMR1 mutation in Taiwan assessed by large-scale screening of newborn boys and analysis of DXS548 FRAXAC1 haplotype. Am J Med Genet A 133:37–43
Grigsby JP, Kemper MB, Hagerman RJ, Myers CS (1990) Neuropsychological dysfunction among affected heterozygous fragile X females. Am J Med Genet 35:28–35
Grigsby J, Kemper MB, Hagerman RJ (1992) Verbal learning and memory among heterozygous fragile X females. Am J Med Genet 43:111–115
Mazzocco MM, Pennington BF, Hagerman RJ (1993) The neurocognitive phenotype of female carriers of fragile X: additional evidence for specificity. J Dev Behav Pediatr 14:328–335
Verkerk AJMH, Pieretti M, Sutcliffe JS et al (1991) Identification of a gene (FMR1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65:905–914
Yu S, Pritchard M, Kremer E et al (1991) Fragile-X genotype characterized by an unstable region of DNA. Science 252:1179–1181
Van der Molen M, Huizinga M, Huizenga HM et al (2010) Profiling fragile X syndrome in males: strengths and weaknesses in cognitive abilities. Res Dev Disabil 31:426–439
Mandel JL, Biancalana V (2004) Fragile X mental retardation syndrome: from pathogenesis to diagnostic issues. Growth Horm IGF Res 14(Suppl A):S158–S165
Hagerman R, Hagerman P (eds) (2002) Fragile X syndrome: diagnosis, treatment and research, 3rd edn. John Hopkins University Press, Baltimore, pp 3–109
Meryash DL, Cronk CE, Sachs B, Gerald PS (1984) An anthropometric study of males with the fragile-X syndrome. Am J Med Genet 17:159–174
Hagerman RJ (2002) Physical and behavioural phenotype. In: Hagerman RJ, Hagerman P (eds) Fragile X syndrome: diagnosis, treatment and research, 3rd edn. The Johns Hopkins University Press, Baltimore, pp 3–109
Chonchaiya W, Schneider A, Hagerman RJ (2009) Fragile X: a family of disorders. Adv Pediatr 56:165–186
Berry-Kravis E (2002) Epilepsy in fragile X syndrome. Dev Med Child Neurol 42:724–728
Jacquemont S, Hagerman RJ, Hagerman P, Leehey M (2007) Fragile-X syndrome and fragile X-associated tremor/ataxia syndrome: two faces of FMR1. Lancet Neurol 6:45–55
Reiss AL, Freund LS (1990) Fragile X syndrome, DSM-III-R, and autism. J Am Acad Child Adolesc Psychiatry 29:885–891
Scharfenaker S, O’Connor R, Stackhouse R et al (1996) An integrated approach to intervention. In: Hagerman R, Cronister A (eds) Fragile X syndrome: diagnosis treatment and research. The Johns Hopkins University Press, Baltimore, pp 349–411
Levitas A (1996) Neuropsychiatric aspects of fragile X syndrome. Semin Clin Neuropsychiatry 1:154–167
Lachiewicz AM, Spiridigliozzi GA, Gullion CM et al (1994) Aberrant behaviours of young boys with fragile X syndrome. Am J Ment Retard 98:567–579
Hatton D, Sideris J, Skinner M et al (2006) Autistic behaviour in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. Am J Med Genet 140:1804–1813
Kaufmann WE, Cortell R, Kau AS et al (2004) Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. Am J Med Genet 129A:225–234
Rogers SJ, Wehner EA, Hagerman RJ et al (2001) The behavioural phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. J Dev Behav Pediatr 22:409–417
Harris S, Hessl D, Goodlin-Jones B et al (2008) Autism profiles of males with fragile x syndrome. Am J Ment Retard 113:427–438
Demark JL, Feldman MA, Holden JJ (2003) Behavioural relationship between autism and fragile X syndrome. Am J Ment Retard 108:314–326
Bailey DB, Mesibov GB, Hatton DD et al (1998) Autistic behaviour in young boys with fragile X syndrome. J Autism Dev Disord 28:6
Hessl D, Tassone F, Cordeiro L et al (2008) Brief report: aggression and stereotypic behaviour in males with fragile X syndrome—moderating secondary genes in a “single gene” disorder. J Autism Dev Disord 38:184–189
Tsiouris JA, Brown WT (2004) Neuropsychiatric symptoms of fragile X syndrome: pathophysiology and pharmacotherapy. CNS Drugs 18:687–703
Hagerman RJ, Hills J, Scharfnaker S et al (1999) Fragile X syndrome and selective mutism. Am J Med Genet 83:313–317
Cordeiro L, Ballinger E, Hagerman R, Hessl D (2011) Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: prevalence and characterization. J Neurodev Disord 3:1–11
Hagerman R, Kemper M, Hudson M (1985) Learning disabilities and attentional problems in boys with the fragile X syndrome. Am J Dis Child 139:674–678
Hessl D, Nguyen DV, Green C et al (2008) A solution to limitations of cognitive testing in children with intellectual disabilities: the case of fragile X syndrome. J Neurodev Disord 1:33–45
Dykens EM, Hoddap RM, Leckman JF (1987) Strengths and weaknesses in the intellectual functioning of males with fragile X syndrome. Am J Med Genet 28:13–15
Freund LS, Reiss AL (1991) Cognitive profiles associated with the fragile X syndrome in males and females. Am J Med Genet 38:542–547
Kemper MB, Hagerman RJ, Ahmad RS, Mariner R (1986) Cognitive profiles and the spectrum of clinical manifestations in heterozygous fragile X females. Am J Med Genet 23:139–156
Cornish KM, Munira F, Cross G (1999) Spatial cognition in males with fragile X syndrome: evidence for a neuropsychological phenotype. Cortex 35:263–271
Shapiro MB, Murphy DGM, Hagerman RJ et al (1995) Adult fragile X syndrome: neuropsychology, brain anatomy and metabolism. Am J Med Genet 60:480–493
Munir F, Cornish K, Wilding J (2000) A neuropsychological profile of attention deficits in young males with fragile X syndrome. Neuropsychologia 38:1261–1270
Kaufman PM, Leckman JF, Ort SI (1990) Delayed response performance in males with fragile X syndrome. J Clin Exp Neuropsychol 12:69
Munir F, Cornish KM, Wilding J (2000) Nature of the working memory deficit in fragile X syndrome. Brain Cogn 44:387–401
Escalante JA, Grunspun H, Frota-Pessoa O (1971) Severe sex-linked mental retardation. J Genet Hum 19:137–140
Loesch DZ, Hay DA (1988) Clinical features and reproductive patterns in fragile X female heterozygotes. J Med Genet 25:407–414
Hagerman RJ, Jackson C, Amiri K et al (1992) Girls with fragile X syndrome: physical and neurocognitive status and outcome. Pediatrics 89:395–400
Moore PS, Chudley AE, Winter JS (1990) True precocious puberty in a girl with the fragile X syndrome. Am J Med Genet 37:265–267
Riddle JE, Cheema A, Sobesky WE et al (1998) Phenotypic involvement in females with the FMR1 gene mutation. Am J Ment Retard 102:590–601
Tassone F, Hagerman RJ, Ikle DN et al (1999) FMR1 protein expression as a potential prognostic indicator in fragile X syndrome. Am J Med Genet 84:250–261
Loesch DZ, Huggins RM, Hagerman RJ (2004) Phenotypic variation and FMR1 protein levels in fragile X. Med Retard Dev Disabil Res Rev 10:31–41
Dyer-Friedman J, Glaser B, Hessl D et al (2002) Genetic and environmental influences on the cognitive outcomes of children with fragile X syndrome. J Am Acad Child Adolesc Psychiatry 41(3):237–244
Reiss AL, Freund LS, Baumgardner TL, Abrams MT, Denckla MB (1995) Contribution of the FMR1 gene mutation to human intellectual dysfunction. Nat Genet 11:331–334
Freund L, Reiss AL, Abrams M (1993) Psychiatric disorders associate with fragile X in the young female. Paediatrics 91:321–329
Kaplan SC, Hong GK, Weinhold C (1986) Epidemiology of depressive symptomatology in adolescents. Am J Psychiatry 18:343–354
Roberts JE, Bailey DB Jr, Mankowski J et al (2009) Mood and anxiety disorders in females with the FMR1 premutation. Am J Med Genet 150B:130–139
Reiss AL, Hagerman RJ, Vingradov S, Abrams M, King RJ (1988) Psychiatric disability in female carriers of the fragile X chromosome. Arch Gen Psychiatry 45:25–30
Freund LS, Reiss AL (1992) Chromosome fragility and psychopathology in obligate female carriers of the fragile X chromosome. Arch Gen Psychiatry 49:54–60
Hagerman RJ, Staley LW, O’Connor R et al (1996) Learning disabled males with a fragile X CGG expansion in the upper premutation range. Paediatrics 97:122–126
Loesch DZ, Hay DA, Mulley JC (1994) Transmitting males and carrier females in fragile X revisited. Am J Med Genet 51:392–399
Moore CJ, Daly EM, Tassone F et al (2004) The effect of pre-mutation of X chromosome CGG trinucleotide repeats on brain anatomy. Brain 127:2672–2681
Dorn MB, Mazzocco MM, Hagerman RJ (1994) Behavioural and psychiatric disorders in adult male carriers of fragile X. J Am Acad Child Adolesc Psychiatry 33:256–264
Farzin F, Perry H, Hessl D et al (2006) Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr 27(2):S137–S144
Aziz M, Stathopulu E, Callias M et al (2003) Clinical features of boys with fragile X premutations and intermediate alleles. Am J Med Genet 121B(1):119–127
Goodlin-Jones B, Tassone F, Gane LW, Hagerman RJ (2004) Autistic spectrum disorder and the fragile X premutation. J Dev Behav Pediatr 25(6):392–398
Cornish KM, Kogan C, Turk J et al (2005) The emerging fragile X premutation phenotype: evidence from the domain of social cognition. Brain Cogn 57(1):53–60
Bailey DB Jr, Hatton DD, Mesibov GB, Ament N, Skinner M (2000) Early development, temperament and functional impairment in autism and fragile X syndrome. J Autism Dev Disord 30:49–59
Bourgeois JA, Coffey SM, Rivera SM et al (2009) A review of fragile X premutation disorders: expanding the psychiatric perspective. J Clin Psychiatry 70(6):852–862
Hull C, Hagerman RJ (1993) A study of the physical, behavioural, and medical phenotype, including anthropometric measures of females with fragile X syndrome. Am J Dis Child 147:1236–1241
Bennetto L, Pennington BF (2002) Neuropsychology. In: Hagerman RJ, Hagerman PJ (eds) Fragile X syndrome: diagnosis, treatment and research, 3rd edn. The Johns Hopkins University Press, Baltimore
Franke P, Leboyer M, Gansicke M et al (1998) Genotype-phenotype relationship in female carriers of the premutation and full mutation of FMR-1. Psychiatry Res 80:113–127
Rodriguez-Revenga L, Madrigal I, Pagonabarraga J et al (2009) Penetrance of FMR1 premutation associated pathologies in fragile X syndrome families. Eur J Hum Genet 17:1359–1362
Constant EL, Adam S, Seron X et al (2005) Anxiety and depression, attention, and executive functions in hypothyroidism. J Int Neuropsychol Soc 11:535–544
Larisch R, Kley K, Nikolaus S et al (2004) Depression and anxiety in different thyroid function states. Horm Metab Res 36:650–653
Hundscheid RD, Braat DD, Kiemeney LA et al (2001) Increased serum FSH in female fragile X premutation carriers with either regular menstrual cycles or on oral contraceptives. Human Reprod 16:457–462
Allingham-Hawkins DJ, Brown CA, Babul R et al (1999) Fragile X premutation is a significant risk factor for premature ovarian failure. The international collaborative POF in fragile X study—preliminary data. Am J Med Genet 83:322–325
Hagerman RJ, Leehey M, Heinrichs W et al (2001) Intention tremor, Parkinsonism and generalized brain atrophy in older male carriers of fragile X. Neurology 57:127–130
Jacquemont S, Hagerman RJ, Leehey M et al (2003) Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet 72:869–878
Leehey MA, Munhoz RP, Lang AE et al (2003) The fragile X premutation presenting as essential tremor. AMA Arch Neurol 60:117–121
Coffey SM, Cook K, Tartaglia N et al (2008) Expanded clinical phenotype of women with the FMR1 premutation. Am J Med Genet 146A:1009–1016
Cornish KM, Kogan CS, Li L et al (2009) Lifespan changes in working memory in fragile X premutation males. Brain Cogn 69:551–558
Hunter JE, Allen EG, Ann Abramowitz A et al (2008) No evidence for a difference in neuropsychological profile among carriers and non-carriers of the FMR1 premutation in adults under the age of 50. Am J Hum Genet 83:692–702
Conway GS, Hettitarachein S, Murray A, Jacobs PA (1995) Fragile X premutations in familial premature ovarian failure. Lancet 346:309–310
Sherman SL (2000) Premature ovarian failure in the fragile X syndrome. Am J Med Genet 97:189–194
Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ (2000) Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet 66:6–15
Stevenson RE, Schwartz CE, Schroer RJ (2000) X-linked mental retardation. Oxford University Press, New York
Lubs H (1969) A marker X chromosome. Am J Hum Genet 21:231–244
Sutherland GR (1977) Fragile sites on human chromosomes: demonstration of their dependence on the type of tissue culture medium. Science 197:265–266
Merenstein SA, Sobesky WE, Taylor AK et al (1996) Molecular clinical correlations in males with an expanded FMR1 mutation. Am J Med Genet 64:388–394
Reddy KS (2005) Cytogenetic abnormalities and fragile-X syndrome in autism spectrum disorder. BMC Med Genet 18:3
Nolin SL, Brown WT, Glicksman A et al (2003) Expansion of the fragile X CGG repeat in females with premutation or intermediate alleles. Am J Hum Genet 72:454–464
Zalfa F, Bagni C (2004) Molecular insights into mental retardation: multiple functions for the fragile X mental retardation protein? Curr Issues Mol Biol 6:73–88
Abitbol M, Menini C, Delezoide AL et al (1993) Nucleus basalis magnocellularis and hippocampus are the major sites of FMR1 expression in the human fetal brain. Nat Genet 4:147–153
Hinds HL, Ashley CT, Sutcliffe JS et al (1993) Tissue specific expression of FMR1 provides evidence for a functional role in fragile X syndrome. Nat Genet 3:36–43
Zhang Y, O’Connor P, Siomi M et al (1995) The fragile X mental retardation syndrome protein interacts with novel homologs FXR1 and FXR2. EMBO J 14:5358–5366
Eberhart DE, Malter HE, Feng Y, Warren ST (1996) The fragile X mental retardation protein is a ribonucleoprotein containing both nuclear localization and nuclear export signals. Hum Mol Genet 5:1083–1091
Adinolfi S, Bagni C, Musco G et al (1999) Dissecting FMR1, the protein responsible for fragile X syndrome, in its structural and functional domains. RNA 5:1248–1258
Schaeffer C, Beaulande M, Ehresmann C et al (2003) The RNA binding protein FMRP: new connections and missing links. Biol Cell 95:221–228
Ceman S, Brown V, Warren ST (1999) Isolation of an FMRP-associated messenger ribonucleoprotein particle and identification of nucleolin and the fragile X related proteins as components of the complex. Mol Cell Biol 19:7925–7932
Carola JM, Mcllwain KL, Nieuwenhuien IM et al (2002) Knockout mouse model for FXR2: a model for mental retardation. Hum Mol Genet 11:487–498
Broadie V, Pan L (2005) Translational complexity of the fragile X mental retardation protein: insights form the fly. Mol Cell 17:757–759
Bilousova TV, Dansie L, Ngo M et al (2009) Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J Med Genet 46:94–102
Antar LN, Li C, Zhang H, Carroll RC, Bassell GC (2006) Local functions for FMRP in axon growth cone motility and activity-dependent regulation of filopodia and spine synapses. Mol Cell Neurosci 32:37–48
Gantois I, Vandesompele J, Speleman F et al (2006) Expression profiling reveals underexpression of the GABAA receptor subunit delta in the fragile X knockout mice model. Neurobiol Dis 21:346–357
Mihalek RM, Banerjee PK, Korpi ER et al (1999) Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci USA 96:12905–12910
D’Hulst C, De Geest N, Reeve SP et al (2006) Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res 1121:238–245
D’Hulst C, De Geestb N, Reeveb S et al (2009) Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res 1253:176–183
Huber KM, Kayser MS, Bear MF (2000) Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science 288:1254–1257
Huber KM, Roder JC, Bear MF (2001) Chemical induction of mGluR5- and protein synthesis-dependent long-term depression in hippocampal area CA1. J Neurophysiol 86:321–325
Greenough WT, Klintsova AY, Irwin SA et al (2001) Synaptic regulation of protein synthesis and the fragile X protein. Proc Natl Acad Sci USA 98:7101–7106
Weiler IJ, Irwin SA, Klintsova AY et al (1997) Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc Natl Acad Sci USA 94:5395–5400
Grossman AW, Aldridge GM, Weiler IJ, Greenough WT (2006) Local protein synthesis and spine morphogenesis: fragile X syndrome and beyond. J Neurosci 26:7151–7155
Aschrafi A, Cunningham BA, Edelman GM, Vanderklish PW (2005) The fragile X mental retardation protein and group I metabotropic glutamate receptors regulate levels of mRNA granules in brain. Proc Natl Acad Sci USA 102:2180–2185
Bear MF, Dolen G, Osterweil E, Nagarajan N (2008) Fragile X: translation in action. Neuropsychopharmacology 33:84–87
Bear MF, Huber KM, Warren ST (2004) The mGluR theory of fragile X mental retardation. Trends Neurosci 27:370–377
Silverman JL, Tolu S, Barkan C, Crawley J (2010) Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology 35:976–989
Jacquemont S, Curie A, des Portes V et al (2011) Epigenetic modification of the FMR1 gene in fragile X syndrome is associated with differential response to the mGluR5 antagonist AFQ056. Sci Transl Med 3(64):64ra1
Friedmann C, Davis L, Ciccone P, Rubin R (1980) Phase II double-blind controlled study of a new anxiolytic, fenobam (McN-3377) vs placebo. Curr Ther Res 27:144–151
Pecknold JC, McClure DJ, Appeltauer L, Wrzesinski L, Allan T (1982) Treatment of anxiety using fenobam (a nonbenzodiazepine) in a double-blind standard (diazepam) placebo-controlled study. J Clin Psychopharmacol 2:129–133
Berry-Kravis E, Hessl D, Coffey S et al (2009) A pilot open label, single dose trial of fenobam in adults with fragile X syndrome. J Med Genet 46:266–271
Paribello C, Tao L, Folino A et al (2010) Open-label add-on treatment trial of minocycline in fragile X syndrome. BMC Neurol 10:91
Utari A, Chonchaiya W, Rivera SM et al (2010) Side effects of minocycline treatment in patients with fragile X syndrome and exploration of outcome measures. Am J Intellect Dev Disabil 115:433–443
Irwin SA, Idupulapati M, Gilbert ME et al (2002) Dendritic spine and dendritic field characteristics of layer V pyramidal neurons in the visual cortex of fragile-X knockout mice. Am J Med Genet 111:140–146
Irwin SA, Patel B, Idupulapati M et al (2001) Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am J Med Genet 98:161–167
Hinton VJ, Brown WT, Wisniewski K, Rudelli RD (1991) Analysis of neocortex in three males with fragile X syndrome. Am J Med Genet 41:289–294
Irwin SA, Galvez R, Greenough WT (2000) Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb Cortex 10:1038–1044
Koukoui SD, Chaudhuri A (2007) Neuroanatomical, molecular, genetic, and behavioural correlates of fragile X syndrome. Brain Res Rev 53:27–38
Pfeiffer BE, Huber KM (2007) Fragile X mental retardation protein induces synapse loss through acute postsynaptic translational regulation. J Neurosci 27:3120–3130
Pfeiffer BE, Zang T, Wilkerson JR et al (2010) Fragile X mental retardation protein is required for synapse elimination by the activity-dependent transcription factor MEF2. Neuron 66:191–197
Greco CM, Berman RF, Martin RM et al (2006) Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS). Brain 129:243–255
Tassone F, Iwahahi C, Hagerman PJ (2004) FMR1 RNA within the intranuclear inclusions of Fragile X associated tremor/ataxia syndrome. RNA Biol 1:103–105
Greco C, Hagerman RJ, Tassone F et al (2002) Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain 125:1760–1771
Reiss AL, Abrams MT, Greenlaw R, Freund L, Denckla MB (1995) Neurodevelopmental effects of the FMR1 full mutation in humans. Nat Med 1:159–167
Eliez S, Blasey CM, Freund LS et al (2001) Brain anatomy, gender and IQ in children and adolescents with fragile X syndrome. Brain 124:168–1610
Gothelf D, Furfaro JA, Hoeft F et al (2008) Neuroanatomy of fragile X syndrome is associated with aberrant behaviour and the fragile X mental retardation protein (FMR1 PROTEIN). Ann Neurol 63:40–51
Hoeft F, Lightbody AA, Hazlett HC et al (2008) Morpohometric spatial patterns differentiating boys with fragile X syndrome, typically developing boys, developmentally delayed boys aged 1 to 3 years. Arch Gen Psychiatry 65:1087–1097
Hazlett HC, Poe MD, Lightbody AA et al (2009) Teasing apart the heterogeneity of autism: same behaviour, different brains in toddlers with fragile X syndrome and autism. J Neurodev Disord 1:181–190
Hallahan B, Craig M, Toal F et al (2010) In vivo brain anatomy of adult males with fragile X syndrome: an MRI study. Neuroimage 54:16–24
Wilson LB, Tregellas JR, Hagerman RJ et al (2009) A voxel-based morphometry comparison of regional gray matter between fragile X syndrome and autism. Psychiatry Res 174:138–145
Mostofsky SH, Mazzocco MM, Aakalu G et al (1998) Decreased cerebellar posterior vermis size in fragile X syndrome: correlation with neurocognitive performance. Neurology 51:121–130
Reiss AL, Aylward E, Freund LS et al (1991) Neuroanatomy of fragile X syndrome: the posterior fossa. Ann Neurol 29:26–32
Reiss AL, Abrams MT, Greenlaw R et al (1995) Neurodevelopmental effects of the FMR-1 full mutation in humans. Nat Med 1:159–167
Guerreiro MM, Camargo EE, Kato M et al (1998) Fragile X syndrome, Clinical, electroencephalographic and neuroimaging characteristics. Aq Neuropsiquiatr 56:18–23
Reiss AL, Lee J, Freund L (1994) Neuroanatomy of fragile X syndrome: the temporal lobe. Neurology 44:1317–1324
Kates WR, Abrams MT, Kaufmann WE, Breiter SN, Reiss AL (1997) Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Res 76:15–27
Jäkälä P, Hänninen T, Ryynänen M et al (1997) Fragile-X: neuropsychological test performance, CGG triplet repeat lengths, and hippocampal volumes. J Clin Invest 100:331–338
Cornish KM, Munira F, Cross G (2001) Differential impact of the FMR1 full mutation on memory and attention functioning: a neuropsychological perspective. J Cogn Neurosci 13:144–150
Hoeft F, Hernandez A, Sudharshan P et al (2007) Fronto-striatal dysfunction and potential compensatory mechanisms in male adolescents with fragile X syndrome. Hum Brain Map 28:543–554
Allen G, Buxton R, Wong EC, Courchesne E (1997) Attentional activation of the cerebellum independent of motor involvement. Science 275:1940–1943
Townsend J, Courchesne E, Covington J et al (1999) Spatial attention deficits in patients with acquired or developmental cerebellar abnormality. J Neurosci 19:5632–5643
Ronning C, Sundet K, Due-Tonnessen B et al (2005) Persistent cognitive dysfunction secondary to cerebellar injury in patients treated for posterior fossa tumors in childhood. Pediatr Neurosurg 41:15–21
Brunberg JA, Jacquemont S, Hagerman RJ et al (2002) Fragile X permutation carriers: characteristic MR imaging findings in adult males with progressive cerebellar and cognitive dysfunction. Am J Neuroradiol 23:1757–1766
Loesch DZ, Litewka L, Brotchie P et al (2005) Magnetic resonance imaging study in older fragile X premutation male carriers. Ann Neurol 58:326–330
Cohen S, Masyn K, Adams J et al (2006) Molecular and imaging correlates of the fragile X-associated tremor/ataxia syndrome. Neurology 67:1426–1431
Adams JS, Adams PE, Nguyen D et al (2007) Volumetric brain changes in females with fragile X-associated tremor/ataxia syndrome (FXTAS). Neurology 69:851–859
Koldewyn K, Hessl D, Adams J et al (2008) Reduced hippocampal activation during recall is associated with elevated FMR1 mRNA and psychiatric symptoms in men with the fragile X premutation. Brain Imaging Behav 2:105–116
Hessl D, Rivera S, Koldewyn K et al (2007) Amygdala dysfunction in men with the fragile X premutation. Brain 130:404–416
Adams PE, Adams JS, Nguyen DV et al (2010) Psychological symptoms correlate with reduced hippocampal volume in fragile X premutation carriers. Am J Med Genet Part B 153B:775–785
Oberle I, Rousseau F, Heitz D et al (1991) Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science 252:1097–1102
Willemsen R, Oosterwijk JC, Los FJ et al (1996) Prenatal diagnosis of fragile X syndrome. Lancet 348:967–968
Iwahashi C, Tassone F, Hagerman RJ et al (2009) A quantitative ELISA assay for the fragile X mental retardation 1 protein. J Mol Diagn 11:281–289
McConkie-Rosell A, Finucane B, Cronister A, Abrams L, Bennett RL, Pettersen BJ (2005) Genetic counselling for fragile X syndrome: updated recommendations of the NSGC. J Genet Couns 14:249–270
Fragile X (1994) syndrome: diagnostic and carrier testing (policy statement). Am J Med Genet 53:380–381
Hall DA, Berry-Kravis E, Hagerman RJ et al (2006) Symptomatic treatment in the fragile X associated tremor/ataxia syndrome. Mov Disord 21:1741–1744
Hagerman RJ, Murphy MA, Wittenberger MD (1988) A controlled trial of stimulant medication in children with fragile X syndrome. Am J Med Genet 30:377–392
Torroi MG, Vernacotola S, Mariotti P et al (1999) Double-blind placebo controlled study of L-acetylcarnitine for the treatment of hyperactive behaviour in fragile x syndrome. Am J Med Genet 87:366–368
Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP (2005) Suppression of two major fragile X syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology 49:1053–1066
McBride SM, Choi CH, Wang Y et al (2005) Pharmacological rescue of synaptic plasticity, courtship behaviour, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron 45:753–764
Restivo L, Ferrari F, Passimo E et al (2005) Enriched environment promotes behavioural and morphological recovery in a mouse model for the fragile X syndrome. Proc Natl Acad Sci USA 102:11557–11562
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gallagher, A., Hallahan, B. Fragile X-associated disorders: a clinical overview. J Neurol 259, 401–413 (2012). https://doi.org/10.1007/s00415-011-6161-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-011-6161-3