Abstract
Our objective was to evaluate amyloid deposition in posterior cortical atrophy (PCA), using both cerebrospinal fluid (CSF) biomarker analysis and amyloid imaging. Five PCA patients, selected based on their neuropsychological profile and atrophic changes in posterior regions on MRI, underwent CSF analysis. CSF amyloid-beta 1–42, total tau, and phosphorylated tau at threonine 181 levels were determined. They also had positron emission tomography (PET) with Pittsburgh Compound B ([11C]PIB). [11C]PIB ratio images were assessed with visual, regional and voxel-based analyses and compared to eight typical Alzheimer's disease (AD) patients and eight controls. The biological profile in the five PCA patients, resulting from CSF and [11C]PIB images analysis, was consistent with AD. Individual comparisons of PCA patients’ [11C]PIB images with the AD group with Statistical Parametric Mapping (SPM) revealed a distinctive posterior uptake in four out of the five patients showing increased amyloid deposition in occipital, temporal, and/or parietal regions. ROI group analysis showed a tendency for higher amyloid deposition in occipital and temporal regions. However, this pattern was not found with SPM group analysis when the global level of [11C]PIB uptake was used as a covariate. Our results indicate that amyloid burden can be demonstrated in vivo in PCA suggesting a diagnosis of AD. PCA patients may present a higher global amyloid load than AD that was not related to age at onset, disease severity, disease duration, or educational level in our study. Combined CSF and PET biomarkers seem helpful for in vivo diagnosis of this focal syndrome with underlying AD pathology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Posterior cortical atrophy (PCA) is a rare neurodegenerative syndrome. It is characterized by progressive development of higher-order visuoperceptive deficits, in addition to atrophic or metabolic changes in the posterior regions of the brain [1–4]. Clinical features may include Balint’s syndrome, Gerstmann’s syndrome, alexia, visual object agnosia, visuospatial neglect, prosopagnosia, environmental disorientation and apraxia. Homonymous hemianopia may also be present [5]. Magnetic resonance imaging (MRI) usually shows cortical atrophy in the parietal, occipital and posterior temporal regions [6], while [18F]fluorodeoxyglucose positron emission tomography (FDG-PET) may reveal hypometabolism in the posterior cerebral hemispheres, even in the absence of concomitant atrophy [7, 8]. This syndrome almost universally progresses to more diffuse cognitive impairment and dementia.
Histologically, PCA is most often associated with Alzheimer’s disease (AD) pathology [9]. Neurofibrillary tangles (NFT), corresponding to tau protein deposits, are preferentially located in the posterior cortex, including primary visual cortex and visual associative areas [2, 3]. Senile plaques (SPs), corresponding to amyloid deposits, may also predominate posteriorly but this finding is more controversial [2, 10].
Cerebrospinal fluid (CSF) levels of amyloid-beta 1–42 (A42) are decreased in AD, and those of total tau (T-tau) and tau phosphorylated at threonine 181 (P-tau181) are increased. When levels of all three are combined into a single biomarker, both sensitivity and specificity for diagnosing AD are in the region of 90% [11, 12].
The PET imaging radioligand Pittsburgh Compound B ([11C]PIB) allows in vivo assessment of Aβ plaque burden. PIB is a derivative of thioflavin T that binds specifically to fibrillar Aβ deposits in human brain [13]. Human PET studies have shown significantly higher neocortical retention of [11C]PIB in AD than controls [14, 15], reflecting the presence of Aβ plaques and amyloid angiopathy [16–18].
We studied amyloid deposition in five PCA patients, using CSF biomarker analysis and [11C]PIB imaging with visual, regional and voxel-based analyses. Eight typical AD patients and eight controls also had [11C]PIB imaging for comparison. Our objective was to demonstrate in vivo amyloid burden in PCA and to reveal any specific pattern of cortical deposit in comparison to typical AD.
Materials and methods
Subjects
PCA subjects
Five PCA patients (mean age: 65.2 ± 7.6 years; MMSE [19]: 20.4 ± 5; CDR [20]: 1 ± 0; Table 1) were prospectively recruited from our memory clinic. PCA was diagnosed according to the following diagnostic criteria [Renner et al. (2004), McMonagle et al. (2006)]: (1) History of progressive acquired visual impairment, interfering with daily life, and not due to ophthalmological abnormalities. (2) Evidence of one or several disorders reflecting dysfunction of occipito-parieto-temporal association cortices, such as Balint’s syndrome, Gerstmann’s syndrome or apraxia. (3) Absent or minor memory impairment, executive dysfunction or language deficits. (4) Evidence of atrophy of the posterior cortex on MRI, or of reduced regional blood flow on [99Tc]HMPAO SPECT. A visual analysis of MRI and, when available, SPECT images was performed for each patient at their inclusion in the study. Patients showing major vascular lesions on MRI were excluded from the study. All PCA patients underwent full ophthalmological testing, including visual field determination. They all underwent a lumbar puncture for CSF biomarkers analysis and amyloid PET imaging with [11C]PIB.
AD group
Eight patients (mean age: 74.6 ± 6.2 years; MMSE: 21.4 ± 5.9, CDR: 1.2 ± 0.4) with mild or moderate AD were recruited from our memory clinic as a comparison group for [11C]PIB analysis (Table 2). They had a history of progressive cognitive impairment with mild or moderate weakening of autonomy; memory complaint; and memory impairment at neuropsychological testing. The other cognitive functions could be variably affected. AD patients met the NINCDS-ADRDA diagnostic criteria for probable AD [21]. They underwent the same neuropsychological tests and imaging as PCA patients. Visual analysis of amyloid PET imaging showed increased [11C]PIB binding consistent with probable AD in all.
Control group
Eight normal volunteers (mean age: 73.9 ± 6.1 years, MMSE: 29.4 ± 0.7, CDR: 0) without cognitive complaints were recruited from the community by advertisement as a comparison group for [11C]PIB analysis. All were free of relevant medical illness. Control subjects were screened via an evaluation that included a medical history, functional assessment, neurological examination and neuropsychological testing. Only cognitively intact subjects were included. They all had normal [11C]PIB binding on PET according to visual analysis.
All patients and controls gave their written informed consent to participate. The study was approved by the institutional ethics committee. [11C]PIB PET scanning in humans was approved by the French regulatory authorities (AFSSAPS).
Statistical analysis
Demographic and neuropsychological data were compared between PCA and AD groups using a non-parametric test (Mann–Whitney U) because of the small sample size; p values <0.05 were considered significant (Table 3). Analysis was performed with MedCalc® version 11.1.1.0 (Frank Schoonjans, MedCalc Software, Mariakerke, Belgium).
Neuropsychological evaluation
Verbal episodic memory was assessed with the Free and Cued Selective Recall Reminding Test (FCSRT) [22] or the French version of the California Verbal Learning Test [23]. We explored verbal fluency [24] and naming with the Bachy-Langedock denomination task [25]. Executive functions were assessed with the Stroop Test [26] or the Trail Making Test [27]. Visuospatial, visuoperceptive and visuoconstructive abilities were assessed with the Visual Object and Space Perception battery [28], the clock drawing test [29], and complex figure copying tests. Praxis were most often evaluated with informal personal procedures. These neuropsychological tests and PIB-PET were performed within the same month, and both within 1 year after the lumbar puncture.
CSF data
CSF sampling and storage
CSF sampling was performed according to a standard protocol. In total, 10 mL were collected in polypropylene vials and then centrifuged immediately. Supernatant was sampled in polypropylene vials and aliquots were stored at −80°C until analysis.
CSF analysis
CSF T-tau, P-tau181 and Aβ42 levels were determined using a commercially available ELISA kit (INNOTEST htau-Ag, INNOTEST Phospho-Tau(181), INNOTEST β-amyloid(1–42), respectively, Innogenetics®, Gent, Belgium) according to the manufacturer’s instructions. All biomarker levels were measured in duplicate. The inter-assay coefficient of variation was less than 10%. Cut-off values were determined from literature data and experience of our neurobiology department, and were T-tau >350 pg/mL, P-tau181 >60 pg/mL and Aβ42 <500 pg/mL [30–32]. If all three levels lay beyond these cut-off values, subjects were considered to have a typical AD profile. CSF pattern was said atypical for patients with either elevated T-tau >350 pg/mL and P-tau181 >60 pg/mL or decreased Aβ42 <500 pg/mL.
PET imaging
Radiochemistry
[11C]PIB was produced by methylation of 1.2 mg of its desmethyl precursor 6-OH-BTA-0 (ABX Germany) with [11C]methyl triflate in 200 μL acetone for 3 min at 100°C, with a Bioscan MeI + synthesizer, starting from [11C]O2 produced via a 14N(p,α)11C nuclear reaction using an IBA Cyclone 18/9 cyclotron. The reaction product was purified using high-performance liquid chromatography with a SymetryPrep column (Waters) using water/acetonitrile (50:50) at 3 mL/min. The collected fraction was loaded on a C18 SepPak, washed with 10 mL of water and eluted with 1 mL absolute ethanol. After addition of NaCl 0.9% the product was sterilized through a 0.22 μm Millipore sterile filter (Waters). The radioligand purity was assessed by analytical radio-HPLC (Beckman 32 K). Radiochemical purity was in excess of 95%, and specific activity in the range of 10–40 GBq/μmol.
PET imaging
PET scans were performed on a CTI-Siemens HR + scanner (Knoxville, TN, USA). For tracer injections, an intravenous catheter was placed in a vein of the left forearm. Before emission acquisition, a 10-min transmission scan was performed using three 68Ge rod sources for the measurement of tissue and head support attenuation. After i.v. injection of a bolus of [11C]PIB (470 ± 12 MBq), a dynamic emission scan consisting of 34 frames of increasing duration (20 s–5 min) was acquired over 90 min. The PET scanner was operating in 3D mode. Images were corrected for scatter and attenuation, and reconstructed using a filtered back projection (Hanning filter, cut-off 0.5 cycles/pixels) to provide a 3D volume of 63 slices (2.42 mm thickness) with 128 × 128 voxels in plane (2.06 mm2).
MRI
Structural brain MRI was performed using a 1.5 T Siemens Magnetom scanner (Siemens AG, Erlangen, Germany), with a 3D anatomical T1-weighted sequence (TR: 9.7 ms, TE: 4 ms) covering the whole brain volume with 1 mm3 cubic voxels.
Visual analysis
Voxel based [11C]PIB parametric RATIO images were computed by dividing, for each voxel, the integrated activity over the 40–90 min interval by integrated activity of the reference region (cerebellar gray matter) for the same interval. [11C]PIB-RATIO images were read by a neurologist unaware of the clinical diagnosis. Images were graded as normal (similar to controls), doubtful or probable AD. Transverse, sagittal, and coronal views were available for viewing. Before reading the [11C]PIB images, a demonstration was given using two AD images and one typical HC [11C]PIB-RATIO image. The reader was advised to use a rainbow color scale. Subjects with cortical binding (yellow or red) in frontal, cingulate, parietal and temporal cortex were classified as having probable AD, depending on intensity and extent of [11C]PIB uptake.
ROI analysis
Five neocortical ROIs were considered [33]: the cingulate gyrus, and the frontal, lateral temporal, parietal and occipital cortices. These ROI were defined on individual PET scans using the Hammers maximum probability atlas [34] and SPM5 software (Wellcome Trust Centre for Neuroimaging, UCL, London, UK) as follows. First, T1 MRIs were coregistered to summed activity PIB-PET images without reslicing, then spatially normalized to the MNI/ICBM152 T1 stereotactic template using deformations obtained via Unified Segmentation [35]. The inverse deformation fields were applied to the Hammers atlas to fit the individual T1 anatomy. Probabilistic images of the grey and white matter and CSF tissue classes were obtained in the segmentation step. Gray matter images were thresholded at a probability of >0.5 and multiplied with the atlas resampled in the individual space. Finally, regional activities of the selected ROIs were extracted from 40 to 90 min [11C]PIB RATIO images. Regional values were compared between the three groups using Kruskall-Wallis testing with MedCalc®. If significant differences (p < 0.05) were observed then intergroup comparisons were performed using Mann–Whitney U tests.
Parametric image analysis
Using SPM5, [11C]PIB-RATIO images were spatially normalized to a customized template created from PCA and AD patients’ MRIs. Pairwise comparisons were performed between groups (control, PCA and AD), and PCA patients compared individually with the AD group. A voxel based ANCOVA with age as a covariate was used to identify clusters of voxels that significantly differed in these comparisons. Global cerebral mean was used as group specific covariate in a second analysis. Statistical parametric maps resulting from contrasts of comparisons were thresholded at p uncorr <0.001 at the voxel level. Further thresholding at p uncorr <0.001 at the cluster level, with a minimum cluster extent of 350 voxels, led to a joint probability significance level of p corr <0.01 corrected for multiple comparisons at the cluster level.
Results
Demographic and clinical data
Simultanagnosia, alexia, acalculia, agraphia, ideomotor and constructional apraxia were the most frequent signs (Table 1). Two patients had a left hemianopia (cases 2 and 5). Mild signs of symmetrical parkinsonism were noticed in two patients (cases 1 and 3). One patient had visual hallucinations (case 5). None had fluctuation of cognitive or motor symptoms. No patient had more than one of these three cardinal symptoms, and therefore, none fulfilled clinical criteria for dementia with Lewy bodies (DLB). None showed evidence of motor signs such as asymmetrical or unilateral parkinsonism with gestural apraxia or dystonia consistent with corticobasal degeneration (CBD). PCA patients were younger than AD patients (p < 0.05). PCA patients’ symptoms had been progressing for 1 to 4 years at the clinical evaluation, compared with 1 to 5 years for AD patients (n.s). Level of education was also comparable in the two groups (Table 3). MRI most often showed asymmetrical cortical or subcortical atrophy in the parietal and occipital regions. No PCA patient had medial temporal lobe or hippocampal atrophy suggesting typical AD.
Neuropsychological deficits
Cognitive functions of PCA patients were variably affected with a mean MMSE ranging from 15 to 28. Impact of cognitive disorders on autonomy was also variable from one subject to another with a CDR ranging from 0.5 to 1 (Table 1). PCA and AD patients were not significantly different in terms of global cognitive function as measured with MMSE and CDR. Visuoperceptive, visuospatial and visuoconstructive abilities were systematically affected for the five PCA patients. Their visuoconstructive apraxia as assessed with figure copying was more important than that of AD patients (p = 0.008). Verbal memory was preserved in two PCA patients (cases 1 and 2), mildly impaired in one patient (case 5) and moderately altered in two patients (cases 3 and 4). Because of his visual impairment, patient 4 could not perform FCSRT. His memory performance as assessed using the French version of the California Verbal Learning Test was moderately reduced (data not shown). Memory deficits evaluated with the FCSRT in the four other PCA patients was less severe than in AD patients (free delayed recall, p = 0.02; total delayed recall, p = 0.048). Executive functions were systematically but variably affected. PCA patients were slower than AD patients during the TMT-A (p = 0.02). Naming was preserved in two PCA cases (1 and 3) and impaired in the others (Table 3).
CSF data
Among our five PCA patients (Table 4), three fulfilled biological criteria for typical AD with abnormal levels of the three CSF markers (cases 1, 2 and 5). Two PCA patients were classified as atypical AD due to abnormalities of either tau or amyloid protein levels (cases 3 and 4). None had normal CSF biomarkers. Thus, all of PCA patients showed a biological profile consistent with AD, either typical or atypical.
[11C]PIB data
Visual analysis
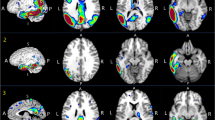
Visual inspection of the quantitative data showed neocortical retention of [11C]PIB in all PCA patients (Table 3), as in AD subjects, suggesting underlying amyloid pathology. No [11C]PIB-RATIO scans of our PCA patients were considered as normal or doubtful (Table 4, Fig. 1).
[11C]PIB-PET images of PCA patients. [11C]PIB-RATIO images of all PCA patients show an extensive but variable cortical [11C]PIB uptake in frontal, temporal, parietal, occipital and cingulate cortices consistent with amyloid deposition and probable AD. [11C]PIB uptake in posterior regions is variable from one PCA subject to another
ROI analysis (Fig. 2)
PCA, AD versus controls. ROI group analysis showed that AD and PCA patients had significantly higher RATIO values than controls in all five neocortical regions investigated (p < 0.001). PCA versus AD. There was no statistical difference of [11C]PIB uptake in the frontal cortex (p = 0.24), parietal cortex (p = 0.14) and cingulate cortex (p = 0.88) between PCA and AD patients. PCA patients had a significantly higher RATIO of [11C]PIB uptake than AD patients in the occipital cortex (p = 0.03). A trend for higher binding in PCA was also noticed in the lateral temporal cortex (p = 0.05).
ROI analysis of PCA group versus typical AD group. AD and PCA patients have significantly higher RATIO values than controls in all five investigated neocortical regions (p < 0.001). There is no statistical difference of [11C]PIB retention in the frontal (p = 0.24), parietal (p = 0.14) and cingulate cortices (p = 0.88) between PCA and AD patients. PCA patients have a significantly higher RATIO of [11C]PIB uptake than AD in the occipital cortex (p = 0.03). A trend is also noticed in lateral temporal cortex (p = 0.05)
Parametric image analysis (Fig. 3)
PCA, AD versus controls. Both PCA and AD patients had significantly higher [11C]PIB uptake in neocortical brain regions than control subjects (data not shown). PCA versus AD. When global mean was not included as a covariate in the analysis, PCA subjects showed a focally higher [11C]PIB uptake in the visual cortex, the bilateral occipito-temporal junction, the left parietal cortex and the right temporal cortex, and to a lesser degree in the frontal cortex (Fig. 3, no global). These differences were no longer significant when global cerebral mean was included as a covariate (Fig. 3, global). AD versus PCA. There were no regions of higher [11C]PIB RATIO in AD than in PCA patients. Individual comparison of PCA patients with the AD patient group revealed a great heterogeneity of [11C]PIB-RATIO patterns. In four PCA patients (cases 1, 2, 3 and 5), the posterior regions showed a significantly higher [11C]PIB-RATIO retention than in the AD group. Retention of [11C]PIB could encompass the visual cortex on both sides (case 1 and 2), with more variable extension to temporal and parietal cortices (cases 2, 3 and 5). However, two of the four PCA patients (cases 1 and 5) also showed a higher [11C]PIB retention in the frontal cortex. Finally, one PCA patient (case 4) did not show higher [11C]PIB retention in any region as compared to the AD group, i.e. his [11C]PIB-RATIO pattern was similar to that of typical AD subjects (Fig. 3).
SPM analysis of individual PCA patients and PCA group vs typical AD group. Individual analysis. Four PCA patients (cases 1, 2, 3 and 5) show a significantly higher retention of [11C]PIB than typical AD in posterior regions with a variable pattern including occipital, temporal and/or parietal cortices. Two of these four PCA patients (cases 1 and 5) also show a higher [11C]PIB retention in the anterior regions. One case (case 4) presents a [11C]PIB uptake pattern similar to that of typical AD subjects. Group analysis. When global mean is not included as a covariate in the analysis, PCA subjects, in comparison to AD patients, show a focally higher [11C]PIB retention in the visual cortex, the bilateral occipito-temporal junction, the left parietal and the right temporal cortices but also in frontal cortex at a lesser degree. These differences are no longer found with global cerebral mean as a covariate
Discussion
In this study, the combination of CSF biomarker analysis and [11C]PIB PET scanning in five consecutive prototypical PCA patients demonstrated in vivo a profile overall suggestive of AD both for CSF biomarkers and cerebral amyloid imaging. A posterior cortical distribution of [11C]PIB involving parietal, temporal and occipital cortices, particularly visual cortex, was inconsistently found in individual PCA cases, as well as in the group analyses, but was not significant when global means were taken into account. Our imaging and CSF data suggestive of AD diagnosis are concordant with neuropathological studies showing AD pathology in most PCA cases [2, 3, 9]. There are few studies comparing CSF and PIB results in AD and we found only a single PCA case report in the literature where both biomarkers were available, results of which were consistent with our findings [36]. Our combined biomarker study reinforces the hypothesis that the PCA syndrome is most often a focal form of AD.
Only four case reports, including a total of six patients, have been published to date. They have shown abnormal [11C]PIB retention in PCA using visual inspection [36, 37], ROI analysis [38], or SPM analysis [39], but none combined these three types of analysis.
In our study, SPM and ROI group comparisons showed higher [11C]PIB retention in PCA patients compared with AD patients, with a posterior emphasis (Figs. 2, 3). Visual inspection of scans showed that the five PCA patients had abnormal cortical retention of [11C]PIB of similar magnitude as patients with typical AD (Fig. 1). However, individual SPM analyses showed a pattern of cortical [11C]PIB retention that significantly differed from the AD group, with higher amyloid burden in the primary visual cortex, in the parietal or the temporal cortices. Two patients also had a higher [11C]PIB uptake in anterior regions. Only one PCA patient had a pattern not distinguishable from AD, i.e. 4/5 did show differences, indicating that such analyses may be clinically relevant.
ROI and SPM individual analyses also showed a great interregional variability in [11C]PIB uptake in patients compared to control subjects, particularly for the PCA group (Figs. 2, 3). The posterior-anterior gradient of amyloid load seen in a majority of our PCA patients may be related to a particular distribution pattern of lesions as shown in autopsied cases [2, 10]. Indeed, these authors found more numerous SPs in occipital [2] or occipito-parietal regions [10] than in frontal regions [10] and hippocampus [2]. However, these data seem less robust than those related to NFTs, showing higher density in posterior regions in PCA compared to AD. Thus, the pattern of posterior predominance of PIB uptake was no longer found when the overall level of PIB binding was included as a covariate in the SPM group comparison (Fig. 3). This discrepancy is likely to be due to a global higher [11C]PIB retention in PCA compared to AD (Fig. 2).
Some previous PIB-PET studies showed a positive correlation between [11C]PIB uptake, severity of cognitive deficit in AD [40], hypometabolism [41] and level of education [42]. These variables, as well as disease duration, were, however, not different between groups in our sample, and hence do not explain the higher amyloid load in our PCA group. Age is unlikely to underlie the global difference, even if the patients in the PCA group were younger, this variable having been included as a covariate in the SPM analysis. In the same way, another study did not show any correlation between [11C]PIB uptake and age at onset, clinical status or glucose metabolism in AD [43]. Higher NFT density and more severe neuronal loss were shown in early-onset PCA compared to late-onset cases [10] whereas differences in amyloid load were not reported.
Increased [11C]PIB retention in neocortical regions in our AD group compared to controls is consistent with the literature [14, 15, 42, 44].The five PCA patients had neocortical [11C]PIB retention comparable to AD patients. PIB positivity is highly suggestive of amyloid deposition [16]. It correlates with the presence and the concentration of neuritic plaques characteristic of AD [18]. It is indicative of amyloid brain pathology, but not pathognomonic of AD. Amyloid deposits can also be found in DLB [45, 46], and in non-demented aged controls [47, 48]. Based on [11C]PIB data, our five PCA patients have increased amyloid concentrations and are likely to have AD pathology. None of them have symptoms consistent with DLB or CBD, which is also in line with this hypothesis.
CSF showed decreased Aβ42 levels in four patients and elevated levels of T-tau and P-tau181 in a partially overlapping group of four others. According to our results, three of the five PCA patients (cases 1, 2 and 5) showed a typical AD CSF profile. The two others (cases 3 and 4) with either elevated CSF tau or decreased Aβ42 showed a less typical profile, which is nevertheless consistent with AD. Our study thus identified a dominant AD type biological profile in PCA. These findings are in accordance with published studies with CSF analysis in PCA [49] (Seguin et al., in press). Decreased CSF Aβ42 and elevated phosphorylated tau at threonine 199 levels have been reported in a single case of PCA [36].
Decreased levels of CSF Aß42, which have been linked to trapping of the protein in senile plaques [50], is a reliable marker of AD pathology, with a variable diagnostic sensitivity (58–96%) and specificity (61–86%). Taking into account increased levels of T-tau protein and P-tau181 protein also helps to identify AD patients with a sensitivity of 65–80% and specificity of 60–92% in different studies with post-mortem confirmation of diagnosis [12, 51, 52]. Combining the three biomarkers achieves sensitivities and specificities of about 90% [30, 53]. Cut-off values used in our study are compatible with normative data of most laboratories [30–32, 54], and we are confident in the AD diagnosis of our patients with typical AD CSF profiles.
Atypical CSF patterns have previously been described in patients with autopsy-based diagnosis of AD [55] and remain consistent with underlying AD pathology. However, these CSF profiles are less specific of AD and can be found in other neurodegenerative diseases, sometimes associated with AD. CSF profiles with low Aβ42 and normal tau proteins level have been found in particular in patients showing AD lesions in association with DLB [56], vascular dementia, or Parkinson's disease with dementia [55]. Similarly, CSF profiles with normal Aβ42 and elevated tau and P-tau181 levels were described in autopsied cases, either with vascular dementia or DLB [55]. None of our patients, even those with atypical CSF patterns, fulfilled diagnostic criteria for DLB. They are therefore most likely to have underlying AD, but DLB associated with AD pathology remains a differential diagnosis.
Although histological data were not available and the sample size was small, our results indicated that a combination of biomarkers could demonstrate amyloid burden in vivo in PCA, suggesting a diagnosis of AD in these patients. We could not formally exclude associated DLB pathology, but our PET and CSF data were consistent with those of neuropathological studies in PCA. Our results also showed a posterior regional pattern of amyloid deposits in PCA. Although this finding was reminiscent of the regional distribution of histological lesions, it was partially explained by a globally higher [11C]PIB retention in PCA as compared to AD in our sample. This higher amyloid burden in PCA compared to AD was not related to age at onset, disease severity, disease duration or level of education.
Although post mortem examination remains the only way to determine the whole spectrum of pathological events related to this clinical condition, we found combined CSF and PET biomarkers helpful in the in vivo diagnosis of PCA with underlying AD pathology.
References
Benson DF, Davis RJ, Snyder BD (1988) Posterior cortical atrophy. Arch Neurol 45(7):789–793
Tang-Wai DF et al (2004) Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology 63(7):1168–1174
Renner JA et al (2004) Progressive posterior cortical dysfunction: a clinicopathologic series. Neurology 63(7):1175–1180
McMonagle P et al (2006) The cognitive profile of posterior cortical atrophy. Neurology 66(3):331–338
Formaglio M et al (2009) Homonymous hemianopia and posterior cortical atrophy. Rev Neurol (Paris) 165(3):256–62
Whitwell JL et al (2007) Imaging correlates of posterior cortical atrophy. Neurobiol Aging 28(7):1051–1061
Nestor PJ et al (2003) The topography of metabolic deficits in posterior cortical atrophy (the visual variant of Alzheimer’s disease) with FDG-PET. J Neurol Neurosurg Psychiatry 74(11):1521–1529
Schmidtke K, Hull M, Talazko J (2005) Posterior cortical atrophy: variant of Alzheimer’s disease? A case series with PET findings. J Neurol 252(1):27–35
Alladi S et al (2007) Focal cortical presentations of Alzheimer’s disease. Brain 130(Pt 10):2636–2645
Hof PR et al (1997) Atypical form of Alzheimer’s disease with prominent posterior cortical atrophy: a review of lesion distribution and circuit disconnection in cortical visual pathways. Vision Res 37(24):3609–3625
Hulstaert F et al (1999) Improved discrimination of AD patients using beta-amyloid(1–42) and tau levels in CSF. Neurology 52(8):1555–1562
Shaw LM et al (2009) Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 65(4):403–413
Mathis CA et al (2002) A lipophilic thioflavin-T derivative for positron emission tomography (PET) imaging of amyloid in brain. Bioorg Med Chem Lett 12(3):295–298
Klunk WE et al (2004) Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 55(3):306–319
Engler H et al (2006) Two-year follow-up of amyloid deposition in patients with Alzheimer’s disease. Brain 129(Pt 11):2856–2866
Bacskai BJ et al (2007) Molecular imaging with Pittsburgh Compound B confirmed at autopsy: a case report. Arch Neurol 64(3):431–434
Lockhart A et al (2007) PIB is a non-specific imaging marker of amyloid-beta (Abeta) peptide-related cerebral amyloidosis. Brain 130(Pt 10):2607–2615
Ikonomovic MD et al (2008) Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain 131(Pt 6):1630–1645
Folstein MFFS, McHugh PR (1975) Mini-mental state. A pratical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Morris JC (1993) The clinical dementia rating (CDR): current version and scoring rules. Neurology 43(11):2412–2414
McKhann G et al (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology 34(7):939–944
Grober E, Buschke H (1987) Genuine memory deficits in dementia. Dev Psychol 3:13–36
Delis D et al (1987) The California Verbal Learning Test (research Ždition). Psychological corporation, New York
Cardebat DDB, Puel M, Goulet P, Joanette Y (1990) Formal and semantic lexial evocation in normal subjects. Performance and dynamics of production as a function of sex, age and educational level. Acta Neurol Belg 90:207–217
Bachy-Langedock N (1989) Batterie d’examen des troubles en denomination, Editest, Bruxelles
Stroop (1935) Studies of interferences in serial verbal reactions. J Exp Psychol 18:643–662
Reitan RM, Wolfson D (2004) The Trail Making Test as an initial screening procedure for neuropsychological impairment in older children. Arch Clin Neuropsychol 19(2):281–288
Rapport LJ, Millis SR, Bonello PJ (1998) Validation of the Warrington theory of visual processing and the visual object and space perception battery. J Clin Exp Neuropsychol 20(2):211–220
Sunderland T et al (1989) Clock drawing in Alzheimer’s disease. A novel measure of dementia severity. J Am Geriatr Soc 37(8):725–729
Hansson O et al (2006) Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol 5(3):228–234
Tapiola T et al (2009) Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol 66(3):382–389
Mattsson N et al (2009) CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. Jama 302(4):385–393
Lopresti BJ et al (2005) Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med 46(12):1959–1972
Hammers A et al (2003) Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp 19(4):224–247
Ashburner J, Friston KJ (2005) Unified segmentation. Neuroimage 26(3):839–851
Kambe T et al (2010) Posterior cortical atrophy with [11C] Pittsburgh compound B accumulation in the primary visual cortex. J Neurol 257(3):469–71
Migliaccio R et al (2009) Clinical syndromes associated with posterior atrophy: early age at onset AD spectrum. Neurology 73(19):1571–1578
Tenovuo O et al (2008) Posterior cortical atrophy: a rare form of dementia with in vivo evidence of amyloid-beta accumulation. J Alzheimers Dis 15(3):351–355
Ng SY et al (2007) Evaluating atypical dementia syndromes using positron emission tomography with carbon 11 labeled Pittsburgh Compound B. Arch Neurol 64(8):1140–1144
Grimmer T et al (2009) Clinical severity of Alzheimer’s disease is associated with PIB uptake in PET. Neurobiol Aging 30(12):1902–1909
Edison P et al (2007) Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PIB and [18F]FDG PET study. Neurology 68(7):501–508
Kemppainen NM et al (2007) PET amyloid ligand [11C]PIB uptake is increased in mild cognitive impairment. Neurology 68(19):1603–1606
Rabinovici GD et al (2010) Increased metabolic vulnerability in early-onset Alzheimer’s disease is not related to amyloid burden. Brain 133(Pt 2): p 512–28
Forsberg A et al (2008) PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging 29(10):1456–1465
Gomperts SN et al (2008) Imaging amyloid deposition in Lewy body diseases. Neurology 71(12):903–910
Edison P et al (2008) Amyloid load in Parkinson’s disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. J Neurol Neurosurg Psychiatry 79(12):1331–1338
Pike KE et al (2007) Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain 130(Pt 11):2837–2844
Villemagne VL et al (2008) Abeta deposits in older non-demented individuals with cognitive decline are indicative of preclinical Alzheimer’s disease. Neuropsychologia 46(6):1688–1697
Baumann TP et al (2010) CSF-Tau and CSF-Abeta(1–42) in posterior cortical atrophy. Dement Geriatr Cogn Disord 29(6):530–533
Strozyk D et al (2003) CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology 60(4):652–656
Roher AE et al (2009) Proteomics-derived cerebrospinal fluid markers of autopsy-confirmed Alzheimer’s disease. Biomarkers 14(7):493–501
Koopman K et al (2009) Improved discrimination of autopsy-confirmed Alzheimer’s disease (AD) from non-AD dementias using CSF P-tau(181P). Neurochem Int 55(4):214–218
Mulder C et al (2010) Amyloid-beta(1-42), total tau, and phosphorylated tau as cerebrospinal fluid biomarkers for the diagnosis of Alzheimer disease. Clin Chem 56(2):248–53
Hort J et al (2010) Use of cerebrospinal fluid biomarkers in diagnosis of dementia across Europe. Eur J Neurol 17(1):90–6
Engelborghs S et al (2008) Diagnostic performance of a CSF-biomarker panel in autopsy-confirmed dementia. Neurobiol Aging 29(8):1143–1159
Iqbal K et al (2005) Subgroups of Alzheimer’s disease based on cerebrospinal fluid molecular markers. Ann Neurol 58(5):748–757
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Formaglio, M., Costes, N., Seguin, J. et al. In vivo demonstration of amyloid burden in posterior cortical atrophy: a case series with PET and CSF findings. J Neurol 258, 1841–1851 (2011). https://doi.org/10.1007/s00415-011-6030-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-011-6030-0