Abstract
It is widely assumed that the thalamus is not involved in olfaction. The ventrolateral thalamus is, however, closely connected to the contralateral cerebellum, which is involved in the sense of smell based on findings from functional imaging studies and findings of olfactory deficits in patients with cerebellar disease. We hypothesized that olfactory deficits following lesions of the ventrolateral thalamus may be similar to olfactory deficits following cerebellar lesions. Fifteen patients with a focal thalamic lesion involving the ventrolateral thalamus were examined and compared to 15 patients with a focal cerebellar lesion and 15 healthy controls. A detailed olfactory test (“Sniffin’ Sticks”) was used to assess different olfactory functions separately for each nostril. In the group of patients with a lesion of the ventrolateral thalamus, an impairment of the odor threshold was found at the ipsilateral nostril, consistent with the unilateral orientation of the olfactory system in the telencephalon. In the group of patients with a cerebellar lesion, an olfactory deficit at the contralesional nostril emerged. In controls, no significant side difference was found. The involvement of the ventrolateral thalamus in olfaction is comparable to that of the cerebellum in respect to odor threshold. Further study is needed to assess if these findings are related to an impairment of an olfactomotor loop. Present evidence for this hypothesis is indirect. Effects were subclinical as none of the patients reported olfactory disturbance. The results suggest that the cerebello-thalamic axis plays an adjuvant role in olfaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
At first glance, the thalamus does not appear to be involved in olfaction, as olfactory information reaches the cortex without an obligatory thalamic relay, which is in contrast to all other sensory modalities [1–3]. However, several lines of evidence point to an indirect involvement of the mediodorsal (MD) thalamus in the sense of smell [4–9]. In contrast, the role of the ventrolateral (VL) thalamus in olfaction is unclear at present. Exploring the involvement of the VL thalamus in the sense of smell is of interest to obtain a better insight into the neuronal network of olfaction, which is still an understudied area of clinical research.
The VL thalamus and the cerebellum are traditionally considered to be part of the motor-control system. Yet, involvement of the cerebellum in olfaction is suggested by cerebellar activation described in functional imaging studies of olfaction [10–13] and olfactory impairment in patients with cerebellar disease [14–16]. The VL thalamus is functionally closely connected to the contralateral cerebellum, and is therefore also referred to as the “cerebellar thalamus” [17–19]. For example, lesions of the VL thalamus lead to ataxia of the contralesional limbs reminiscing motor deficits ipsilateral to focal cerebellar disease [20–22]. Comparable to motor deficits, we hypothesized that olfactory deficits following a lesion of the VL thalamus may similarly be found to olfactory deficits with cerebellar lesions. In contrast to other sensory modalities, the olfactory system has an ipsilateral orientation at the telencephalon [3]. Therefore, we anticipated olfactory deficits at the nostril ipsilateral to a lesion of the VL thalamus. To investigate this hypothesis, olfactory function was tested in patients with a lesion of the VL thalamus and compared to patients with a focal cerebellar lesion and healthy controls.
Methods
Patients with a single vascular lesion involving the VL thalamus or the cerebellum were included. Additionally, two patients following surgical removal of a benign cerebellar tumor were examined. Exclusion criteria were past or present tobacco smoking, use of medication that act on the central nervous system, deformities, infections, or inflammatory diseases of the nose or sinuses, psychiatric diseases, involuntary movements, or brain lesions outside the thalamus or the cerebellum. Altogether, 15 patients with a thalamic lesion (Table 1), 15 patients with a cerebellar lesion (Table 2), and 15 healthy controls matched for age and sex (4 women, 11 men; age 60.3 ± 9.6 years) were examined. Major pathology of the nasal cavities was excluded with anterior rhinoscopy. Furthermore, rhinomanometry was used to assess air flow through the nostrils during normal breathing, which was found not to be different between groups (Table 3 in Online Resource). The examiner was blinded towards the participants’ group and side of the brain lesion. Additionally, participants were informed about the background of this study but not given details related to the hypothesis assessed. Furthermore, during olfactory testing the participants did not receive any feedback about their performance.
Olfactory testing
The “Sniffin’ Sticks” [23, 24] were administered separately for each nostril to assess odor threshold, odor discrimination, and odor identification (for details of the olfactory testing see Online Resource).
Brain imaging
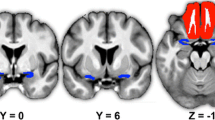
The axial images of the magnetic resonance tomography or the computer tomography made during routine care were used to localize the brain lesion (for examples please see Fig. 1). The location and extent of the lesion was manually entered into MRIcroN software (Lazarus INC, USA, Version 15).
Examples of the brain scans that were used to identify the thalamic lesions. a Magnet resonance image (MRI) of patient T2 in Table 1 with a lesion of the VL thalamus only, b Computer tomography of patient T4 in Table 1 showing a lesion of the VL thalamus, which extends into the anterior thalamus, c. MRI of patient T10 in Table 1 showing a thalamic lesion involving the VL thalamus and the posterior thalamus. The thalamic strokes were only seen on one slice, as they extended primarily into the horizontal direction [41]
Neuropsychological testing
Basic memory functioning was assessed using the logical memory tests, and the digit span tests [25]. The Multiple Choice Vocabulary Intelligence test [26] was also performed.
Statistics
Non-parametric Kruskal–Wallis test for repeated measurements was used to compare the side difference between nostrils in the olfactory tests in the three groups. Additionally, the Wilcoxon rank sum test was applied to compare results of the ipsilesional nostril with those of the contralesional nostril in each group. P values <0.05 were regarded as significant. The study was performed in accordance with the ethical standards laid down in the Declaration of Helsinki (1964). The local ethical committee approved this study. All participants gave written informed consent before testing.
Results
In the group of patients with a thalamic lesion, an olfactory impairment at the ipsilesional nostril was found as indicated by a lower score of the ipsilesional nostril compared to the score of the contralesional nostril (Fig. 2a–c). In contrast, the patient group with a cerebellar lesion had an olfactory deficit at the contralesional nostril, while no side difference was found in the group of healthy controls.
Results from the Sniffin’ Sticks test. a Odor discrimination task, b odor threshold task, and c identification task. Dashed lines depict the tenth percentile, indicative for hyposmia [19]. Controls, group of healthy controls; cerebellum, group of patients with a cerebellar lesion; thalamus, group of patients with a thalamic lesion involving the VL thalamus; *p < 0.05 and **p < 0.01 revealed by Wilcoxon rank sum test
The Kruskal–Wallis test yielded a significant difference between the three groups for odor threshold (p < 0.01) and odor identification (p = 0.04), while there was no difference in odor discrimination (p = 0.13). The Wilcoxon rank sum test revealed a significant difference between the two nostrils for odor threshold in patients with a cerebellar lesion (p < 0.01) and in patients with a thalamic lesion (p < 0.01), while a significant difference between the two nostrils on the odor identification task was only found in patients with a cerebellar lesion (p = 0.01).
In the analysis of the imaging data, patients with a focal lesion of the thalamus and an ipsilesional odor threshold deficit were found to have their largest overlap in the VL thalamus, while cerebellar patients with a contralesional odor threshold deficit were found to have the largest overlap of their lesion in the cerebellar hemispheres (Fig. 3 in Online Resource). Lesion size (number of voxels) did not correlate with the olfactory deficits in either patient group (p > 0.25, Spearman rank correlation). For subgroup analysis, the patients with a thalamic lesion were divided into three groups: (1) patients with a lesion restricted to the VL thalamus, (2) patients with a lesion of the VL thalamus that extended into the posterior thalamus, and (3) patients with a lesion of the VL thalamus that extended into the anterior thalamus (Table 1). None of the three subgroups exhibited a distinguishable pattern (Table 1; odor discrimination: p = 0.76, odor threshold: p = 0.71 or odor identification: p = 0.12; Kruskal–Wallis test). Although thalamic patients had their stroke in the territory of the thalamo-geniculate arteries [27], findings upon clinical examination varied, i.e. they may have ataxia, hypesthesia, or a slight paresis [20–22]. Subgroup analysis indicated that patients with a thalamic lesion and ataxia but without a paresis had a larger odor threshold impairment at the ipsilesional nostril (side difference 2.1 ± 2.2) than did patients without ataxia and without paresis (side difference 0.3 ± 0.8) (p = 0.04, Wilcoxon rank sum test), while all other comparisons (with/without hypesthesia or paresis) were not different (p values >0.15).
The analysis of the neuropsychological test revealed a significant difference on the digit span forward task (p = 0.04) and digit span backward task (p = 0.01) where cerebellar patients performed the worst (Table 4 in Online Resource). Spearman rank correlation comparing performance in the olfactory tests and performance with the neuropsychological assessment, septal orientation (towards the contralesional or ipsilesional nostril as assessed with anterior rhinoscopy), or air flow with normal breathing did not reveal any significant correlations.
Discussion
Using a standardized approach, this study demonstrates involvement of the VL thalamus in olfaction. In the group of patients with a lesion of the VL thalamus, an olfactory deficit was found at the ipsilateral nostril consistent with the unilateral orientation of the olfactory system in the telencephalon [3, 28]. Patients with a cerebellar lesion had the opposite, an olfactory deficit at the contralesional nostril, which is in accordance with the functional bond of the cerebellum to the contralateral VL thalamus [17–19].
Involvement of the cerebellum and the VL thalamus in olfaction appears surprising at first. However, the cerebellum receives information from different sensory modalities, to monitor and modulate behavior in order to optimize sampling of sensory information [29]. This may also be true for the role of the cerebellum with respect to the sense of smell [10, 16, 30]. The cerebellum receives projections from the primary olfactory cortex [31], as well as the trigeminal nerve [32] and projects via the contralateral VL thalamus to multiple cortical areas involved in olfaction [18, 33, 34]. The major termination zones of cerebellar projections to the thalamus are the nucleus ventrointermedius and the nucleus ventrooralis posterior of the human VL thalamus [18]. These nuclei project to the motor cortex [33, 34] where the pyramidal tracts originate. These tracts are also important for the movements of the diaphragm [35], which, as the principal muscle of inspiration, is also important for sniffing.
These brain structures may be part of an olfactomotor loop. Within this circuit, the cerebellum is believed to maintain a feedback loop that regulates sniff volume inversely proportional to odor concentration allowing for optimal collection of olfactory information [10, 16, 36]. Impaired functioning of this loop may have hampered patients taking more vigorous sniffs at low n-butanol concentrations to detect the odor resulting in the odor threshold impairment found. In this study, we were not able to control or assess sniff volume on-line. Evidence that the odor threshold impairment in patients with a lesion of the VL thalamus may be related to an impairment of a cerebellar (olfactomotor) loop is indirect. Subgroup analyses suggest that the odor threshold impairment in patients with a thalamic lesion is related to ataxia, a cerebellar motor deficit. Additionally, this odor threshold impairment is not related to hypesthesia. In patients with a thalamic lesion, hypesthesia (if present) was at the contralesional side while the odor threshold impairment was found at the ipsilateral nostril. Furthermore, the olfactory deficits are not caused by a blockade of the nostrils because airflow through the nostrils with normal breathing was not different between groups. In future studies, the assessment of olfactomotor functioning through the use of a fixed-sniff method [16] and the assessment of olfactory event-related potentials [37] may provide further support for this view. Another experiment may be to puff an odor into the nostril without sniffing. Following this line of reasoning, the odor threshold deficit should disappear with this test.
The odor identification impairment in the group of patients with a cerebellar lesion is consistent with reports of olfactory deficits in patients with cerebellar disease [14–16]. This impairment may be related to a disturbance of cerebellar projections to cortical areas involved in processing of cognitive functioning. These pathways were possibly spared from the thalamic lesions as we did not find an odor identification deficit in patients with a thalamic lesion. Cerebellar projections to subcortical structures such as the hippocampus [38, 39] may be involved in odor identification and are therefore not affected by a thalamic lesion. Deficits in the odor discrimination task were not found in any of the two patient groups. As a possibly less complex task, it involves the allocation of cognitive resources that can compensate for the subtle memory impairments found.
The comparison of the three thalamic subgroups revealed no difference of the olfactory deficits. This is possibly because all patients had a lesion that involved the VL thalamus. Lesion size did not correlate with olfactory impairment, which is in accordance with past findings with respect to cerebellar lesions [16]. The location rather than the size of a cerebellar lesion is crucial for causing a deficit [40]. Therefore, the comparison of patients with and without a lesion of the VL thalamus may disclose a correlation between location of a thalamic lesion and olfactory deficit. A case report seems to support this idea. A lesion of the MD thalamus lead to a disturbance of odor discrimination [7], while a lesion of the VL thalamus (this study) lead to odor threshold impairment.
In conclusion, the findings suggest involvement of the VL thalamus in olfaction. As none of the patients reported olfactory disturbance, the cerebello-thalamic axis may play an adjuvant role in olfaction.
References
Schünke M, Schulte E (2006) Prometheus – Kopf und Neuroanatomie. Thieme, Stuttgart
Kay LM, Sherman SM (2007) An argument for an olfactory thalamus. Trends Neurosci 30:47–53
Gottfried JA (2006) Smell: central nervous processing. In: Hummel T, Welge-Lüssen A (eds) Taste and smell. An update. Adv Otorhinolaryngol, vol 63. Karger, Basel, pp 44–69
Motokizawa F (1974) Olfactory input to the thalamus: electrophysiological evidence. Brain Res 67:334–337
Price JL (1985) Beyond the primary olfactory cortex: olfactory-related areas in the neocortex, thalamus and hypothalamus. Chem Senses 10:239–258
Plailly J, Howard JD, Gitelman DR, Gottfried JA (2008) Attention to odor modulates thalamocortical connectivity in the human brain. J Neurosci 28:5257–5267
Potter H, Butters N (1980) An assessment of olfactory deficits in patients with damage to prefrontal cortex. Neuropsychologia 18:621–628
Rousseaux M, Muller P, Gahide I, Mottin Y, Romon M (1996) Disorders of smell, taste, and food intake in a patient with a dorsomedial thalamic infarct. Stroke 27:2328–2330
Tham WW, Stevenson RJ, Miller LA (2009) The functional role of the medio dorsal thalamic nucleus in olfaction. Brain Res Rev 62:109–126
Sobel N, Prabhakaran V, Hartley CA et al (1998) Odorant-induced and sniff-induced activation in the cerebellum of the human. J Neurosci 18:8990–9001
Ferdon S, Murphy C (2003) The cerebellum and olfaction in the aging brain: a functional magnetic resonance imaging study. Neuroimage 20:12–21
Yousem DM, Williams SC, Howard RO et al (1997) Functional MR imaging during odor stimulation: preliminary data. Radiology 204:833–838
Qureshy A, Kawashima R, Imran MB et al (2000) Functional mapping of human brain in olfactory processing: a PET study. J Neurophysiol 84:1656–1666
Abele M, Riet A, Hummel T, Klockgether T, Wüllner U (2003) Olfactory dysfunction in cerebellar ataxia and multiple system atrophy. J Neurol 250:1453–1455
Connelly T, Farmer JM, Lynch DR, Doty RL (2003) Olfactory dysfunction in degenerative ataxias. J Neurol Neurosurg Psychiatry 74:1435–1437
Mainland JD, Johnson BN, Khan R, Ivry RB, Sobel N (2005) Olfactory impairments in patients with unilateral cerebellar lesions are selective to inputs from the contralesional nostril. J Neurosci 6:6362–6371
Stanton GB (1980) Topographical organization of ascending cerebellar projections from the dentate and interposed nuclei in Macaca mulatta: an anterograde degeneration study. J Comp Neurol 190:699–731
Sakai ST, Inase M, Tanji J (1996) Comparison of cerebellothalamic and pallidothalamic projections in the monkey (Macaca fuscata): a double anterograde labeling study. J Comp Neurol 368:215–228
Krack P, Dostrovsky J, Ilinsky I et al (2002) Surgery of the motor thalamus: problems with the present nomenclatures. Mov Disord 17:S2–S8
Bastian AJ, Thach WT (1995) Cerebellar outflow lesions: a comparison of movement deficits resulting from lesions at the levels of the cerebellum and thalamus. Ann Neurol 38:881–892
Melo TP, Bogousslavsky J, Moulin T, Nader J, Regli F (1992) Thalamic ataxia. J Neurol 239:331–337
Solomon DH, Barohn RJ, Bazan C, Grissom J (1994) The thalamic ataxia syndrome. Neurology 44:810–814
Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G (1997) ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 22:39–52
Hummel T, Kobal G, Gudziol H, Mackay-Sim A (2007) Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol 264:237–243
Härting H, Markowitsch HJ, Neufeld H, Calabrese P, Deisinger K, Kessler J (2000) Wechsler Gedächtnistest–Revidierte Fassung. Hans Huber, Bern
Lehrl S (2005) Mehrfachwahl-Wortschatz-Intelligenztest MWT-B. Spitta Verlag, Balingen
Cosson A, Tatu L, Vuillier F, Parratte B, Diop M, Monnier G (2003) Arterial vascularization of the human thalamus: extra-parenchymal arterial groups. Surg Radiol Anat 25:408–415
Lascano AM, Hummel T, Lacroix JS, Landis BN, Michel CM (2010) Spatio-temporal dynamics of olfactory processing in the human brain: an event-related source imaging study. Neuroscience (in press)
Bower JM (1997) Control of sensory data acquisition. Int Rev Neurobiol 41:489–513
Johnson BN, Mainland JD, Sobel N (2003) Rapid olfactory processing implicates subcortical control of an olfactomotor system. J Neurophysiol 90:1084–1094
Ikai Y, Takada M, Shinonaga Y, Mizuno N (1992) Dopaminergic and non-dopaminergic neurons in the ventral tegmental area of the rat project, respectively, to the cerebellar cortex and deep cerebellar nuclei. Neuroscience 51:719–728
Yatim N, Billig I, Compoint C, Buisseret P, Buisseret-Delmas C (1996) Trigeminocerebellar and trigemino-olivary projections in rats. Neurosci Res 25:267–283
Darian-Smith C, Darian-Smith I, Cheema SS (1990) Thalamic projections to sensorimotor cortex in the macaque monkey: use of multiple retrograde fluorescent tracers. J Comp Neurol 299:17–46
Middleton FA, Strick PL (2000) Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Rev 31:236–250
Colebatch JG, Adams L, Murphy K et al (1991) Regional cerebral blood flow during volitional breathing in man. J Physiol 443:91–103
Laing DG (1983) Natural sniffing gives optimum odour perception for humans. Perception 12:99–117
Hummel T, Kobal G (2001) Olfactory event-related potentials. In: Simon SA, Nicolelis MAL (eds) Methods and frontiers in chemosensory research. CRC Press, Boca Raton, pp 429–464
Newman PP, Reza H (1979) Functional relationships between the hippocampus and the cerebellum: an electrophysiological study of the cat. J Physiol 287:405–426
Oganesian EA, Melik-Musian AB, Fanardzhian VV, IuKh Grigorian (1980) Morpho-functional analysis of the nature of cerebello-hippocampal connections. Fiziol Zh SSSR Im I M Sechenova 66:1632–1639
Konczak J, Schoch B, Dimitrova A, Gizewski E, Timmann D (2005) Functional recovery of children and adolescents after cerebellar tumour resection. Brain 128:128–1441
Miller Fischer C (1978) Thalamic pure sensory stroke: a pathologic study. Neurology 28:1141–1144
Schaltenbrand G, Wahren W (1977) Atlas for stereotaxy of the human brain. Thieme, Stuttgart
Acknowledgments
This project was supported by the START-Program, Faculty of Medicine, RWTH Aachen. We thank Professor Dr. Willmes (Division of Neuropsychology, RWTH Aachen) for his statistical advice. Additionally, we thank Professor Coenen (Division of Neurosurgery, University Bonn) for critical comments on the imaging data.
Conflict of interest statement
There are no conflicts of interest to be disclosed.
Author information
Authors and Affiliations
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zobel, S., Hummel, T., Ilgner, J. et al. Involvement of the human ventrolateral thalamus in olfaction. J Neurol 257, 2037–2043 (2010). https://doi.org/10.1007/s00415-010-5656-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-010-5656-7