Abstract
Neurosarcoidosis (NS) represents an important differential diagnosis of multiple sclerosis (MS). However, thus far no reliable laboratory marker of neurosarcoidosis exists. The objective of this study was to evaluate whether cerebrospinal fluid (CSF) levels of soluble interleukin 2 receptor (sIL2-R) distinguish NS and other inflammatory disorders of the central nervous system. For this purpose, 139 paired CSF and serum samples from 11 patients with NS, 21 with MS, 10 with CNS vasculitis, 22 with bacterial meningitis, 17 with viral meningitis/encephalitis, seven with neurotuberculosis, and 18 healthy donors were assessed for sIL2-R using an enzyme-linked immunosorbent assay. We found that sIL2-R CSF levels above 150 pg/ml identified untreated NS patients with an overall accuracy of 93% against a group of non-infectious CNS-diseases. Furthermore, an increase in sIL2-R in the CSF was associated with and preceded the outbreak of new neurological symptoms. In conclusion, these findings suggest that sIL2-R measurement in the CSF may be a valuable tool in the diagnosis and follow-up of patients with suspected and proven neurosarcoidosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcoidosis, a systemic disease of putative autoimmune etiology, is characterised by the formation of granulomas potentially in almost every tissue, but preferentially in the lung, lymph nodes, liver and other organs. Although the prevalence is said to be 10–50 in 100,000 depending on the ethnical background, the disease might well be underdiagnosed as a post mortem study with an estimated prevalence of more than 600 in 100,000 suggests [1, 2]. The nervous system is involved in 5–27% of cases with a broad range of symptoms including headache, aseptic meningitis, visual disturbances, cranial nerve palsy, spinal cord symptoms, brainstem or cerebellar signs and pituitary gland dysfunction [3–7]. However, the diagnosis of neurosarcoidosis remains difficult, especially in the absence of an established diagnosis of systemic sarcoidosis, which is the case in almost half of the patients with NS [5, 7]. Furthermore, laboratory tests such as the detection of angiotensin-converting enzyme (ACE) in the CSF have a poor sensitivity of 24–55% in the diagnosis of NS [8, 9]. According to a recent suggestion, the definite diagnosis of neurosarcoidosis is only to be made histologically [7]. The differentiation from other chronic inflammatory diseases such as MS or CNS vasculitis is especially difficult since they share common clinical, CSF and magnetic resonance imaging (MRI) findings [10]. Hence, a test able to differentiate between neurosarcoidosis and other chronic inflammatory central nervous system (CNS) diseases would be welcome.
One of the most prominent immunologic features of sarcoidosis is the accumulation of activated CD4+ T helper cells at the sites involved. Interleukin 2 (IL2) plays a crucial role in T cell activation and the IL2 receptor (IL2-R), although partially released into the microenvironment, is mainly expressed on activated T cells as a heterotrimeric protein with relative molecular weight of 55,000. Therefore, measurement of soluble IL2 receptor in serum is regarded as a method to evaluate ongoing T cell activation. Recent studies suggest that serum levels of the sIL2-R in patients with sarcoidosis may serve as a diagnostic and prognostic parameter [11, 12]. An increase of sIL2-R was detected in sarcoidosis patients compared to normal controls and in patients with active versus inactive disease [13, 14]. Increased levels of sIL2-R have also been detected in bronchoalveolar lavage (BAL) of patients with sarcoidosis of the lungs [12, 14]. sIL2-R levels in CSF have not been evaluated thus far.
To assess whether elevated sIL2-R levels represent a marker of NS, we analysed 28 serum and CSF samples from patients with NS and compared them to suitable controls.
Subjects and methods
Subjects
The diagnosis of neurosarcoidosis was made according to previously established criteria [7]. sIL2-R levels of paired CSF and serum samples from 11 NS patients were compared with those of 21 patients with MS, 10 with CNS vasculitis (VS) and 46 with CNS infection. Additionally, 18 healthy volunteers (HC), 11 women and seven men, with a mean age of 26 years (range 20–30 years) gave their informed consent for a spinal tap and consecutive analysis of the CSF.
The NS group consisted of eight male and three female patients with a mean age of 42 years (range 24–75 years). The multiple sclerosis group comprised 18 patients with a relapsing-remitting and three with secondary progressive course of the disease. Twelve patients were female. The mean age was 37 years (range 22–74 years). Diagnosis was established according to the McDonald criteria. The mean disease duration was 6.5 years. Lumbar puncture was performed during acute exacerbation or deterioration of the disease.
In the vasculitis group, 20 samples from eight female and two male patients with a mean age of 33 years (range 19–55 years) were analysed. Except for two patients in whom the CNS vasculitis could not be attributed to a systemic disease, all patients suffered from secondary CNS disease due to systemic lupus erythematosus in five patients and Morbus Behçet in three patients. In most cases, the diagnosis of lupus or Behçet’s disease was established before neurological symptoms appeared by a rheumatologist. Current diagnostic criteria like those of the Behçet Syndrome Research Committee of Japan (1972) or the American College of Rheumatology, revised version (1997) were used to make the respective diagnosis.
The group of patients with infectious CNS disorder consisted of 22 patients with bacterial meningitis (BM) or meningoencephalitis, 13 samples from seven patients with neurotuberculosis (TB) and 17 patients with viral meningitis, meningoencephalitis or myelitis (VM). The mean age in the bacterial meningitis group was 51 years ranging from 16 to 78 years. The 13 female and nine male patients suffered for an average of 2.5 days from typical symptoms when the diagnostic lumbar puncture was done and three patients already were on antibiotic treatment. In 12 cases Streptococcus pneumonia, in four cases Neisseria meningitidis, in two cases Staphyloccocus aureus, in one case Streptococcus salivarius and in three cases no bacterium was detected.
The neurotuberculosis group consisted of five men and two women with a mean age of 57 years (range 29–82 years). In all cases Mycobacterium DNA was detected by PCR. The viral CNS infection group consisted of 10 women and seven men with a mean age of 42 years (range 17–81 years). The mean time between first symptoms and diagnostic spinal tap was 9.3 days. In all patients either herpes simplex virus or varicella zoster virus was identified as infectious agent.
Soluble IL2 receptor ELISA
Soluble IL2 receptor concentrations in paired CSF and serum samples of patients and healthy volunteers were measured with a commercially available enzyme linked immunosorbent assay (ELISA) kit (sIL2-R/CD24 Eli-pair, Diaclone, Besançons, France) according to the manufacturer’s instructions, with slight modifications. In brief, 96-well microtiter plates (Nunc immuno plates maxisorp F96, VWR International, Darmstadt, Germany) were coated with 100 μl of a solution containing a capture anti-sIL2-R antibody in phosphate-buffered saline (Dulbecco’s PBS, Sigma-Aldrich Chemie Gmbh, Steinheim, Germany) and stored overnight at 4°C. The following day the plates were washed twice with 300 μl of PBS 0.05% polyoxyethylene sorbitan monolaurate v/v (Tween 20, Merck KG, Darmstadt, Germany) and incubated with saturation buffer containing PBS with 5% bovine serum albumin w/v (BSA, Sigma) for 2 h at room temperature. A standard curve was constructed with sIL2-R concentrations ranging from 2,200 to 34 pg/ml. PBS with 1% BSA w/v was used to dilute samples and standards as recommended by the manufacturer. Dilutions of samples ranged between undiluted and 1:100. All standards and samples were added to the plate in duplicate, 100 μl to each well followed by addition of 50 μl of a solution containing biotinylated detection anti-sIL2-R antibody which was reconstituted with distilled water and diluted in PBS with 1% BSA w/v. The plates were incubated at room temperature for 3 h, shaken at 200 rpm, and then washed three times. 100 μl of horseradish peroxidase-streptavidin dilutions containing a diluent buffer consisting of PBS with 0.1% Tween 20 plus 1% BSA w/v was added. The plates were then incubated for 20 min at room temperature and again washed three times. Colour development was permitted for 10 min, shaken at 200 rpm in the dark after distribution of 100 μl of ready-to-use 3,3′,5,5′-tetramethylbenzidin (TMB) and then stopped with the same amount of 1 M sulfuric acid (Merck KG, Darmstadt, Germany). The absorbance of the colour reaction was read at 450 nm versus appropriate blank wells containing only diluent buffer by a V max Kinetic microplate reader (Molecular Devices, Ismaning/Munich, Germany). The optical densitometry readings of the test samples were plotted against the standard curve, and sIL2-R concentrations were expressed in pg/ml using the software SOFTmax PRO 3.0 (Molecular Devices, Ismaning/Munich, Germany).
To test for possible inhibitory substances in the CSF samples, 1,100 pg of standard sIL2 receptor were added to samples of CSF of healthy control. The measured amount of sIL2-R diluted in CSF was 101% of the expected value, indicating that there were no interfering substances that could inhibit the measurement of the sIL2-R in the sample.
To test for influences of storing conditions on the stability of sIL2-R, serum samples were frozen and defrosted up to four times before measurement. While samples which had been frozen and thawed once or twice showed stable amounts of sIL2-R, further freezing and thawing lead to significant elevation of sIL2-R. Thus, all samples of patients and healthy controls used for tests have been frozen and thawed only once.
sIL2-R index
To correct the CSF sIL2-R level for serum-derived sIL2-R crossing the blood-CSF-barrier, we calculated the sIL2-R index according to the IgG index as ratio of (CSF sIL2-R:serum sIL2-R):(CSF albumin:serum albumin). To our knowledge, normal values for sIL2-R index have not yet been established.
Statistical analysis
For statistical analysis all samples with no detectable sIL2-R were set to the detection limit of the test (34 pg/m). Mann–Whitney-U-test was used to compare sIL2-R levels and index in each of the study groups. Due to multiple comparisons, Bonferroni adjustment was performed and a p value below 0.007 was regarded as statistically significant. Statistical analysis was performed using SPSS 13.0 software program (SPSS, Chicago, IL, USA).
Sensitivity, specificity and overall accuracy of CSF sIL2-R for the identification of neurosarcoidosis patients among patients with chronic CNS inflammation were determined. Sensitivity was calculated as ratio of sIL2-R positive neurosarcoidosis patients and all patients with neurosarcoidosis. Specificity is defined as ratio of sIL2-R negative patients (MS, VS) and all patients with MS or vasculitis. To assess the overall accuracy, the sum of sIL2-R positive neurosarcoidosis patients plus sIL2-R negative controls was divided by the number of all patients (MS, VS).
Results
Clinical presentation of patients with neurosarcoidosis
Twenty-eight paired CSF and serum samples of 11 patients with neurosarcoidosis were tested. The mean age in the neurosarcoidosis patients did not significantly differ from the mean age of the control groups. Only the healthy volunteers were younger (26 ± 0.9 years) and the bacterial meningitis patients older (52 ± 3.5 years) than the NS patients. In four patients, the diagnosis of systemic sarcoidosis was established only when neurological signs appeared. In all patients but one an extracerebral manifestation of sarcoidosis was detected before or during the clinical work-up. The diagnosis of sarcoidosis was ascertained by biopsy in six patients, one of which presented as isolated neurosarcoidosis. One patient had a possible, nine patients had a probable and one patient had a definite diagnosis of neurosarcoidosis according to the Zajicek criteria [7]. Affected organs and presenting CNS symptoms are listed in Table 1.
CSF analysis
Most patients with NS showed abnormalities in routine CSF analysis. In 18 of 28 samples, the cell count was elevated (>4 cells/μl). However, CSF cell count did not exceed 357 cells/μl (31 ± 12.9, mean ± std error). Total protein in the CSF was elevated (>45 mg/dl) in 16 out of 28 samples. In five of the 28 samples, CSF glucose was below 50% of serum glucose. CSF lactate was elevated (>2.1 mmol/l) in two out of 22 samples. ACE was measured in eight CSF samples, but was elevated in only one single CSF sample. Oligoclonal bands were detected in six CSF samples of NS patients without corresponding bands in the serum samples. The mean cell count and total protein in the CSF are given in Table 2.
Soluble IL2 receptor
sIL2-R levels in the CSF of patients with NS differed significantly from those in the CSF of healthy volunteers (Fig. 1; Table 2). Equally, sIL2-R was significantly higher in the CSF of neurosarcoidosis patients than in patients with multiple sclerosis or CNS vasculitis. CSF sIL2-R did not differ significantly between the neurosarcoidosis and both meningitis groups. Although sIL2-R was significantly higher in neurotuberculosis than in neurosarcoidosis (p = 0.001), sIL2-R values of 10 of 13 neurotuberculosis samples and of most active neurosarcoidosis samples were found within a common range (400–20,000 pg/ml). However, the CSF cell count in neurosarcoidosis patients differed significantly from those in all three meningitis groups (p < 0.001 in all cases, Fig. 2).
In the CSF of normal controls, sIL2-R was below the detection limit of 34 pg/ml in all cases, whereas 14 of 21 samples in the MS group, and 12 of 20 samples in the CNS vasculitis group were below the detection limit. In the neurosarcoidosis group, six of 28 samples were below the detection limit, all of which were assessed during treatment with steroids or in a clinically silent period. In the meningitis groups, one of 17 (VM), one of 22 (BM) and none of TB samples, respectively, were below the detection limit.
Introducing a cut-off value with best discriminative properties, only one MS and three CNS vasculitis samples were above and two active neurosarcoidosis samples below the cut-off value of 150 pg/ml.
However, in contrast to the CSF levels, sIL2-R serum levels did not differ significantly between the neurosarcoidosis and any of the five control groups (Fig. 3).
sIL2-R index
Mean sIL2-R index values in the NS, NT and VM were significantly higher than in the healthy control group (p < 0.01). The highest mean sIL2-R index was found in patients with viral meningitis. In the CNS vasculitis and BM group, mean sIL2-R index did not exceed the value measured in healthy controls.
As with the absolute CSF sIL2-R levels, differences of the sIL2-R index between the NS group and the MS, VS, HC groups were significant (multiple sclerosis p = 0.005, healthy controls p = 0.002, CNS vasculitis p < 0.001). No statistical significant difference between neurosarcoidosis and viral meningitis was seen. Compared to the assessment of absolute CSF sIL2-R levels, determination of sIL2-R index values failed to show a significant difference between neurosarcoidosis and neurotuberculosis. On the other hand, the sIL2-R index clearly distinguished between neurosarcoidosis and bacterial meningitis (p < 0.001). Mean sIL2-R levels for all groups are given in Table 2.
Sensitivity and specificity of CSF sIL2-R levels
A sIL2-R level in the CSF exceeding 150 pg/ml classified a patient with neurosarcoidosis correctly with a sensitivity of 61% and a specificity of 93% against patients with other chronic inflammatory diseases of the CNS (MS and vasculitis control group) or healthy controls. The positive predictive value (PPV) was 0.81, the negative predictive value (NPV) was 0.83 and the overall accuracy (OA) 0.83. If neurosarcoidosis patients undergoing steroid treatment or with silent disease were excluded, the sensitivity rose to 94% with an unchanged specificity and an overall accuracy of 0.93. Under these conditions, PPV was 0.8 and NPV 0.98.
However, sIL2-R levels in the CSF above 150 pg/ml did not discriminate neurosarcoidosis and meningitis due to viruses or bacteria (sensitivity 61%, specificity 13%, OA 0.3).
Since neurosarcoidosis and neurotuberculosis may present with similar clinical and CSF characteristics we looked for a cut-off sIL2-R value that would distinguish the two diseases. A sIL2-R level in the CSF of more than 1,500 pg/ml identified a patient with neurotuberculosis with a sensitivity of 80% and a specificity of 67%, resulting in an overall accuracy of 0.73. However, as mentioned above, the CSF sIL2-R levels of 10 of 13 neurotuberculosis and most active neurosarcoidosis patients were found in a common range.
Repetitive sIL2-R measurement
In six patients with neurosarcoidosis, repetitive spinal taps were done and 2–8 CSF samples from each patient were available for analysis. Since sIL2-R levels fluctuated substantially during the course of the disease depending on disease activity and treatment status, three patients are presented in more detail.
Patient 1
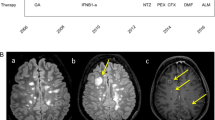
A 32 year old female of African descent presented with recurrent headache, sinusitis and swelling of the lacrimal gland. The neurological examination was normal. CSF analysis revealed a slight lymphocytic pleocytosis of 14 cells/μl, a slight disturbance of the blood-CSF-barrier and an intrathecal IgG, IgA and IgM synthesis. Some non-enhancing white matter lesions were seen on magnetic resonance imaging (MRI) (see Fig. 4a). Schirmer’s test was normal and no other evidence of Sjögren’s syndrome was found. Wegener’s granulomatosis was ruled out histologically by lip biopsy. X ray of the lung and ACE in serum and CSF were normal. Symptoms remitted after treatment with steroids was initiated. After glucocorticoids were tapered very slowly to a dose of 6 mg methylprednisolone, the patient deteriorated with weight loss and nightly sweating. Later, left-sided retroorbital pain and visual loss occurred. On MRI, an enhancing lesion beneath the left wing of the sphenoid and the left orbita was detected in exactly the same position where a Gallium radioisotope scan revealed enhanced Gallium uptake (Fig. 4b). A biopsy from this area showed histologically granulomatous tissue. After glucocorticoid treatment was increased, symptoms and MRI findings improved. Extensive investigations could not detect any extracerebral tissue involvement. Under low-dose glucocorticoid treatment the patient is now stable for several years. The results of the repetitive sIL2-R measurement in serum and CSF are given in Fig. 5.
Representative MRI images of patient 1 (a and b) and patient 2 (c and d). In a white matter lesions (FLAIR sequence), in d an ischemic infarction of the pons (T2 weighted sequence), in b an enhancing retro-orbital mass, and in c Gadolinium enhancing meninges (c and d Gadolinium enhanced T1 weighted scans) are shown
Patient 2
A formerly healthy 24 year old male of Caucasian descent presented with headache, vomitus, confusion and gait disturbance in the absence of fever. CSF analysis revealed moderate lymphocytic pleocytosis and severe disturbance of the blood-CSF-barrier without intrathecal immunoglobulin synthesis. Lactate was elevated with 7.1 mmol/l and the CSF glucose was 15% of the serum glucose. Infection with mycobacterium tuberculosis could be ruled out by PCR and CSF culture as well as the presence of other infectious agents by appropriate laboratory tests. MRI showed a nodular basal Gadolinium enhancement of the meninges (see Fig. 4c). On X ray, an enlargement of the mediastinal lymph nodes was seen. Lymphocyte subsets in the bronchioalveolar lavage were changed in favour of the CD4 positive cells with a CD4/CD8 ratio of 4.1. The diagnosis of meningoencephalitis due to sarcoidosis was made and a glucocorticoid treatment initiated. Thereafter, the patient improved as did the CSF and MRI findings. However, 5 weeks after the first symptoms the patient suffered an ischemic infarction of the right pons with hemiparesis on the left side (Fig. 4d). Meanwhile the patient is in a stable phase under a treatment with cyclophosphamide and steroids. Serum and CSF sIL2-R levels are given in Fig. 6.
Patient 3
A 72 year old male of Caucasian descent developed an incomplete myelitis with saddle block hypesthesia and exaggerated tendon reflexes of the right lower limb within several months. CSF analysis revealed a mild lymphocytic pleocytosis and a slight disturbance of the blood-CSF-barrier. The MRI detected a myelitic lesion at Th7. Under treatment with glucocorticoids, a slight improvement was seen. However, 6 months after the first symptoms, a transverse syndrome with sensomotor, bladder and bowel dysfunction occurred. Additionally, X ray of the lung revealed bilateral enlarged mediastinal lymph nodes that contained granuloma with epithelioid cells at the histological analysis. Steroid treatment achieved a partial improvement. Serum and CSF sIL2-R levels during the course of the disease are given in Fig. 7.
Discussion
To our knowledge, this is the first report on increased sIL2-R levels in the CSF of patients with neurosarcoidosis. We found that sIL2-R levels in CSF from patients with neurosarcoidosis are significantly higher than sIL2-R levels in MS, CNS vasculitis and healthy controls. Moreover, sIL2-R CSF concentrations exceeding 150 pg/ml were found to distinguish between patients with untreated neurosarcoidosis and patients with MS or vasculitis with an overall accuracy of 93%. The sensitivity of sIL2-R testing found in our study was higher than that of any other previously suggested test for NS, including ACE [8, 9]. Determination of sIL2-R levels might thus facilitate the early distinction of NS from other chronic inflammatory CNS disorders such as MS and autoimmune vasculitis, which is of importance since treatment options and long term prognosis differ substantially.
In contrast to what we found in patients with chronic inflammatory CNS diseases, sIL2-R levels in the CSF did not differentiate well between bacterial or viral meningitis and NS. However, bacterial meningitis may be differentiated easily from neurosarcoidosis by clinical signs, CSF cell count and type of pleocytosis. Difficulties may arise in differentiating a patient with aseptic meningitis due to neurosarcoidosis and a patient with lymphocytic meningitis. In this rare case, the measurement of sIL2-R in the CSF will be of little value.
Although patients with neurotuberculosis tend to show higher CSF sIL2-R levels than patients with neurosarcoidosis, sIL2-R will not be of great value in distinguishing between the two diseases due to largely overlapping levels. However, a cut-off value of 1,500 pg/ml had a sensitivity of 80% and a specificity of 67% in differentiating neurotuberculosis from neurosarcoidosis.
Determination of the sIL2-R index did not add largely to the diagnostic value of sIL2-R measurement. This marker distinguished NS from MS, as well as NS from CNS vasculitis, with similar accuracy as absolute sIL2-R CSF levels. Viral CNS infection and neurotuberculosis could not be differentiated from neurosarcoidosis by means of sIL2-R index. Interestingly, the mean sIL2-R index in the BM group was not higher than that of the healthy controls. The latter finding supports the hypothesis that high CSF sIL2-R levels in bacterial meningitis originate from the serum and cross the highly disturbed blood-CSF-barrier rather than being released from CSF immune cells. In contrast, in diseases with modest blood-CSF-barrier disturbance, such as viral meningitis, and those with predominantly lymphocytic pleocytosis, such as neurosarcoidosis and neurotuberculosis, activated CSF immune cells are probably the source of sIL2-R in the CSF since activated lymphocytes are known to shed sIL2-R from their surfaces [15].
Histologically, granulomatous lesions in patients with NS are characterised by an accumulation of activated T cells and macrophages. Even though the exact etiopathogenesis of the disease is still unknown, it is assumed that activated macrophages and T cells release proinflammatory cytokines such as TNFα and IL2 that subsequently attract and activate other mononuclear cells. IL-2 receptors are expressed on activated T cells and, in a soluble form, are released into the microenvironment.
Serum levels of sIL2-R are thought to correlate with disease activity in patients with systemic sarcoidosis, rendering them a potentially useful marker both for diagnosis and during treatment and clinical follow-up [11]. Similarly, in neurosarcoidosis patients sIL2-R levels in the CSF were found to fluctuate during the course of the disease in our study. Mainly treatment with steroids reduced sIL2-R levels in the CSF (and serum). In our patients, an increase in CSF sIL2-R was associated with onset of the CNS disease and even preceded the appearance of new neurological symptoms. In one case sIL2-R levels in the CSF even exceeded those in the serum (patient 2). This finding highlights the role of IL2 in the disease process. Measuring sIL2-R in the CSF thus might be helpful in monitoring CNS disease activity.
Although sarcoidosis is mostly regarded as following a benign course of disease, involvement of the CNS represents a potentially life threatening complication that requires immunosuppressive treatment. Measuring sIL2-R in the CSF may aid making appropriate treatment decisions in neurosarcoidosis.
Due to the limited number of patients, our results have to be regarded as preliminary and should be confirmed by further studies. If our findings are reproducible, elevated sIL2-R in the CSF might turn out to be a powerful tool improving diagnostic options and treatment control in neurosarcoidosis patients.
References
Kitaichi M (1998) Prevalence of sarcoidosis around the world. Diffuse Lung Dis 15:16–18
Hagerstrand I, Linell F (1964) The prevalence of sarcoidosis in the autopsy material from a Swedish town. Acta Med Scand Suppl 425:171–174
Manz HJ (1983) Pathobiology of neurosarcoidosis and clinicopathologic correlation. Can J Neurol Sci 10:50–55
Mayock RL, Bertrand P, Morrison CE, Scott JH (1963) Manifestation of sarcoidosis: analysis of 145 Patients, with a review of nine series selected in the literature. Am J Med 35:67–89
Stern BJ, Krumholz A, Johns C et al (1985) Sarcoidosis and its neurological manifestations. Arch Neurol 42:909–917
Vinas FC, Rengachary S (2001) Diagnosis and management of neurosarcoidosis. J Clin Neurosci 8:505–513
Zajicek JP, Scolding NJ, Foster O et al (1999) Central nervous system sarcoidosis-diagnosis and management. Q J Med 92:103–117
Khoury J, Wellik KE, Demaerschalk BM, Wingerchuk DM (2009) Cerebrospinal fluid angiotensin-converting enzyme for diagnosis of central nervous system sarcoidosis. Neurologist 15:108–111
Marangoni S, Argentiero V, Tavolato B (2006) Neurosarcoidosis: clinical description of 7 cases with a proposal for a new diagnostic strategy. J Neurol 253:488–495
Joseph FG, Scolding NJ (2009) Neurosarcoidosis: a pilot study of 30 new cases. J Neurol Neurosurg Psychiatry 80:297–304
Ziegenhagen MW, Benner UK, Zissel G et al (1997) Sarcoidosis: TNF-alpha release from alveolar macrophages and serum level of sIL-2R are prognostic markers. Am J Respir Crit Care Med 156:1586–1592
Grutters JC, Fellrath JM, Mulder L et al (2003) Serum soluble interleukin-2 receptor measurement in patients with sarcoidosis: a clinical evaluation. Chest 124:186–195
Keicho N, Kitamura K, Takaku F, Yotsumoto H (1990) Serum concentration of soluble interleukin-2 receptor as a sensitive parameter of disease activity in sarcoidosis. Chest 98:1125–1129
Muller-Quernheim J, Pfeifer S, Strausz J, Ferlinz R (1991) Correlation of clinical and immunologic parameters of the inflammatory activity of pulmonary sarcoidosis. Am Rev Respir Dis 144:1322–1329
Rubin LA, Kurman CC, Fritz ME et al (1985) Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol 135:3172–3177
Acknowledgments
We thank Hendrik Lindeman for excellent technical assistance.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petereit, HF., Reske, D., Tumani, H. et al. Soluble CSF interleukin 2 receptor as indicator of neurosarcoidosis. J Neurol 257, 1855–1863 (2010). https://doi.org/10.1007/s00415-010-5623-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-010-5623-3