Abstract
Prior work has shown that adiabatic T1ρ and T2ρ relaxation time constants may have sensitivity to cellular changes and the presence of iron, respectively, in Parkinson’s disease (PD). Further understanding of these magnetic resonance imaging (MRI) methods and how they relate to measures of disease severity and progression in PD is needed. Using T1ρ and T2ρ on a 4T MRI scanner, we assessed the substantia nigra (SN) of nine non-demented moderately affected PD and ten gender- and age-matched control participants. When compared to controls, the SN of PD subjects had increased T1ρ and reduced T2ρ. We also found a significant correlation between asymmetric motor features and asymmetry based on T1ρ. This study provides additional validation of T1ρ and T2ρ as a means to separate PD from control subjects, and T1ρ may be a useful marker of asymmetry in PD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pathogenesis of Parkinson’s disease (PD) is influenced by genetic and/or environmental factors. Iron is increased in the substantia nigra (SN) in PD and is stored as ferritin or neuromelanin in neurons and glia [1, 2]. Release of iron could facilitate oxidative reactions, thereby leading to oxidative stress and subsequent neurodegeneration [3, 4]. It remains to be determined if iron deposition is affected by heavy metal environmental exposure, varying dietary metal intake, polymorphisms, or mutations in metal regulatory proteins or PD causative genes, or if it is a result of the disease process and not necessarily causative [5]. Also, recent post-mortem work has demonstrated increased expression of a divalent metal transporter (DMT1/Nramp2/Slc11a2) isoform in the SN of PD brains, which suggests that this may enhance iron’s entry into nigral neurons and hasten or facilitate PD pathogenesis [6].
Meanwhile, several research groups, including our own, have utilized iron sensitive magnetic resonance imaging (MRI) methods to separate individuals with PD from healthy controls [7–9]. Some techniques have also shown a relationship with the SN and lateralized motor scores but only with the more severely affected contralateral side [10, 11]. In a different study, Gorell et al. [9] presented a correlation of asymmetry of simple reaction time with asymmetries of SN iron-related contrasts generated with R *2 and R2′.
The present study was designed to: (1) replicate that neuronal-sensitive and iron-sensitive measures, T1ρ and T2ρ, respectively, can separate PD from controls in a cross-sectional study; (2) determine if there is a correlation between clinical measures of PD and MRI relaxation times; and (3) evaluate if MRI methods could provide correlations with clinical asymmetry.
Methods
Patient and control subjects were recruited from the University of Minnesota Movement Disorders Clinic or through posted advertisements. Interested and eligible participants provided informed consent in this University of Minnesota Institutional Review Board-approved study. In addition to age- and gender-matched controls, subjects with a diagnosis of PD [12] who were taking and responding to antiparkinsonian medication were enrolled. All PD subjects were rated in their “on” motor state by the same neurologist (PT) using the complete Unified Parkinson’s Disease Rating Scale (UPDRS), including the Hoehn and Yahr (H & Y) rating scale [13]. Individuals with dementia (as ascertained clinically and with a Mini-Mental State Examination score <24) and those unable to undergo a brain MRI were excluded from this study [14].
All scans were performed on an MRI system using a Varian Unity INOVA console (Varian Associates, CA) interfaced to a 90-cm bore 4T magnet (Oxford Magnet Technology, Oxford, UK). Transverse multislice images were obtained with a Rapid Acquisition with Relaxation Enhancement (RARE) sequence [repetition time (TR) = 4 s, echo train length = 8, echo spacing = 15 ms, echo time = 60 ms, seven slices, two averages]. Slices were positioned perpendicular to the longitudinal axis of the brainstem. The image of midbrain including the center of the red nucleus (RN) and mammillary body was selected for the SN analysis [8]. T1ρ and T2ρ measurements were performed as described in prior work [15]. For T1ρ and T2ρ, TurboFLASH imaging readout (four segments) was used [16]. T1ρ and T2ρ were assessed using 0.70 mm2 in-plane resolution, FOV = 20 cm2, 2562 matrix, and slice thickness = 3 mm. Thus, the digital pixel area is 0.49 mm2, and voxel volume is 1.5 μL. T1ρ and T2ρ measurements were performed using variable numbers (m) of hyperbolic secant adiabatic full passage (AFP) HS1 pulses using pulse time duration 0.006 s and calibrated to the peak power ω max1 = 1.3 kHz [17].
For T2ρ measurements, the AFP pulse train was placed after coherence excitation by an adiabatic half passage (AHP) pulse. Magnetization was returned back to the Z′ axis using AHP pulse, and the TurboFLASH imaging readout was used. For T1ρ measurements, the AFP pulse train was placed prior to the coherence excitation by an AHP pulse.
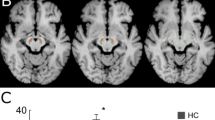
Relaxation T1ρ and T2ρ maps were generated from pixel-by-pixel analysis using MATLAB software package (MATLAB 7.0, Mathworks, Natick, MA, USA). Region-of-interest (ROI) analyses were performed by one of the authors in a blinded manner. The process of segmenting acquired images of the SN into the pars reticularis (SNr) and pars compacta (SNc) is demanding as there is often interdigitation of tissue [10, 18]. From our images, a low signal intensity area which merges anteriorly into the cerebral peduncle corresponds to SNr and a relatively brighter crescent-shaped region located between SNr and RN may be SNc. In this study, we placed a ROI centrally in the hyperintense area of the region that we designate the SNc (Fig. 1). It was first placed in the RARE image and then copied to matching T1ρ and T2ρ maps. Statistical analyses were performed on the mean values of the relaxation time constants obtained from the ROIs. For each PD patient the degree of asymmetry of motor dysfunction [19] was determined according to the following formula:
UPDRS right and UPDRS left correspond to the sum of each side’s UPDRS part III (motor UPDRS) ratings for rigidity, tremor, and bradykinesia (i.e., finger taps, hand grips, hand pronate/supinate, leg agility). The more negative scores imply more severe left-sided motor findings, and more positive scores correspond to more severe right-sided motor findings. Values of 0 represent symmetric motor impairment. The identical equation was used for calculation of T1ρ and T2ρ relaxation time constant asymmetries, respectively. A correlation between clinical and MRI asymmetry scores were calculated regarding a relation between the given SN and motor symptoms on the contralateral body side.
Statistical comparisons were based on results of the two-sided Student t test. In addition, a discriminatory ability of T1ρ and T2ρ was investigated using receiver operating characteristics (ROC) curves. As a measure of correlations between clinical and MRI findings, the Pearson product–moment correlation coefficient (r) was used. Only p values less than 0.05 were considered to indicate a significant difference.
Results
Nine patients with moderate PD and disease duration greater than 5 years [4 females (F) and 5 males (M)]; 59.0 ± 7.1 years [mean age ± standard deviation (SD)] and ten age- and gender-matched healthy volunteers [5 F and 5 M; 59.3 ± 5.5 years (mean age ± SD)] were included in this prospective study. The mean “ON state” UPDRS motor score was 31 ± 13 (mean age ± SD), and the mean “on” state H&Y score was 2.5. Duration of PD after diagnosis was 7.67 ± 1.9 years (mean ± SD). In Fig. 2, T1ρ versus T2ρ relaxation time constants of SNc are plotted which shows a clear separation between the PD and control groups. ROC curves revealed good-to-excellent ability to differentiate between PD patients and healthy controls. The area under the ROC curve (AUC) for T1ρ is 0.989 (0.016), and for T2ρ the AUC is 0.956 (0.048). Both (T1ρ, T2ρ) together in a linear discriminant rule yields perfect classification for AUC = 1.0. Picking optimal cutoffs, the sensitivity of T1ρ was 1.00 with 95% (likelihood ratio) CI (0.81–1.00) and specificity 0.90 with 95% CI (0.63–0.99). The sensitivity of T2ρ was 0.89 (0.59–0.99) and specificity 1.00 (0.83–1.00). If both (T1ρ, T2ρ) are used together, the sensitivity and specificity reached 1.00 with 95% CI (0.81–1.00) for sensitivity and (0.83–1.00) for specificity. Figure 3 demonstrates a correlation between T1ρ-derived imaging asymmetry of the SNc and UPDRS III-based clinical asymmetry. No asymmetry was detected with T2ρ. Significant differences for both T1ρ and T2ρ relaxation time constants between PD and control subjects are shown in Table 1. In the PD group, there were no significant correlations between the H&Y stage, duration of the disease, UPDRS scores (total score or its subscores), and either contralateral T1ρ or T2ρ contrasts.
Discussion
This study showed that T1ρ and T2ρ of the “SNc” can distinguish patients with moderate PD from healthy age- and gender-matched controls, which further validates these MRI methods [8]. Compared to standard MRI techniques, utilization of adiabatic pulses at high magnetic fields enhances sensitivity of MRI to molecular motion in the local susceptibility gradients that arises from non-heme iron and tissue water–protein interactions. These methods have been shown to provide enhanced sensitivity to molecular dynamic processes over conventional T1 and T2 MRI methods as demonstrated in our prior work [8]. T2ρ provides information about diffusion and exchange of water protons in environments with different local susceptibilities, and as a result, the shortening of T2ρ is an indicator of iron content in tissue [8, 20]. Our results are in agreement with evidence of increased iron in the SN in PD [10, 11, 21]. Meanwhile, T1ρ contrast is considered to reflect differences in cell density by its specificity to water spin dynamics such as chemical exchange of protons between water associated with macromolecules and free water [8, 22–24]. The linkage of T1ρ and cell integrity is assumed from animal and human MRI studies [8, 23, 24]. Marked T1ρ increase in SNc as compared to controls was revealed in Pitx3-aphakia mice [23, 25]. In Pitx3 deficient mice SNc neurons vanish during development, which models loss of nigrostriatal neurons but not the gliosis in PD [23]. Human in vivo T1ρ-weighted MRI studies have shown changes in the hippocampus of Alzheimer’s patients [24] and in the SN of PD patients [8]. Both studies showed increased T1ρ in the diseased structures in patients, which suggests this measure is reflective of cellular density. Likewise, in this study we found increased T1ρ in the SN of PD versus controls. Although we evaluated a different cohort of PD subjects with longer duration of disease, we did not find a significant correlation between T1ρ or T2ρ and duration of disease, UPDRS scores, or stage of disease. This finding is congruent with previously published cross-sectional MRI studies looking at iron in the SN [9–11] and with studies using transcranial sonography (TCS) of the SN [26]. As all PD subjects were scanned on medication, one cannot appreciate if there were medication effects on imaging findings, or if there would have been imaging correlates to “off” UPDRS scores. Presently it remains to be determined if these methods provide a measure of disease severity or a means for tracking progression.
Another aspect that this study focused on is the asymmetric nature of disease. With nuclear tracer imaging, the ability to demonstrate laterality of disease is well established [27–29]. In the present study we demonstrated a correlation with T1ρ but not T2ρ measures of the SN with an asymmetry ratio calculated from UPDRS motor scores. Moreover and similar to the Gorel et al. paper, no relationship was found between means of lateralized motor scores and both T1ρ or T2ρ measures of respective SNcs [9]. This finding might be due to the age range and severity of subjects evaluated in this study, which was different from Martin et al. where they evaluated untreated patients and from the Wallis et al. study where they included patients with a greater range of disease duration and all H&Y stages [10, 11].
In conclusion, we have demonstrated the ability of MRI methods to separate PD from controls. T1ρ may be a useful marker of asymmetry in mild-moderate PD.
References
Zecca L, Stroppolo A, Gatti A, Tampellini D, Toscani M, Gallorini M et al (2004) The role of iron and copper molecules in the neuronal vulnerability of locus ceruleus and substantia nigra during aging. Proc Natl Acad Sci USA 101(26):9483–9848
Faucheux BA, Martin ME, Beaumont C, Hauw JJ, Agid Y, Hirsch EC (2003) Neuromelanin associated redox-active iron is increased in the substantia nigra of patients with Parkinson’s disease. J Neurochem 86(5):1142–1148
Kaur D, Lee D, Ragapolan S, Andersen JK (2009) Glutathione depletion in immortalized midbrain-derived dopaminergeric neurons results in increases in the labile iron pool: implications for Parkinson’s disease. Free Radic Biol Med 46(5):593–598
Zecca L, Casella L, Albertini A, Bellei C, Zucca FA, Engelen M et al (2008) Neuromelanin can protect against iron-mediated oxidative damage in system modeling iron overload of brain aging and Parkinson’s disease. J Neurochem 106(4):1866–1875
Rhodes SL, Ritz B (2008) Genetics of iron regulation and the possible role of iron in Parkinson’s disease. Neurobiol Dis 32(2):183–195
Salazar J, Mena N, Hunot S, Prigent A, Alvarez-Fischer D, Arrendondo M et al (2008) Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson’s disease. Proc Natl Acad Sci USA 105(47):18578–18583
Hutchinson M, Raff U (2008) Detection of Parkinson’s disease by MRI: Spin–lattice distribution imaging. Mov Disord 23(14):1991–1997
Michaeli S, Oz G, Sorce DJ, Garwood M, Ugurbil K, Majestic S et al (2007) Assessment of brain iron and neuronal integrity in patients with Parkinson’s disease using novel MRI contrasts. Mov Disord 22(3):334–340
Gorell JM, Ordidge RJ, Brown GG, Deniau JC, Buderer NM, Helpern JA (1995) Increased iron-related MRI contrast in the substantia nigra in Parkinson’s disease. Neurology 45(6):1138–1143
Martin WR, Wieler M, Gee M (2008) Midbrain iron content in early Parkinson disease: a potential biomarker of disease status. Neurology 70(16):1411–1417
Wallis LI, Paley MN, Graham JM, Grunewald RA, Wignall EL, Joy HM et al (2008) MRI assessment of basal ganglia iron deposition in Parkinson’s disease. J Magn Reson Imaging 28(5):1061–1067
Gelb D, Oliver E, Gilman S (1999) Diagnostic criteria for Parkinson disease. Arch Neurol 56(1):33–39
Fahn S, Elton RL, members of the UPDRS Development Committee (1987) Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M (eds) Recent developments in Parkinson’s disease. Macmillan Healthcare Information, Florham Park, pp 153–163
Folstein M, Folstein S, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198
Michaeli S, Grohn H, Grohn O, Sorce DJ, Kauppinen R, Springer CS Jr et al (2005) Exchange-influenced T2rho contrast in the human brain images measured with adiabatic radio frequency pulses. Magn Reson Med 53(4):823–829
Haase Snapshot A, MRI FLASH (1990) Applications to T1, T2, and chemical-shift imaging. Magn Reson Med 13(1):77–89
Silver MS, Joseph RI, Chen CN, Sank VJ, Hoult DI (1984) Selective population inversion in NMR. Nature 310(5979):681–683
Damier P, Hirsch E, Agid Y, Graybiel A (1999) The substantia nigra of the human brain II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 122:1437–1448
Foster ER, Black KJ, Antenor-Dorsey JA, Perlmutter JS, Hershey T (2008) Motor asymmetry and substantia nigra volume are related to spatial delayed response performance in Parkinson’s disease. Brain Cogn 67(1):1–10
Haacke EM, Cheng NYC, House MJ, Liu Q, Neelavalli J, Ogg RJ et al (2005) Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging 23(1):25
Sofic E, Riederer P, Heinsen H, Beckmann H, Reynolds GP, Hebenstreit G et al (1988) Increased iron (III) and total iron content in postmortem substantia nigra of parkinsonian brain. J Neural Transm 74(3):199–205
Grohn OH, Lukkarinen JA, Silvennoinen MJ, Pitkanen A, van Zijl PC, Kauppinen RA (1999) Quantitative magnetic resonance imaging assessment of cerebral ischemia in rat using on-resonance T(1) in the rotating frame. Magn Reson Med 42(2):268–276
Michaeli S, Burns TC, Kudishevich E, Hanson T, Sorce DJ, Garwood M et al (2009) Detection of neuronal loss using T1rho MRI assessment of 1H2O spin dynamics in the aphakia mouse. J Neurosci Methods 177(1):160–167
Borthakur A, Sochor M, Davatzikos C, Trojanowski JQ, Clark CM (2008) T1rho MRI of Alzheimer’s disease. Neuroimage 41(4):1199–1205
Michaeli S, Burns TC, Kudishevich E, Harel N, Hanson T, Sorce DJ et al (2009) Detection of neuronal loss using T1rho MRI assesment of 1H2O spin dynamics in the aphakia mouse. J Neurosci Methods 177(1):160–167
Berg D, Merz B, Reiners K, Naumann M, Becker G (2005) Five-year follow-up study of hyperechogenicity of the substantia nigra in Parkinson’s disease. Mov Disord 20(3):383–385
Leenders KL, Salmon EP, Tyrrell P, Perani D, Brooks DJ, Sager H et al (1990) The nigrostriatal dopaminergic system assessed in vivo by positron emission tomography in healthy volunteer subjects and patients with Parkinson’s disease. Arch Neurol 47(12):1290–1298
Pirker W, Holler I, Gerschlager W, Asenbaum S, Zettinig G, Brucke T (2003) Measuring the rate of progression of Parkinson’s disease over a 5-year periods with beta-CIT SPECT. Mov Disord 18(11):1266–1272
Benamer HT, Patterson J, Wyper DJ, Hadley DM, Macphee GJ, Grosset DG (2000) Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov Disord 15(4):692–698
Acknowledgments
This study was supported by the University of Minnesota Academic Health Center, the University of Minnesota Undergraduate Research Opportunities Program, the University of Minnesota Medical Foundation, the National Institute of Dental & Craniofacial Research (T32DE007288), the National Institutes of Health (R01NS061866), and the Department of Veterans’ Affairs. We would like to acknowledge Heidi Vander Velden for her work as study coordinator and Dr. Michael A. Kuskowski (Minneapolis VA Medical Center, GRECC) for his assistance with the statistical analyses. T. Liimatainen was funded by the Instrumentarium Science Foundation, Orion Corporation Research Foundation, Finnish Cultural Foundation Northern Savo, and NIH grants P30 NS057091, P41 RR008079, R01NS061866 and R21NS059813.
Conflict of interest statement
The authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nestrasil, I., Michaeli, S., Liimatainen, T. et al. T1ρ and T2ρ MRI in the evaluation of Parkinson’s disease. J Neurol 257, 964–968 (2010). https://doi.org/10.1007/s00415-009-5446-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-009-5446-2