Abstract
Dopaminergic dysfunction is thought to play a pivotal role in human immunodeficiency virus (HIV)-related dementia. Decreased dopamine (DA) levels in the cerebrospinal fluid (CSF) and neuronal loss in the substantia nigra (SN) have been reported in HIV-infected patients, suggesting nigrostriatal damage. Structural changes detectable as hyperechogenicity in transcranial ultrasound (TCS) scans of the SN have been reported in patients with Parkinson’s disease (PD) and other neurological conditions. In this study, we assessed the echomorphology of the SN in 40 HIV-positive patients compared to 40 age- and sex-matched healthy controls and correlated these findings with CSF levels of DA and the metabolites homovanillic acid (HVA) and 3,4-dihydroxy phenylacetic acid (DOPAC) and with neuropsychologic performance. We observed that the SN of HIV-infected patients was hyperechogenic relative to that of controls (0.07 ± 0.05 vs. 0.04 ± 0.07 cm2; mean ± SEM; P < 0.001) and that this SN hyperechogenicity was correlated with decreased DA levels in the CSF, decreased CD4 cell count, and an impaired performance in the psychopathology assessment scale (AMDP) subtest for drive and psychomobility. An association to CDC stage, duration of HIV infection, or presence of HIV dementia was not observed. Our results indicate changes in the nigrostriatal system in HIV-infected patients that are detectable as hyperechogenic SN precede prominent extrapyramidal symptoms and cognitive dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinical manifestations of dopaminergic dysfunction are prominent findings in patients with human immunodeficiency virus (HIV) infection and consecutive HIV dementia. This symptom-complex consisting of subcortical dementia and extrapyramidal features, such as bradykinesia, postural and gait abnormalities, and hypomimia, was recognized in the 1980s and represents primary HIV-related neuronal damage involving the nigrostriatal pathway. Even in the absence of cognitive impairment, HIV-infected patients may exhibit subclinical motor disturbances [11]. Cerebrospinal fluid (CSF) studies also reflect dopaminergic dysfunction in HIV patients, with HIV-positive patients with or without neurological deficits showing significantly reduced levels of CSF dopamine (DA) than control subjects [12]. Using stereological techniques, Reyes et al. [25] estimated the volume density of melanin for quantitative assessment of pigmented cells in the pars compacta of the substantia nigra (SN) of 12 autopsied patients with acquired immunodeficiency syndrome (AIDS) without parkinsonism and 12 age-matched controls. There was a 25% neuronal decline in AIDS patients (P < 0.01), with evidence of reactive astrocytosis, extracellular melanin, and small neuronal cell bodies. These observations indicate injury of the nigrostriatal pathway prior to the development of extrapyramidal symptoms. Structural changes detectable as hyperechogenicity in transcranial ultrasound (TCS) of the SN were reported in patients with Parkinson’s disease (PD) [3, 8]. In this study, we assessed the echomorphology of the SN in different stages of HIV infection and correlated these findings with DA levels in the CSF, including its metabolites, and with neuropsychological performance.
Methods
Subjects
We investigated 40 male patients with HIV infection [mean age 45.9 ± 8.6 (SD) years; mean duration of HIV infection 9.8 ± 6.5 years (range 1–21 years); mean CD4 lymphocyte count 500.85 ± 273.5; mean plasma HIV RNA 8362.1 ± 10,544 copies/μl (range 50–122,040 copies/μl); mean T4/T8 ratio 0.5] and 40 healthy controls (35 males, mean age 46.6 ± 10.2) without any neurological limitation (Table 1). All patients were prospectively recruited from the outpatient clinic for sexually transmitted diseases at the University Hospital Essen. Inclusion criteria were informed consent and an age >18 years. Exclusion criteria were opportunistic systemic or CNS infection, CNS neoplasm, active alcohol or drug abuse, aphasia, and concomitant neurological disease, such as dementia caused by factors other than HIV, schizophrenia or other severe psychiatric disease. Thirty patients (75%) received highly active antiretroviral therapy (HAART). All patients and controls underwent a careful neurological examination, and all participants gave their informed consent according to the Declaration of Helsinki prior to study inclusion. The study was approved by the local ethics committee.
Neuropsychological and clinical testing
Neuropsychological assessments were performed using the following tests: trail making test A and B, Wechsler memory scale-R (figural and logical memory), mosaic test, digit symbol test, multiple word choice test form b (MWCT-b), Raven standard progressive matrices, Regensburg word fluency test, syndrome short test (SKT), Wisconsin card sorting test (WCST), and digit-span memory test. All patients were also evaluated by the Hamilton depression rating scale (HAM-D), unified PD rating scale (UPDRS), HIV dementia scale and psychopathology assessment scale (AMDP).
Transcranial sonography
Transcranial sonographic examination was performed as described by Berg et al. [8].
For TCS, a color-coded, phase array ultrasound system equipped with a 2.5-MHz transducer was used (Siemens Sonoline Elegra). Two investigators (MM, OK), blinded for the results of the neuropsychological testing and patients’ diagnosis, performed transcranial sonography via a preauricular acoustic bone window with a penetration depth of 16 cm and a dynamic range of 45 dB. The SN was identified within the mesencephalic brainstem, with scanning from both temporal bone windows. Since the signal brightness (echogenicity) is not quantifiable by ultrasound, the area of hyperechogenic signals in the SN region was encircled and measured. The average measurement values from both sides (left and right) were used for further analysis.
Measurement of DA, homovanillic acid, and 3,4-dihydroxy phenylacetic acid
Lumbar puncture was performed in 20 patients. Prior to analysis, the CSF samples were centrifuged at 10,000 rpm. (Hettich, Micro Rapid, Tuttlingen, Germany) through molecular weight filters (cut-off 5 kDa; Milipore, Bedford, MA) at 4°C to remove high-molecular-weight components. Filtrate (20 μl) was injected into a Rheodyne injector (model G1313 A, 1100; Agilent, Waldbronn, Germany) and analyzed for DA, and its metabolites DOPAC (3,4-dihydroxy phenylacetic acid) and HVA (homovanillic acid) were analyzed by a reverse-phase high-performance liquid chromatography system with electrochemical detection. The HPLC system (model 1100; Agilent) consisted of a solvent delivery pump, an electrochemical detector (Bio-Rad model 1640; Bio-Rad, Hercules, CA) with a glassy carbon electrode. Peaks were recorded by means of an integrator (HP Chem Station 2/3; Agilent). All settings, columns, and solutions were the same as described elsewhere [17]. Qualitative and quantitative analyses were performed by comparing retention times and peak heights with those obtained with commercially available standards (Sigma, St. Louis, MO).
Statistics
We used a simple t test for independent samples to make a group comparison of SN echogenicity between healthy controls and HIV-positive patients. The statistical level of significance was set to P < 0.05. We also performed a simple regression analysis using Pearson test for parametric correlation of SN echogenicity, CSF and serum analysis results, and clinical and neuropsychological testing results (SPSS for Windows ver. 14; SPSS, Chicago, IL).
Results
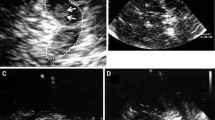
Figure 1 shows an example of an SN with a hyperechogenic area in contrast to a normal SN.
The TCS scan revealed a mean (± SD) area of SN hyperechogenicity of 0.07 ± 0.05 cm2 in HIV-infected patients in contrast to 0.04 ± 0.07 cm2 in the control group (P < 0.001) (Fig. 2a). The interrater variability of SN measurements between MM and OK was r = 0.81.
A neurological deficit was present in 13 patients and included sensory disturbances of the lower limb (seven patients), sensory disturbances of the upper limb (two patients), facial asymmetry after peripheral facial palsy (one patient), horizontal gaze nystagmus (one patient), gait ataxia (one patient), and resting tremor of the right side (one patient). There was no difference in SN echogenicity between patients with and without neurological deficits (P = 0.26). Highly active antiretroviral therapy did not influence SN echogenicity when patients on HAART were compared with those not on HAART (P = 0.46). The eight patients (10%) with HIV dementia scale results ≤10 had a lower CD4 cell count (361.6 ± 180.7 cells/μl; P = 0.046) than the other HIV patients, but there was no difference in SN echogenicity or DA level in the CSF.
In the CSF, the mean DA level was 29.6 ± 13.6 ng/ml, the mean HVA level was 54.4 ± 15.4 ng/ml, and the mean DOPAC level was 6.2 ± 3.9 ng/ml. Dopamine levels in the CSF were negatively correlated to the area of SN hyperechogenicity (r = −0.52; P = 0.018) (Fig. 2b). No correlation was found for HVA and DOPAC levels or CSF viral load (HIV RNA). The CD4 cell count was negatively correlated with SN hyperechogenicity (r = −0.35; P = 0.029) (Fig. 2c). Apart from CD4 cell count, we found no correlation to disease duration or disease severity (i.e., CDC stage, HIV RNA blood, etc.).
The psychopathology assessment scale (AMDP) disorders of drive and psychomotor speed and SN hyperechogenicity (r = 0.41; P = 0.006) were positively correlated. In contrast, the remaining neuropsychological tests correlated with neither SN hyperechogenicity or with CSF analysis results.
Discussion
Transcranial sonography revealed that HIV-infected patients have an enlarged area of hyperechogenic SN compared to healthy controls and that the SN hyperechogenicity is correlated with decreased levels of DA in the CSF, a decreased CD4 cell count, and impaired performance in the AMDP subtest for drive and psychomotor speed.
An increased hyperechogenicity of the SN, as evidenced on TCS scans, was first described in PD [3]. Subsequently, a hyperechogenic SN was reported in several other conditions, such as depression [28], parkinsonism induced by neuroleptic medication [8], spinocerebellar ataxia type 3 [23], and Huntington’s disease [24]. In contrast, a hypoechogenic SN was shown in patients with restless legs syndrome [26]. Substantia nigra hyperechogenicity is assumed to be caused by binding of an increased amount of iron to iron-metabolizing proteins or structural alteration of iron-binding proteins leading to differences in the reflection of the ultrasound beam, which is then displayed as hyperechogenic signals [4]. However, a correlation between SN hyperechogenicity and the degeneration of presynaptic dopaminergic neurons could not be demonstrated in a study combining TCS and single-photon emission computed tomography (SPECT) [27].
Motivational drive and psychomotor speed (as assessed by AMPD) was the only clinical feature that showed a correlation to SN hyperechogenicity in our patients, in contrast to extrapyramidal symptoms. In patients with PD, the size of the hyperechogenic area in the SN remains stable throughout the course of the disease, despite increased motor slowing [9].
In one study, a hyperechogenic SN was found in 46% of 33 healthy elderly subjects (age range 86–95 years); this hyperechogenic SN was strongly correlated with the presence of subtle extrapyramidal motor dysfunctions that were not prominent enough for clinical diagnosis of PD without cognitive deficits [5]. Studies in healthy younger subjects (mean age 77 years, n = 79) showed comparable results [10]. The authors proposed SN hyperechogenicity as a risk factor for nigral injury, which is supported by the fact that 9% of healthy young subjects (median age 30 years, n = 330) had hyperechogenic SN revealed on their TCS scans [7]. Further work-up in ten subjects did not find a deficit in comprehensive motor testing and neuropsychological assessment, but it did detect dopaminergic dysfunction with a marked decrease in the accumulation of [18F]-DOPA in the caudate nucleus and putamen using positron emission tomography (PET) [7]. Interestingly, in a prospective study, initial hyperechogenicity of the SN was strongly correlated with the development of extrapyramidal side effects in patients receiving their first neuroleptic therapy, indicating subclinical nigrostriatal injury disclosed by antidopaminergic agents [8]. The likelihood of HIV-positive patients developing extrapyramidal side effects due to neuroleptic therapy is two- to fourfold higher than that of HIV-negative subjects, providing reasons to assume subclinical dopaminergic dysfunction [15]. The prevalence of hyperechogenic SN in HIV-infected patients seems to parallel the higher prevalence of extrapyramidal side effects of antidopaminergic agents. Thus, SN hyperechogenicity may be an important risk factor for the development of these side effects in HIV-positive patients.
In our patients, there was no correlation between SN hyperechogenicity, duration of disease, or presence of HIV dementia. Hence, an early subclinical nigrostriatal injury detectable by TCS in HIV-infected patients may predict the development of extrapyramidal symptoms and HIV dementia, but it does not necessarily entail consequent disease progression.
The results from the studies of DA, DOPAC, and HVA in the CSF demonstrate that neurochemical alterations in therapy-naive asymptomatic HIV-infected patients do occur [1]. In our patients, DA levels in the CSF were negatively correlated with SN hyperechogenicity (Fig. 2b), indicating increased SN echogenicity as a marker for dopaminergic hypofunction. Before HAART was available, Larsson et al. [19] reported reduced CSF dopamine metabolite (e.g., HVA) levels in patients with AIDS compared to those at other stages of HIV and healthy controls. The stage of AIDS dementia was not associated with lower HVA levels in the CSF [19], but an association between lower HVA levels with impaired performance in motor speed, attention, concentration, and executive control in ten HIV patients was found [14]. Even though DA metabolites may allow conclusions to be drawn on DA behavior, our CSF results based on 20 HIV patients at different stages did not reveal an association between HVA levels in the CSF and neuropsychological testing.
The fact that SN hyperechogenicity negatively correlated to CD4 cell count may suggest that patients with a low CD4 cell count are at risk of developing structural changes in the SN following increased HIV neurotoxicity. Neurotoxicity in HIV is exhibited via the envelope protein glycoprotein (gp) 120, which causes apoptosis in in vitro and in vivo models [2, 13, 20]. With respect to the dopaminergic system, gp 120 reduces DA uptake in mesencephalic neuronal cultures in vitro [6], and the intrastriatal injection of gp 120 leads to specific loss of dopamine [21]. Tat is another HIV protein with neurotoxic potential for dopaminergic neurons via excitatory amino acid receptors [22] and mitochondrial dysfunction [18].
Neuronal loss in the SN of HIV-infected patients was described pathoanatomically by Reyes et al. [25] and Itoh et al. [16]. Unfortunately, the accumulation of tissue iron content—the presumed underlying mechanism for SN hyperechogenicity—was not examined in these studies. Pathological alterations of the SN were not accompanied by clinical signs of parkinsonism in patients undergoing autopsy, providing evidence that structural changes of the SN occur before the onset of extrapyramidal symptoms [25]. However, it has to be remembered that SN changes in HIV disease seem to result from primary viral protein neurotoxicity and inflammation leading to secondary degenerative processes, whereas SN changes in PD are probably induced by primarily degenerative processes.
In conclusion, our results indicate early nigrostriatal damage mediated through increasing HIV neurotoxicity in patients with a low CD4 count, detectable as hyperechogenicity on a TCS scan as DA depletion in the CSF. This leads to subtle clinical impairment of psychomotor speed before cognitive or extrapyramidal symptoms become apparent.
References
Arendt G, Nolting T, Frisch C, Husstedt IW, Gregor N, Koutsilieri E, Maschke M, Angerer A, Obermann M, Neuen-Jacob E, Adams O, Loeffert S, Riederer P, ter Meulen V, Sopper S (2007) Intrathecal viral replication and cerebral deficits in different stages of human immunodeficiency virus disease. J Neurovirol 13:225–232
Bagetta G, Corasaniti MT, Aloe L, Berliocchi L, Costa N, Finazzi-Agro A, Nistico G (1996) Intracerebral injection of human immunodeficiency virus type 1 coat protein gp120 differentially affects the expression of nerve growth factor and nitric oxide synthase in the hippocampus of rat. Proc Natl Acad Sci USA 93:928–933
Becker G, Seufert J, Bogdahn U, Reichmann H, Reiners K (1995) Degeneration of substantia nigra in chronic Parkinson’s disease visualized by transcranial color-coded real-time sonography. Neurology 45:182–184
Behnke S, Berg D, Naumann M, Becker G (2005) Differentiation of Parkinson’s disease and atypical parkinsonian syndromes by transcranial ultrasound. J Neurol Neurosurg Psychiatry 76:423–425
Behnke S, Double KL, Duma S, Broe GA, Guenther V, Becker G, Halliday GM (2007) Substantia nigra echomorphology in the healthy very old: correlation with motor slowing. Neuroimage 34:1054–1059
Bennett BA, Rusyniak DE, Hollingsworth CK (1995) HIV-1 gp120-induced neurotoxicity to midbrain dopamine cultures. Brain Res 705:168–176
Berg D, Becker G, Zeiler B, Tucha O, Hofmann E, Preier M, Benz P, Jost W, Reiners K, Lange KW (1999) Vulnerability of the nigrostriatal system as detected by transcranial ultrasound. Neurology 53:1026–1031
Berg D, Jabs B, Merschdorf U, Beckmann H, Becker G (2001a) Echogenicity of substantia nigra determined by transcranial ultrasound correlates with severity of parkinsonian symptoms induced by neuroleptic therapy. Biol Psychiatry 50:463–467
Berg D, Siefker C, Becker G (2001b) Echogenicity of the substantia nigra in Parkinson’s disease and its relation to clinical findings. J Neurol 248:684–689
Berg D, Merz B, Reiners K, Naumann M, Becker G (2005) Five-year follow-up study of hyperechogenicity of the substantia nigra in Parkinson’s disease. Mov Disord 20:383–385
Berger JR, Arendt G (2000) HIV dementia: the role of the basal ganglia and dopaminergic systems. J Psychopharmacol 14:214–221
Berger JR, Kumar M, Kumar A, Fernandez JB, Levin B (1994) Cerebrospinal fluid dopamine in HIV-1 infection. AIDS 8:67–71
Biard-Piechaczyk M, Robert-Hebmann V, Richard V, Roland J, Hipskind RA, Devaux C (2000) Caspase-dependent apoptosis of cells expressing the chemokine receptor CXCR4 is induced by cell membrane-associated human immunodeficiency virus type 1 envelope glycoprotein (gp120). Virology 268:329–344
di Rocco A, Bottiglieri T, Dorfman D, Werner P, Morrison C, Simpson D (2000) Decreased homovanilic acid in cerebrospinal fluid correlates with impaired neuropsychologic function in HIV-1-infected patients. Clin Neuropharmacol 23:190–194
Hriso E, Kuhn T, Masdeu JC, Grundman M (1991) Extrapyramidal symptoms due to dopamine-blocking agents in patients with AIDS encephalopathy. Am J Psychiatry 148:1558–1561
Itoh K, Mehraein P, Weis S (2000) Neuronal damage of the substantia nigra in HIV-1 infected brains. Acta Neuropathol 99:376–384
Koutsilieri E, Gotz ME, Sopper S, Stahl-Hennig C, Czub M, ter Meulen V, Riederer P (1997) Monoamine metabolite levels in CSF of SIV-infected rhesus monkeys (Macaca mulatta). Neuroreport 8:3833–3836
Kruman II, Nath A, Mattson MP (1998) HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol 154:276–288
Larsson M, Hagberg L, Forsman A, Norkrans G (1991) Cerebrospinal fluid catecholamine metabolites in HIV-infected patients. J Neurosci Res 28:406–409
Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ (1998) Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci USA 95:14500–14505
Nath A, Anderson C, Jones M, Maragos W, Booze R, Mactutus C, Bell J, Hauser KF, Mattson M (2000) Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. J Psychopharmacol 14:222–227
Nath A, Psooy K, Martin C, Knudsen B, Magnuson DS, Haughey N, Geiger JD (1996) Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J Virol 70:1475–1480
Postert T, Eyding J, Berg D, Przuntek H, Becker G, Finger M, Schols L (2004) Transcranial sonography in spinocerebellar ataxia type 3. J Neural Transm Suppl 68:123–133
Postert T, Lack B, Kuhn W, Jergas M, Andrich J, Braun B, Przuntek H, Sprengelmeyer R, Agelink M, Buttner T (1999) Basal ganglia alterations and brain atrophy in Huntington’s disease depicted by transcranial real time sonography. J Neurol Neurosurg Psychiatry 67:457–462
Reyes MG, Faraldi F, Senseng CS, Flowers C, Fariello R (1991) Nigral degeneration in acquired immune deficiency syndrome (AIDS). Acta Neuropathol 82:39–44
Schmidauer C, Sojer M, Seppi K, Stockner H, Hogl B, Biedermann B, Brandauer E, Peralta CM, Wenning GK, Poewe W (2005) Transcranial ultrasound shows nigral hypoechogenicity in restless legs syndrome. Ann Neurol 58:630–634
Spiegel J, Hellwig D, Mollers MO, Behnke S, Jost W, Fassbender K, Samnick S, Dillmann U, Becker G, Kirsch CM (2006) Transcranial sonography and [123I]FP-CIT SPECT disclose complementary aspects of Parkinson’s disease. Brain 129:1188–1193
Walter U, Hoeppner J, Prudente-Morrissey L, Horowski S, Herpertz SC, Benecke R (2007) Parkinson’s disease-like midbrain sonography abnormalities are frequent in depressive disorders. Brain 130:1799–1807
Conflict of interest statement
The authors report no conflicts of interests.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Obermann, M., Küper, M., Kastrup, O. et al. Substantia nigra hyperechogenicity and CSF dopamine depletion in HIV. J Neurol 256, 948–953 (2009). https://doi.org/10.1007/s00415-009-5052-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-009-5052-3