Abstract
Introduction
Diffusion-weighted imaging (DWI) is mainly used in acute stroke, and signal evolution in the acute phase has been studied extensively. However, patients with a minor stroke frequently present late. Recent studies suggest that DWI may be helpful at this stage, but only very few published data exist on the evolution of the DW-signal in the weeks and months after a stroke. We performed a follow-up study of DWI in the late stages after a minor stroke.
Methods
28 patients who presented 48 hours to 14 days after a minor stroke underwent serial MRI at baseline, 4 weeks, 8 weeks, 12 weeks, 6 months and ≥9 months after their event. Signal intensity within the lesion was determined on T2-weighted images, DW-images and the Apparent Diffusion Coefficient (ADC) map at each time-point, and ratios were calculated with contralateral normal values (T2r, DWIr, ADCr).
Results
T2r was increased in all patients from the beginning, and showed no clear temporal evolution. ADCr normalized within 8 weeks in 83% of patients, but still continued to increase for up to 6 months after the event. The DW-signal decreased over time, but was still elevated in 6 patients after ≥6 months. The evolution of ADCr and DWIr showed statistically highly significant inter-individual variation (p < 0.0001), which was not accounted for by age, sex, infarct size or infarct location.
Conclusion
The ADC and the DW-signal may continue to evolve for several months after a minor ischaemic stroke. Signal evolution is highly variable between individuals. Further studies are required to determine which factors influence the evolution of the ADC and the DW-signal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diffusion-weighted magnetic resonance imaging (DWI) is helpful in the diagnosis and management of acute stroke [4, 19]. Its main advantages in this setting are that it shows ischaemic lesions earlier than other imaging modalities [20], and that its interpretation is very reliable[11]. The time course of lesion evolution in the acute phase has been studied extensively [6, 18, 29] In contrast, the evolution of the DW-signal in the late phase after stroke has not been studied in detail. Data on signal evolution in the late phase after stroke have either been extrapolated from cross-sectional data [2, 9], or are limited to follow-up studies of acute stroke with a single scan in the late phase [13, 29], which did not allow determination of signal changes over time during the late stage after stroke, or of differences between patients. Although some authors commented that the DW-signal may remain elevated for several weeks or months after a stroke, and that rarely the Apparent Difusion Coefficient (ADC} may remain reduced for longer than 14 days [9, 13], the evolution of the ADC and the DW-signal beyond two weeks after an ischaemic event has not been studied systematically.
Two recent studies have shown that DWI is frequently helpful in the management of patients with minor, non-disabling stroke who present late [17, 28]. It identifies recent infarction more frequently than T2-weighted imaging [17], and provides additional information, such as clarification of the affected vascular territory [28]. However, if DWI is to be used widely in such patients, more detailed knowledge about its time course in the late phase after stroke is required. This information cannot necessarily be extrapolated from previous studies, since patients who present late differ from patients in studies of acute stroke: minor strokes will generally be milder, which may have led the patients to defer seeking medical attention. Minor strokes will generally be smaller than acute strokes which lead to immediate hospital admission, minor strokes may also affect different brain structures, and their appearance and evolution on DWI may be different. In the absence of any previous similar study, our aim was to study the evolution of the ADC and the DW-signal in the late stages after a minor stroke. We performed a follow-up study of patients who were scanned within 14 days after a minor stroke and then underwent repeated follow-up scanning for up to a year after their event.
Methods
In this study, patients were recruited from the Oxford Vascular Study (OXVASC), a population-based study of TIA and stroke [26]. The methodology of the OXVASC study has been published in detail elsewhere [26]. Briefly, through collaboration with nine general practices, an urban and rural population of 91 000 people was studied. General practitioners (GPs) were encouraged to report all patients who might have suffered a TIA or stroke during the study period. Strokes were also identified by daily assessment of hospital registers, hospital diagnostic coding, review of referrals for brain and vascular imaging, and review of all death certificates and coroner’s reports when relevant. A study neurologist assessed all cases as soon as possible after notification. Details of the presenting event, clinical characteristics, and medical history were recorded from the patient, GP records, and hospital records. Stroke and TIA were defined according to WHO criteria (sudden onset of neurological deficit, persisting for >24 hours in case of a stroke, or for <24 hours in case of a TIA) [31]. Within OXVASC, one method of ascertaining patients was the daily study clinic, to which GPs referred patients with a suspected TIA or stroke. Patients for the present study were recruited from this clinic. All patients referred to the clinic were reviewed by a study neurologist. If the study neurologist diagnosed a minor stroke the patients were asked whether they would be willing to participate in the study. Patients who consented were scanned as soon as possible within two weeks after their event. They were included in the follow-up study if a relevant lesion was found on DWI. Subsequent follow-up scans were conducted, where possible, 4 weeks after the event (2 weeks after the first scan), and then at 8 weeks, 12 weeks, 6 months, and ≥9 months after the event. The study was approved by the local ethics committee.
Diffusion-weighted imaging
Scanning was conducted on a 1.5 Tesla Siemens Symphony system with quantum gradients. Diffusion-weighted imaging was conducted with the following imaging parameters: TR 5400 ms, TE 84 ms, 36 slices, slice thickness 4.0 mm, no interslice gap, matrix 128*128, FOV 200*200. The diffusion weighted sequence was acquired with two different b-values (b = 0 and 1000 s/mm2). A positive DWI scan was defined as high signal on the b1000 image. The ADC-map was calculated pixel by pixel according to the following formula: ADC = −ln(Sib1000/Sib0)/1000; with Si describing the signal intensity of the b1000 and the b0-image. To exclude haemorrhage and mass lesions close to the area of interest on DWI, the study protocol also included a T2-weighted turbo gradient spin echo (TGSE) sequence and T1-weighted imaging. To ensure stable imaging quality and signal intensity over time, routine quality assessments of the scanner, which ensured that the scanner and the coils were functioning correctly, were performed daily. Calibrations of the gradients using a test phantom of known dimension were performed regularly and no drift in size was observed. To minimize differences in slice position for serial scans, we recorded head positioning for the first scan. At each follow-up scan, we carefully compared the scout images for the follow-up scans with the first scan, ensuring consistent patient positioning between scans. Scans were analysed with Leonardo Syngo (Siemens TM) software Version 2003 A.
Image analysis
A single observer reviewed all DW-images and ADC-maps. The observer manually drew a region of interest (ROI) around the lesion on the b1000 image. The imaging slice which showed the largest extent of the lesion was used. The imaging software allowed direct transfer of the ROI onto the b0-image, which was used to study T2-effects over time, and the ADC-map. For each ROI the mean signal intensity was determined. To control for any potential residual variation in signal intensity between scans, we calculated ratios with the signal intensities of normal control ROIs in the contralateral hemisphere, which corresponded to the lesion ROI in size and location (DWIR, T2R and ADCR). To minimise intra-observer variation, all measurements were performed twice and the mean of the two values was taken. To determine changes in signal intensity over time, we then copied the ROIs from the first scan onto the follow-up scans and measured signal intensity within the ROIs. We measured the mean signal of the entire lesion because we felt that this was the most reliable way of measuring signal intensity within the same area of the brain on repeated occasions. Although we had taken great care to ensure consistent patient positioning, there may still have been minor misalignment between scans. Small ROIs would have been difficult to position into the same area of the brain at each scan, and minor misalignment could have led to a proportionally large measurement error. While a larger ROI might also not have been positioned in exactly the same place, it was more likely to include the previously measured area.
Statistical analysis
Observer reliability was determined with the intra-class correlation coefficient. To determine the evolution of the ADC and the DW-signal over time, we calculated the mean signal intensities at each time point for the entire patient cohort and tested them for heterogeneity and linearity. To determine whether signal evolution was associated with age, sex, infarct size or infarct location, we fitted a linear model for repeated measures to the data, and consecutively included these variables in the models. To test for the extent of inter-individual variation in signal evolution, we plotted the signal intensities versus each time point for each individual patient and visually inspected the graphs. We also fitted a mixed non-linear model and a fixed effect model to the data and compared the models to determine whether there was significant inter-individual variation in signal evolution. The statistical analyses were performed with SPSS Version 12.0® and with S-Plus®.
Results
Twenty-eight patients (9 female, mean (SD) age = 70.0 (11.6) years, range 39–90) were included in the study. Median (IQR) NIH-score [14] at baseline was 1 (0–3). Mean (SD) time from symptom onset to first scan was 9 (3) days. All patients attended for a second scan 4 weeks after their event, 23 attended for a third scan. Ten patients attended for the final follow-up scan at 9 months or more after the event. Of these, five had missed the follow-up appointment at either 3 or 6 months. (see also Table 1) There were 9 cortical infarcts, 13 subcortical infarcts, 2 brainstem infarcts and 4 infarcts that affected both cortical and subcortical tissue. Stroke aetiology according to TOAST criteria [1] was: large vessel – 7 patients, small vessel – 7 patients, cardioembolic – 1 patient, undetermined aetiology – 13 patients.
Intra-observer agreement for image analysis was high. The intraclass correlation coefficient for the DWI-measurements was 0.96 (95% confidence interval = 0.92–0.98, p < 0.0001), for the ADC it was 0.98 (95% CI = 0.95–0.99, p < 0.0001).
In all patients, the T2-signal was and remained increased from the first scan. Table 1 shows the proportion of scans in which the signal on the b1000-image had normalized or decreased, and the percentage of ADC-maps with normalized or increased signal at each point during follow-up. These proportions are likely to represent underestimates: between each of the scans, some patients withdrew from follow-up. If the DW-signal on their final scan had already normalized or was decreased, we included their scans as “negative” in the analysis. However, if the DW-signal was still increased at the final scan, we excluded them, since it was not possible to estimate if and when the signal would normalize. We proceeded in a similar manner with the ADC-maps, assuming that an increased ADC would remain elevated, but excluding patients with decreased ADC on their final scan. Allowing for these assumptions, Table 1 shows that even ≥ 9 months after the event, the DW-signal was decreased in only 60% of scans, and in some patients who agreed to further follow-up, high signal persisted beyond a year. In contrast, the proportion of scans with a persistently decreased ADC was much lower: the ADC had already normalised or was elevated at baseline in 39% of patients. All of these patients were scanned more than one week after their stroke. Within 8 weeks, the ADC was normal or increased in 83% of patients, and it had normalised or was increased in all but one patient six months after the event.
Table 1 also shows the mean signal intensities for the T2-, DW- and ADC-images averaged across all patients scanned at each time point.The T2-signal did not change significantly over time (phet = 0.52), whereas there was a significant decrease in the intensity of the DW-signal (plin < 0.0001) and a significant increase in the ADC (plin < 0.0001). These findings are confirmed in Figure 1, which shows the temporal evolution of the T2-signal, the DW-signal and the ADC for patients who were scanned at least 4 times. The T2-signal was increased at all times, with no obvious temporal evolution. The DW-signal, which according to the inclusion criteria was increased on all baseline scans, decreased during follow-up. This decrease was most marked between baseline and first follow-up 4 weeks after the event. The DW-signal then continued to decrease more gradually over the ensuing weeks and months. The mean ADC was still reduced at baseline, but elevated 4 weeks after the event. It continued to increase for up to six months after the event. In a linear model for repeated measures, lesion size, infarct location (cortical versus subcortical) and infarct aetiology according to TOAST criteria did not have any significant effect on the time course of the T2-signal, the DW-signal or the ADC.
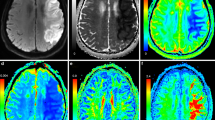
These results indicate that while the ADC normalizes earlier than the DW-signal, both continue to change for several months after an ischaemic event. However, this does not take into account that signal evolution may vary between individuals. Figure 2 gives an example of such variation: while the DW-signal of the medullary infarct shown in Figure 2a was already decreased after 8 weeks, the DW-signal of the subcortical infarct in Figure 2b only normalized after a year. In our cohort, time to normalization of the DW-signal was highly variable: it had normalized within 8 weeks in only 12% of patients, and it was still increased after 6 months in 53% of patients. (Table 1) In contrast, after 8 weeks the ADC had normalised or become elevated in 83% of patients. However, although it was already elevated, between 8 weeks and >9 months the ADC continued to rise in 7 of the 10 patients in whom measurements at this point were available. The extent of interindividual variability in the temporal evolution of the T2-signal, the DW-signal and the ADC is shown in Figure 3.
a: MR-images at baseline, 4 weeks and 8 weeks in a 58 year-old patient who presented with dysarthria and right-sided incoordination. MRI showed a right medullary infarction. The signal on DWI was markedly increased at baseline (4 days after the event), but reduced 8 weeks after the event. ADC was reduced at baseline, pseudo-normal at first follow-up and markedly increased after 2 months. T2-signal was increased on the first and third scan, but showed fogging on the second scan. b: MR-images of a 65-year-old man presenting with right sensori-motor hemiparesis. Signal intensity did not change markedly in the T2-weighted images. The DW-signal progressively decreased over a one-year period. The ADC was slightly decreased at baseline, pseudo-normalised at 4 weeks, and increased further until six months after the event
To analyse the inter-individual variation in signal evolution formally, we fitted a non-linear mixed effect model to the data, which allowed for a fixed effect of time and a random effect of individuals, i.e. the model allowed for inter-individual variation. We compared this with a fixed effect model, which did not allow for inter-individual variation. The models were significantly different (p < 0.0001), which confirms that the temporal evolution of the ADC and of the DW-signal varies significantly between individuals. This variability was not accounted for by age, sex, infarct size, infarct aetiology or infarct location.
Discussion
To our knowledge, our study is the first study of lesion evolution with multiple follow-up scans during the late phase after a minor stroke. We have shown that, although changes are most marked in the first few weeks after an ischaemic event, both the ADC and the DW-signal may continue to evolve for several months. Signal evolution is highly variable between individuals. In our study, this variability was not accounted for by patient age, sex, infarct size or infarct location.
It is generally accepted that the ADC increases to supra-normal values within a few weeks after a stroke [25]. However, since most follow-up studies to date were limited to one scan in the late phase, [17, 18] it has not been demonstrated if and for how long the ADC may continue to change. We have shown that the ADC may continue to increase for several weeks or months after an ischaemic event. The continuing rise of the ADC explains why the DW-signal may also continue to change for months, and why even high DW-signal that has persisted for some months may still normalise. Therefore, even several weeks after a stroke DWI still provides additional information to T2-weighted imaging: DW-lesions are more likely to have occurred over the past few months, whereas on T2-weighted imaging it is not possible to differentiate whether lesions are several months or years old. However, the great interindividual variability in lesion evolution also shows that interpretation of DWI in the subacute phase after an ischaemic event has to be cautious, since there will be false negatives and false positives: a lesion that evolves fast may not be visible any more only a few days after an ischaemic event, whereas the presence of a lesion on DWI does not necessarily imply an acute ischaemic event. DWI therefore has to be interpreted within the context of the clinical presentation. DWI is also increasingly being used as a surrogate outcome measure in trials of stroke. In some studies, presence of a high signal lesion on DWI during follow-up is seen as evidence for recent ischaemic events. However, our findings suggest that high signal lesions may well be several weeks or months old and do not necessarily indicate recent ischaemic activity. This should be borne in mind for any studies that use DWI to monitor for recent ischaemic events. In such studies, a baseline DWI-scan has to be available to which any follow-up scans must be compared in detail.
In this study, the evolution of the DW-signal varied significantly between patients. This may explain the conflicting results of previous studies of the time course of the DW-signal [6, 9, 18]. While one study found that the DW-signal normalized within 14 days [6], other studies found that it remained increased for several months [9, 18]. There has been more agreement that the ADC pseudo-normalises 7–10 days after an ischaemic event [27, 29, 30] but some more recent studies found that it may normalize much later [2, 9, 13]. Most of these studies were small, and some of them tried to extrapolate the time course of the DW-signal from cross-sectional data [2, 9]. However, given the high degree of inter-individual variation the usefulness of a general model of the time course of the DW-signal is doubtful. Any such model should be based on a large number of patients and on follow-up rather than on cross-sectional data.
The DW-signal is influenced by the ADC and by T2-effects [9, 18]. If the T2-signal of a lesion is high, high ADC-values are required to allow normalization of the DW-signal. If T2-effects exceed the contribution of the ADC, the DW-lesion will appear persistently bright. In the presence of vasogenic oedema and infarcted tissue, both the T2-signal and the ADC are high. Therefore strong T2-effects are often associated with a concurrently high ADC. The speed of change of the DW-signal is determined by the speed of increase in the ADC. It is unlikely to be affected by the T2-signal, because this did not change systematically over time. The relative contributions of T2-effects and of the ADC to the DW-signal are complex, and they may at least partially account for the inter-individual variation in the appearance and in the evolution of DW-lesions. In this context, variation in the extent of T2-effects and in the ADC will account for variability in final lesion appearance, whereas variation in lesion evolution will be accounted for mainly by differences in the evolution of the ADC.
The appearance of DWI and of the ADC reflect pathological changes in brain tissue. Animal studies suggest that changes in the ADC and DW-signal may reflect changes predominantly in glial cells rather than neurons [24]. While the reduction of the ADC in acute stroke represents cytotoxic oedema, the ensuing rise in the ADC represents evolving vasogenic oedema, cell necrosis and, at the chronic stage, gliosis and cystic transformation [12]. The continuing change of the ADC provides imaging evidence that repair processes, ongoing cystic transformation and necrotic transformation may continue for several months after an ischaemic event. In contrast, the variable extent with which T2-effects and the ADC contribute to the appearance of the DW-lesion may reflect differences in local tissue composition and in local repair mechanisms. Accordingly some authors have suggested that the ADC may evolve differently in white matter compared with grey matter lesions [21, 22] although this was not confirmed by others [3, 11]. Another study found that the temporal evolution of the ADC differed between watershed infarctions and embolic infarcts [16]. We did not find any differences in signal evolution according to infarct location, and we also did not find any differences in signal evolution according to stroke aetiology. However, our study was not designed to analyse such differences, and patient numbers for such analyses were too small.
The variability in signal evolution in this study was not accounted for by patient age or gender. To date, there has only been one retrospective study of the effect of age on the evolution of the ADC [7]. This found a non-significant trend for the ADC to increase earlier with increasing age. No published studies of the effect of gender on the evolution of the DW-signal exist. However, in normal brain tissue, no association between the ADC and age or sex has been found [15].
In this study, we have been unable to identify any factors accounting for the great interindividual variation in the temporal evolution of the ADC and the DW-signal. However, our findings do indicate that tissue evolution after an ischaemic event varies between individuals. Factors which determine lesion presence on DWI may be of clinical relevance. For example, lesion presence on DWI after a TIA has been associated with a higher risk of future ischaemic events [8]. At least in animal studies, the ADC has been associated with functional outcome [23], although in human studies the value of DWI in predicting outcome is less clear [14]. Identifying which factors determine lesion presence and lesion persistence on DWI may therefore also provide useful prognostic information in patients with cerebral ischaemic events.
While our study provides important new information about the evolution of the ADC and the DW-signal in the late stages after stroke, it also has several potential shortcomings. The frequency of follow-up at the very late stage differed between patients, and many patients only underwent three or four scans in total. However, the aim of this study was to demonstrate that lesions on DWI can persist for several months after an ischaemic event, that they continue to evolve for a considerable amount of time, and that the evolution of ischaemic lesions is highly variable. To a certain extent, our findings are qualitative rather than quantitative, and less frequent follow-up during the later stages did not seem to result in any significant loss if information, in particular since the main changes in ADC and DW-signal intensity occur during the first few months after the event.
A further potential drawback was that we only studied patients who still showed a lesion at a mean time of nine days after their event. Even though we would expect the large majority of patients with a stroke to show a lesion at this stage, we may have missed some patients in whom the lesion on DWI had already normalized. However, our aim was to study lesions occurring in a population representative of patients with minor stroke who are seen sub-acutely. Including DWI-lesions that normalize very early would not have resulted in a cohort representative of the population we wished to study.
Conclusion
To our knowledge this is the first study to clarify the temporal evolution of DWI signal with multiple follow-up examinations during the late stages after a minor stroke. The two main findings are that the ADC and consequently the DW-signal may continue to change for several months after an ischaemic event, and that the extent and speed of this change are highly variable between individuals. Although DWI is helpful in diagnosing ischaemic events that happened several weeks or months ago, this may only be true for selected patients, with false negative imaging occurring in others. Further studies are required to determine which factors influence the evolution of the ADC and the DW-signal to be able to select patients more specifically in whom DWI may show a lesion in the late stage after a stroke.
References
Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24:35–41
Ahlhelm F, Schneider G, Backens M, Reith W, Hagen T (2002) Time course of the apparent diffusion coefficient after cerebral infarction. Eur Radiol 12:2322–29
Bastin ME, Rana AK, Wardlaw JM, Armitage PA, Keir SL (2000) A study of the apparent diffusion coefficient of grey and white matter in human ischaemic stroke. Neuroreport 11:2867–74
Bonati LH, Lyrer PA, Wetzel SG, Steck AJ, Engelter ST (2005) Diffusion weighted imaging, apparent diffusion coefficient maps and stroke etiology. J Neurol 252:1387–93
Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Eberle R, Hertzberg V (1989) Measurements of acute cerebral infarction: a clinical examination scale. Stroke 20:864–70
Burdette JH, Ricci PE, Petitti N, Elster AD (1998) Cerebral infarction: time course of signal intensity changes on diffusion-weighted MR images. Am J Roentgenol 171:791–95
Copen WA, Schwamm LH, Gonzalez RG, Wu O, Harmath CB, Schaefer PW, Koroshetz WJ, Sorensen AG (2001) Ischemic Stroke: Effects of etiology and patient age on the time course of the core apparent diffusion coefficient. Radiology 221:27–34
Coutts SB, Simon JE, Eliasziw M, Sohn CH, Hill MD, Barber PA, Palumbo V, Kennedy J, Roy J, Gagnon A, Scott JN, Buchan AM, Demchuk AM (2005) Triaging transient ischemic attack and minor stroke patients using acute magnetic resonance imaging. Ann Neurol 57:848–54
Eastwood JD, Engelter ST, MacFall JF, Delong DM, Provenzale JM (2003) Quantitative assessment of the time course of infarct signal intensity on diffusion-weighted images. Am J Neuroradiol 24:680–87
Fiebach JB, Jansen O, Schellinger PD, Heiland S, Hacke W, Sartor K (2002) Serial analysis of the apparent diffusion coefficient time course in human stroke. Neuroradiology 44:294–98
Fiebach JB, Schellinger PD, Jansen O, Meyer M, Wilde P, Bender J, Schramm P, Juttler E, Oehler J, Knauth M, Hacke W, Sartor K (2002) CT and diffusion-weighted MR imaging in randomized order: diffusion-weighted imaging results in higher accuracy and lower interrater variability in the diagnosis of hyperacute ischemic stroke. Stroke 33:2206–10
Gass A, Niendorf T, Hirsch JG (2001) Acute and chronic changes of the apparent diffusion coefficient in neurological disorders–biophysical mechanisms and possible underlying histopathology. J Neurol Sci 186(Suppl 1):S15–23
Geijer B, Lindgren A, Brockstedt S (2001) Persistent high signal changes in DW-MRI in late stages of small cortical and lacunar ischemic stroke lesions. Neuroradiology 43:115–22
Hand PJ, Wardlaw JM, Rivers CS, Armitage PA, Bastin ME, Lindley RI, Dennis MS (2006) MR diffusion-weighted imaging and outcome prediction after ischemic stroke. Neurology 66:1159–1163
Helenius J, Soinne L, Perkio J, Salonen O, Kaste M, Carano RA, Tatlisumak T (2002) Diffusion-weighted MR imaging in normal human brains in various age groups. AJNR 23:194–99
Huang IJ, Chen CY, Chung HW, Chang DC, Lee CC, Chin SC, Liou M (2001) Time course of cerebral infarction in the middle cerebral arterial territory: deep watershed versus territorial subtypes on diffusion-weighted MR images. Radiology 221(1):35–42
Keir SL, Wardlaw JM, Bastin ME, Dennis MS. (2004) In which patients is diffusion-weighted magnetic resonance imaging most useful in routine stroke care? J Neuroimaging 14:118–22
Lansberg MG, Thijs VN, O’Brien MW, Ali JO, de Crespigny AJ, Tong DC, Moseley ME, Albers GW (2001) Evolution of Apparent Diffusion Coefficient, Diffusion-weighted, and T2-weighted Signal Intensity of Acute Stroke. AJNR 22:637–44
Lutsep HL, Albers GW, DeCrespigny A, Kamat GN, Marks MP, Moseley ME (1997) Clinical utility of diffusion-weighted magnetic resonance imaging in the assessment of ischemic stroke. Ann Neurol 41:574–80
Moseley ME, Cohen Y, Mintorovitch J, Chileuitt L, Shimizu H, Kucharczyk J, Wendland MF, Weinstein PR (1990) Early detection of regional cerebral ischemia in cats: comparison of diffusion- and T2-weighted MRI and spectroscopy. Magn Reson Med 14:330–346
Mukherjee P, Bahn MM, McKinstry RC, Shimony JS, Cull TS, Akbudak E, Snyder AZ, Conturo TE (2000) Differences between Gray Matter and White Matter Water Diffusion in Stroke: Diffusion-Tensor MR Imaging in 12 Patients. Radiology 215:211–20
Munoz Maniega S, Bastin ME, Armitage PA, Farrall AJ, Carpenter TK, Hand PJ, Cvoro V, Rivers CS, Wardlaw JM (2004) Temporal evolution of water diffusion parameters is different in grey and white matter in human ischemic stroke. J Neurol Neurosurg Psychiatry 75:1714–18
Pillekamp F, Grune M, Brinker G, Franke C, Uhlenkuken U, Hoehn M, Hossmann K (2001) Magnetic resonance prediction of outcome after thrombolytic treatment. Magn Reson Imaging 19:143–152
Rivers CS, Wardlaw JM (2005) What has diffusion imaging in animals told us about diffusion imaging in patients with ischaemic stroke? Cerebrovasc Dis 19:328–36
Roberts TP, Rowley HA (2003) Diffusion weighted magnetic resonance imaging in stroke. Eur J Radiol 45:185–94
Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, Gutnikov SA, Edwards P, Mant D, Sackley CM, Farmer A, Sandercock PA, Dennis MS, Warlow CP, Bamford JM, Anslow P; Oxford Vascular Study (2004) Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study). Lancet 363:1925–33
Schlaug G, Siewert B, Benfield A, Edelman RR, Warach S (1997) Time course of the apparent diffusion coefficient (ADC) abnormality in human stroke. Neurology 49:113–19
Schulz UG, Briley D, Meagher T, Molyneux A, Rothwell PM (2004) Diffusion-weighted MRI in 300 patients presenting late with subacute transient ischemic attack or minor stroke. Stroke 35:2459–65
Schwamm LH, Koroshetz WJ, Sorensen AG, Wang B, Copen WA, Budzik R, Rordorf G, Buonanno FS, Schaefer PW, Gonzalez RG (1998) Time course of lesion development in patients with acute stroke: serial diffusion- and haemodynamic-weighted magnetic resonance imaging. Stroke 29:2268–76
Weber J, Mattle HP, Heid O, Remonda L, Schroth G (2000) Diffusion-weighted imaging in ischaemic stroke: a follow-up study. Neuroradiology 42:184–91
World Health Organisation. (1971) Cerebrovascular diseases – prevention, treatment and rehabilitation. Technical Report Series no 469. Geneva: WHO
Acknowledgements
Dr Schulz was funded by The Wellcome Trust. Dr Flossmann was funded by the UK Medical Research Council. Conflict of Interest: None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Received in revised form: 14 April 2006
Rights and permissions
About this article
Cite this article
Schulz, U., Flossmann, E., Francis, J. et al. Evolution of the diffusion-weighted signal and the apparent diffusion coefficient in the late phase after minor stroke. J Neurol 254, 375–383 (2007). https://doi.org/10.1007/s00415-006-0381-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-006-0381-y