Abstract
Previous MR studies have established that grey matter (GM) atrophy occurs in multiple sclerosis (MS) from clinical onset. However, it is uncertain whether early GM atrophy is global or has certain local predilections: using Voxel-Based Morphometry this study aimed to address this question.
Twenty-one patients with early RRMS (mean age 36 years, mean disease duration from symptom onset 25.8 months) and 10 healthy control subjects (mean age 37 years) were studied. T1-weighted three-dimensional MRI images were acquired at baseline and two year follow-up, and analysed with statistical parametric mapping software (SPM2). Two-sample t-tests (p < 0.05 corrected for multiple comparisons at cluster level) were used to compare GM maps from all patients and controls on a voxel-by-voxel basis. At baseline, no GM region appears significantly atrophic in MS subjects compared with controls. However, during the follow-up period significantly greater atrophy occurred in both thalami and the right lateral prefrontal cortex of MS patients when compared with controls. By year two, cross-sectional group comparison revealed GM atrophy in the thalami of MS patients relative to controls. The rate of thalamic atrophy in MS subjects was correlated with changes in EDSS during the follow-up period.
This study suggests that early in the clinical course of RRMS, the thalamic atrophy is more consistently apparent than regional cortical atrophy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Previous MR studies have established that global brain atrophy occurs in patients with multiple sclerosis (MS) from clinical disease onset [8, 11, 12, 14, 15, 26, 35]. Atrophy in MS appears to be the result of tissue loss in both grey matter (GM) and white matter (WM), and probably underlies a significant degree of irreversible disability [20, 25, 31, 33]. GM atrophy may affect both the cerebral cortex [2, 17, 32] and basal ganglia [13, 36] in patients with longstanding disease. In clinically early Relapsing-Remitting MS (RRMS), studies in two cohorts have demonstrated significant GM atrophy [11, 17, 35], although the localization of this early atrophy within the GM remains unclear. Knowledge of the regional distribution and evolution of GM atrophy may offer useful new perspectives on the pathogenesis of GM atrophy in MS, and in this regard, Voxel Based Morphometry (VBM) may be of value. VBM is a relatively unbiased method by which regional disease effects may be identified, without the need to generate a priori hypotheses [3]. Several studies have previously used statistical mapping analysis in MS to investigate region variation in the thickness of cortical ribbon [32], magnetization transfer ratio (MTR) [4, 30] and GM fraction [28]. Recently, Pagani et al. have applied statistical mapping analysis to brain T1-weighted images in patients with longstanding MS [28]. They demonstrated that ventricular enlargement is prominent in RRMS and that cortical atrophy appears to be more pronounced in the progressive forms of the disease. To date, only one statistical mapping analysis study has been performed at the very early stage of the disease, and this demonstrated MTR decrease mainly located in the deep GM structures [4]. Until now, no VBM study has been performed in patients in the early clinical stages of RRMS.
Using VBM, we aimed to determine: (i) the distribution of GM atrophy in the early stages of RRMS; (ii) the pattern of evolving atrophy during a follow-up period of two years; (iii) the relationship of regional GM atrophy with disability and T2 lesion load.
Methods
Subjects
We studied a group of 21 patients with RRMS (16 women and 5 men; mean age at the time of the first MRI 36 years, range 27 to 55 ) and ten control subjects (6 male, 4 women; mean age at the time of the first MR imaging was 37 years, range 31 to 52 ). In MS subjects, at first assessment, the mean disease duration was 25.8 months (range 14.4 to 45.5) and median Kurtzke’s expanded disability status scale (EDSS) [24] of 1 (range 0 to 3). All subjects were imaged at baseline and two years later. The results of global measurement of GM and WM volume change in this cohort have been previously reported [35]. The patients had not received steroids in the month prior to imaging. EDSS scores and MS functional composite (MSFC) scores [19] were determined during baseline and year two MRI imaging sessions. The study had approval from the National Hospital for Neurology and Neurosurgery Ethic Committee. Informed consent was obtained from all subjects before entry into the study.
MRI protocol
MRI examinations were performed on a 1.5 Tesla machine (Signa; General Electric Medical Systems, Milwaukee, WI), using a standard quadrature head-coil. A single inversion-prepared three-dimensional fast spoiled gradient recall (three-dimensional FSPGR) sequence (repetition time [TR] = 13.3 ms, echo time [TE] = 4.2 ms, inversion time [TI] = 450 ms, number of excitations [NEX] = 1, matrix 256 × 160 interpolated to 256 × 192, field of view [FOV] = 300 × 225 mm, final in-plane resolution = 1.2 × 1.2 mm, with 124 × 1.5 mm slices covering the whole brain) was acquired in all subjects. In a separate scanning session, performed a mean of 10 days before or after acquisition of the volumetric data (during which time none of the subjects reported additional symptoms), a dual echo fast spin echo (FSE) sequence (TR 2,000 ms, TE 19/95 ms, NEX = 2, in-plane resolution 0.9 × 0.9 mm), yielding proton density (PD) and T2-weighted images with 28 contiguous 5 mm slices, covering the whole brain, and a T1-weighted spin echo sequence (TR = 540 ms, TE = 20 ms, NEX = 1, in-plane resolution = 0.9 × 0.9 mm, with 28 × 5 mm slices covering the whole brain) were obtained in all the subjects.
The mean interval between baseline and year two MRI was 24.8 months (range 22.5 to 26.6 months) in the control group and 25 months (range 23.3 to 28.5 months) in the MS cohort. When patients and controls were rescanned, repositioning was achieved with reference to the anterior and posterior commissure, as seen on baseline images.
T2 brain lesion load (T2LL) was measured using a semiautomated contouring technique [22].
Image analysis
MRIs were processed using a modification of the ‘standard’ VBM method [3], developed to prevent misclassification of MS lesions with signal intensities very close to those of GM.
The three-dimensional FSPGR images were first co-registered onto the corresponding T2-weighted images of each subject. Then three-dimensional FSPGR images were spatially normalized into the Montreal Neurological Institute (MNI) space using the T1 anatomical template provided in the SPM2 software (Wellcome Functional Imaging Laboratory, Institute of Neurology, London) and resliced to 2 × 2 × 2 isotropic voxels. The spatial transformation was also applied onto the T2 lesion mask. The normalized T2 lesion mask was applied onto the normalized three-dimensional FSPGR images to obtain normalized three-dimensional FSPGR images of T2 lesions. The difference between normalized three-dimensional FSPGR images and normalized three-dimensional FSPGR images of T2 lesions gave the normal appearing brain (NAB) three-dimensional FSPGR images. After segmentation of the normalized NAB three-dimensional FSPGR images using voxel intensities and a priori knowledge procedures (SPM 2), three maps - representing fractions of GM, normal appearing white matter (NAWM) and CSF - were obtained. Two different GM maps were obtained for each subject: GM fraction map at baseline (GMbas) and GM fraction map at year two (GMfol).
In order to determine the GM atrophy rate during the follow-up period, a further set of GM images was generated by calculating GM decrease maps (GMd) for each subject. These maps represented the degree of GM fraction decrease during the follow-up period. They were obtained using the following formula:
The derived GM map voxel values indicated the degree of GM fraction decrease during the follow-up period.
Statistical mapping analysis
Before statistical comparison, normalised GMbas, GMfol and GMd maps were smoothed using a 6 mm Gaussian filter to minimize remaining spatial differences between subjects and to better satisfy conditions of the random field theory [21].
A two-sample t-test was used to compare GMbas, GMfol and GMd maps between RRMS patients and controls on a voxel-by-voxel basis. Clusters of voxels (corrected for multiple comparisons p < 0.05) that had a T score > 2.46 were considered to be significantly different. MNI coordinates were converted to the Talairach space [34] using a non-linear transformation (http://www.mrc-cbu.cam.ac.uk/imaging/mnispace.html). The mean regional GM fraction and GM fraction decrease were determined for each subject in the region surviving between-group random effect comparisons. Differences between patients and controls were then assessed using a non-parametric Mann-Whitney U-test (p < 0.05). Regional individual data were also used to study correlations between regional GM fraction or GM fraction decrease in patients, and T2 LL, EDSS, MSFC scores. Regional GM fractions were also correlated with fractional measures of whole brain grey matter, white matter and total brain volume (Spearman rank correlation), the latter data having been previously analysed and reported in this patient cohort [35].
Results
Clinical and conventional MRI findings
Patients had a mean T2 lesion load at baseline of 7.8 ml (range 1.4–21.2 ml) compared with 9.8 ml at year two (range 0.9–27.5 ml) (increase of 25.6%) (p = 0.02).
Clinical comparisons between baseline and year two revealed no significant change in the MSFC: MSFC scores were 0.04 at baseline and −0.07 at year two (p = 0.9).
Median EDSS increased from 1 (range 0 to 3) at baseline to 2 (range 0 to 4) at year two (p = 0.02).
Comparisons between patients and controls
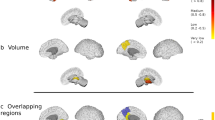
At baseline, no GM region showed significant volume reduction in patients compared with controls. However, patients manifested significantly greater GM volume loss during the follow-up period in both thalami and the right lateral prefrontal cortex when compared to controls (Table 1, Figure 1).
Volex-by volex comparison between patients (n = 21) and controls (n = 11) of the GM fraction decrease between baseline and follow-up time point (Two simple t-test analysis, corrected for multiple comparisons at cluster level P < 0.05, confirmed by individual statistics in the surviving regions P < 0.05 Mann Whitney U-test). GM atrophy rate is higher in patients in the bilateral thalamus and the right lateral prefrontal cortex
By year two, patients with MS had relative GM volume decrease in both thalami compared to controls (Table 2, Figure 2).
Volex-by volex GM fraction maps comparison between patients (n = 21) and controls (n = 11) at the follow-up time point (Two simple t-test analysis, corrected at cluster level P < 0.05, confirmed by individual statistics in the surviving regions P < 0.05 Mann Whitney U-test). GM fraction is lower in patients compared to controls in the bilateral thalamus
Correlations of the observed regional GM volume decreases with T2 lesion loads, global brain, grey matter and white matter volumes, and with clinical measures
No correlation was found of the GM fraction changes during the follow-up period in the thalami or the right prefrontal cortex with T2LL at baseline or changes in T2LL during the follow-up. However, the GM fraction in both thalami at year two was inversely correlated with the T2LL at baseline (rs = −0.824, p = 0.0002) and with changes in T2LL (rs = −0.728, p = 0.001).
The GM fraction in both thalami at year two was also correlated with global white matter fraction (rs = 0.54, p = 0.017) and brain parenchymal fraction (rs = 0.61, p = 0.006) but not with global GM fraction (rs = 0.28, p = 0.21) (the global brain volume data have been previously reported by Tiberio et al. 2005 [35]).
Correlations between clinical data and regional GM fraction are provided in the Table 3.
Discussion
This study was designed to localize GM atrophy in the early clinical stages of RRMS. While at baseline GM atrophy was not observed, significantly greater atrophy occurred during the follow-up period, most consistently in the thalami and in the right lateral prefrontal cortex, and at year two, GM atrophy was observed in the thalami of patients compared with control subjects.
Before discussing the results, several technical aspects and limitations need to be considered. When performing VBM analysis in MS patients, it is important to prevent WM lesions being misclassified as GM (they have similar signal intensities on the volumetric MR images acquired). To address this, lesions identified on the T2 images were masked from the 3D images, and then VBM processing was performed. There is potential that the resulting voids in the images could influence the accuracy of the normalization procedure; however in the present RRMS cohort, the volume of WM T2 lesions was relatively small (mean at baseline = 7.8 ml; mean at year two = 9.8 ml; < 1% of brain volume), and the effect on global normalization accuracy should be therefore negligible. It should also be noted that VBM localizes sites of regional atrophy that are consistently present across a group of subjects, rather than the sites of atrophy whose locations are less consistent and more variable amongst subjects in a group. As such, VBM is not directly equivalent to global atrophy measures (previous application of a global measure of GM fraction in the cohort reported in this study had shown progressive GM atrophy when compared with controls [35]). Another consideration is the gender imbalance between the patient and control group but this is less likely to influence the findings when the differences between groups was seen as a result of the longitudinal study design (i.e. over time and at follow up but not at baseline). Finally, the modest size of the RRMS and the normal control cohorts may have prevented the detection of more subtle changes in GM, and underestimated the extent of regional GM atrophy in patients with RRMS.
GM atrophy has previously been reported early in RRMS suggesting the potential presence of early neuronal loss [2, 11, 12, 15, 17, 35]. A cross-sectional study of T1-weighted MRI scans from twenty six subjects with clinically early RRMS (mean disease duration 1.8 years) found lower global GM fraction compared with twenty seven healthy controls [11]. De Stefano et al. confirmed these results, demonstrating significant global cortical GM atrophy in sixty-five patients with RRMS with short disease duration ( < 5 years) [17]. The current study adds to these earlier observations in demonstrating that in early RRMS ( < 4 years after clinical disease onset), atrophy is consistently present in the thalami. The apparent absence of extensive cortical atrophy does not necessarily mean than there is no cortical atrophy, rather it indicates that it occurs less consistently in the same cortical regions than is the case for the thalami. Indeed, cortical atrophy is hinted at by the longitudinal results, which reveal significant changes in the right lateral prefrontal cortex, and previous studies using global GM measures in this cohort [11, 35] have detected degrees of GM atrophy that cannot readily be accounted for by thalamic volume loss alone [13] as suggested by the absence of any correlation between global GM atrophy measures and regional atrophy inside the thalami. Using a morphometry method optimized for the cortex, but not suitable for the simultaneous assessment of cortical and deep GM structures, Sailer et al. [32] have detected regional atrophy in the frontal and temporal regions.
Previous MR studies in MS have reported abnormalities in the thalamus including, increased water diffusion [18], T2 hypointensity [5], NAA reduction [13], atrophy [36] and decreased MTR [4, 16]. Substantial thalamic neuronal loss (30–35% reduction) is reported in patients with longstanding secondary progressive disease (mean disease duration: 23.5 years) [13]. Using voxel-based comparison of MTR maps, we have recently demonstrated significant morphological changes in the thalamus in patients with clinically isolated syndrome and fulfilling the McDonald imaging criteria for MS [4]. Thus, the thalamus appears vulnerable to disease effects in MS, and it may represent one of the first consistently involved GM regions in patients with RRMS.
In the current study, atrophy of the thalamus at year two follow-up was correlated with T2 LL at baseline, but the rate of atrophy in both thalami during the follow up period was not correlated with the change in T2 LL. These findings suggest the existence of a temporal delay between the process leading to T2 lesion formation and subsequent atrophy. This finding is supported by a previous follow-up study which demonstrated that lesion accumulation early in the clinical course of the disease appeared to be more closely related to subsequent atrophy than later lesion accumulation [10].
There are several potential explanations for the particular sensitivity of the thalamus to MS pathology, and its delayed association with WM lesion loads. Retrograde neuroaxonal degeneration or anterograde transynaptic changes from axonal transection in the WM lesions could be responsible for subsequent atrophy of the thalamus, as suggested by the correlation between atrophy of the thalamus at year two and the T2LL at baseline. Indeed, basal-ganglia-thalamocortical circuits consist of numerous afferent connections, efferent connections and loop connections passing through the periventricular WM [1]. Thus, diffuse periventricular WM damage in RRMS patients affecting these connecting fibre tracts could be responsible for anterograde or retrograde changes in GM regions that have numerous input and output connections, such as the thalami. This potential mechanism is suggested by the positive correlation between atrophy of the thalamus and global white matter fraction at year two. An alternative, but not mutually exclusive, explanation may relate to the relatively high density of demyelinating lesions in the basal ganglia in MS (4% to 7.3% of all brain MS lesions have been reported in these relatively small and, in comparison with other GM regions, richly myelinated structures [9, 27]). Focal GM lesions are associated with neuronal loss [29], and this may contribute towards focal atrophy of the thalamus. In addition, the total volume of such GM lesions may be correlated with WM lesions load, perhaps explaining the relationship between T2LL of the WM at baseline and atrophy of the thalamus at year two in patients. Unfortunately, current MRI methods are largely unable to detect focal GM lesions, although they are well recognized pathologically [7, 23, 29].
The absence of any observable decrease in volume of other GM nuclei may be related to several factors that differentiate the thalamus from the other deep GM structures; compared with other deep GM structures, the thalamus has a greater degree of myelination, and connections from the white matter are more abundant. These factors may induce a more consistent GM decrease in the thalamus for the cohort as a whole, although other GM nuclei are likely to be involved, albeit less consistently - in individual subjects.
In patients, the rate of atrophy in the thalamus was correlated with the change in the EDSS during the follow-up period. However, atrophy of the thalamus at year two was not correlated with EDSS at year two. This apparent discrepancy may relate to the reduction in inter-subject variability inherent in measurements of changes within the same individual over time. The association between the MSFC at year two and the atrophy of the thalamus may reflect a central role of the thalamus in a range of cognitive, motor and sensory functions. In a recent study, Benedict et al. have demonstrated that third ventricle width was strongly associated with neuropsychological impairment and that such finding might be explained in part by atrophy of the thalamus [6].
In conclusion, the present study shows that subtle thalamic atrophy is consistently present in patients with RRMS within four years of clinical disease onset, and that it may be clinically relevant.
References
Alexander G, Crutcher M (1990) Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 13:166–171
Amato MP, Bartolozzi ML, Zipoli V, Portaccio E, Mortilla M, Guidi L, Siracusa G, Sorbi S, Federico A, De Stefano N (2004) Neocortical volume decrease in relapsing-remitting MS patients with mild cognitive impairment. Neurology 63:89–93
Ashburner J, Friston KJ (2000) Voxel-based morphometry–the methods. Neuroimage 11:805–821
Audoin B, Ranjeva JP, My Van AD, Ibarrola D, Malikova I, Confort-Gouny S, Soulier E, Viout P, Ali-Cherif A, Pelletier J, Cozzone PJ (2004) Voxel-based analysis of MTR images: a method to locate gray matter abnormalities in patients at the earliest stage of multiple sclerosis. J Magn Reson Imaging 20:765–771
Bakshi R, Benedict RH, Bermel RA, Caruthers SD, Puli SR, Tjoa CW, Fabiano AJ, Jacobs L (2002) T2 hypointensity in the deep gray matter of patients with multiple sclerosis: a quantitative magnetic resonance imaging study. Arch Neurol 59:62–68
Benedict RH, Weinstock-Guttman B, Fishman I, Sharma J, Tjoa CW, Bakshi R (2004) Prediction of neuropsychological impairment in multiple sclerosis: comparison of conventional magnetic resonance imaging measures of atrophy and lesion burden. Arch Neurol 61:226–230
Bo L, Vedeler CA, Nyland HI, Trapp BD, Mork SJ (2003) Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol 62:723–732
Brex PA, Jenkins R, Fox NC, Crum WR, O’Riordan JI, Plant GT, Miller DH (2000) Detection of ventricular enlargement in patients at the earliest clinical stage of MS. Neurology 54:1689–1691
Brownell B, JT Hughes JT (1962) The distribution of plaques in the cerebrum in multiple sclerosis. J Neurol Neurosurg Psychiatry 25:315–320
Chard DT, Brex PA, Ciccarelli O, Griffin CM, Parker GJ, Dalton C, Altmann DR, Thompson AJ, Miller DH (2003) The longitudinal relation between brain lesion load and atrophy in multiple sclerosis: a 14 year follow up study. J Neurol Neurosurg Psychiatry 74:1551–1554
Chard DT, Griffin CM, Parker GJ, Kapoor R, Thompson AJ, Miller DH (2002) Brain atrophy in clinically early relapsing-remitting multiple sclerosis. Brain 125:327–337
Chard DT, Griffin CM, Rashid W, Davies GR, Altmann DR, Kapoor R, Barker GJ, Thompson AJ, Miller DH (2004) Progressive grey matter atrophy in clinically early relapsing-remitting multiple sclerosis. Mult Scler 10:387–391
Cifelli A, Arridge M, Jezzard P, Esiri MM, Palace J, Matthews PM (2002) Thalamic neurodegeneration in multiple sclerosis. Ann Neurol 52:650–653
Dalton CM, Brex PA, Jenkins R, Fox NC, Miszkiel KA, Crum WR, O’Riordan JI, Plant GT, Thompson AJ, Miller DH (2002) Progressive ventricular enlargement in patients with clinically isolated syndromes is associated with the early development of multiple sclerosis. J Neurol Neurosurg Psychiatry 73:141–147
Dalton CM, Chard DT, Davies GR, Miszkiel KA, Altmann DR, Fernando K, Plant GT, Thompson AJ, Miller DH (2004) Early development of multiple sclerosis is associated with progressive grey matter atrophy in patients presenting with clinically isolated syndromes. Brain 127:1101–1107
Davies GR, Altmann DR, Rashid W, Chard DT, Griffin CM, Barker GJ, Kapoor R, Thompson AJ, Miller DH (2005) Emergence of thalamic magnetization transfer ratio abnormality in early relapsing-remitting multiple sclerosis. Mult Scler 11:276–281
De Stefano N, Matthews PM, Filippi M, Agosta F, De Luca M, Bartolozzi ML, Guidi L, Ghezzi A, Montanari E, Cifelli A, Federico A, Smith SM (2003) Evidence of early cortical atrophy in MS: relevance to white matter changes and disability. Neurology 60:1157–1162
Fabiano AJ, Sharma J, Weinstock-Guttman B, Munschauer FE, 3rd, Benedict RH, Zivadinov R, Bakshi R (2003) Thalamic involvement in multiple sclerosis: a diffusion-weighted magnetic resonance imaging study. J Neuroimaging 13:307–314
Fischer JS, Rudick RA, Cutter GR, Reingold SC (1999) The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler 5:244–250
Fisher E, Rudick RA, Simon JH, Cutter G, Baier M, Lee JC, Miller D, Weinstock-Guttman B, Mass MK, Dougherty DS, Simonian NA (2002) Eight-year follow-up study of brain atrophy in patients with MS. Neurology 59:1412–1420
Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ (1999) Multisubject fMRI studies and conjunction analyses. Neuroimage 10:385–396
Grimaud J, Lai M, Thorpe J, Adeleine P, Wang L, Barker GJ, Plummer DL, Tofts PS, McDonald WI, Miller DH (1996) Quantification of MRI lesion load in multiple sclerosis: a comparison of three computer-assisted techniques. Magn Reson Imaging 14:495–505
Kidd D, Barkhof F, McConnell R, Algra PR, Allen IV, Revesz T (1999) Cortical lesions in multiple sclerosis. Brain 122(Pt 1):17–26
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33:1444–1452
Losseff NA, Wang L, Lai HM, Yoo DS, Gawne-Cain ML, McDonald WI, Miller DH, Thompson AJ (1996) Progressive cerebral atrophy in multiple sclerosis. A serial MRI study. Brain 119(Pt 6):2009–2019
Luks TL, Goodkin DE, Nelson SJ, Majumdar S, Bacchetti P, Portnoy D, Sloan R (2000) A longitudinal study of ventricular volume in early relapsing-remitting multiple sclerosis. Mult Scler 6:332–337
Lumsden CE (1970) The neuropathology of multiple sclerosis. In: Vinken PJ, Bruyn GW (eds) Handbook of clinical neurology, Volume 9. Amsterdam, pp 217–309
Pagani E, Rocca MA, Gallo A, Rovaris M, Martinelli V, Comi G, Filippi M (2005) Regional brain atrophy evolves differently in patients with multiple sclerosis according to clinical phenotype. AJNR Am J Neuroradiol 26:341–346
Peterson JW, Bo L, Mork S, Chang A, Trapp BD (2001) Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol 50:389–400
Ranjeva JP, Audoin B, Duong MV, Ibarrola D, Confort-Gouny S, Malikova I, Soulier E, Viout P, Ali-Cherif A, Pelletier J, Cozzone P (2005) Local tissue damage assessed with statistical mapping analysis of brain magnetization transfer ratio: relationship with functional status of patients in the earliest stage of multiple sclerosis. AJNR Am J Neuroradiol 26:119–127
Rudick RA, Fisher E, Lee JC, Simon J, Jacobs L (1999) Use of the brain parenchymal fraction to measure whole brain atrophy in relapsing-remitting MS. Multiple Sclerosis Collaborative Research Group. Neurology 53:1698–1704
Sailer M, Fischl B, Salat D, Tempelmann C, Schonfeld MA, Busa E, Bodammer N, Heinze HJ, Dale A (2003) Focal thinning of the cerebral cortex in multiple sclerosis. Brain 126:1734–1744
Simon JH, Jacobs LD, Campion MK, Rudick RA, Cookfair DL, Herndon RM, Richert JR, Salazar AM, Fischer JS, Goodkin DE, Simonian N, Lajaunie M, Miller DE, Wende K, Martens-Davidson A, Kinkel RP, Munschauer FE, 3rd, Brownscheidle CM (1999) A longitudinal study of brain atrophy in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Neurology 53:139–148
Talairach J, Tournoux P (1988) Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers, Inc., New York
Tiberio M, Chard DT, Altmann DR, Davies G, Griffin CM, Rashid W, Sastre-Garriga J, Thompson AJ, Miller DH (2005) Gray and white matter volume changes in early RRMS: a 2-year longitudinal study. Neurology 64:1001–1007
Wylezinska M, Cifelli A, Jezzard P, Palace J, Alecci M, Matthews PM (2003) Thalamic neurodegeneration in relapsing-remitting multiple sclerosis. Neurology 60:1949–1954
Acknowledgements
The NMR Research Unit is supported by the MS Society of Great Britain and Northern Ireland. Dr B Audoin is supported by the French Association for Research on Multiple Sclerosis (A.R.S.E.P.). We thank Drs C Griffin and W Rashid for assistance with patient recruitment, Dr J P Ranjeva for methodological advice, and Dr M Tiberio for performing the volumetric analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Received in revised form: 27 March 2006
Rights and permissions
About this article
Cite this article
Audoin, B., Davies, G., Finisku, L. et al. Localization of grey matter atrophy in early RRMS. J Neurol 253, 1495–1501 (2006). https://doi.org/10.1007/s00415-006-0264-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-006-0264-2