Abstract
Background
Previous pharmacokinetic trials with standard levodopa formulations showed a different behaviour of levodopa degradation in plasma of patients with Parkinson’s disease (PD) in various stages.
Objectives
To investigate associations between levodopa plasma levels in relation to the scored intensity of PD.
Subjects and Methods
We administered water soluble 100 mg levodopa and 25 mg benserazide to 50 PD patients, taken off medication for at least 12 hours, and assessed the levodopa plasma concentrations during an 180 minutes period under standardised conditions.
Results
The computed area under the curve (AUC) values of levodopa plasma levels were significant higher in advanced PD patients. PD rating scores significantly correlated to the AUC outcomes and the maximum levodopa plasma concentration.
Conclusions
Levodopa availability improves with progression of PD. This may result from deteriorated peripheral activity of levodopa metabolising enzymes or an increasing enteric dysfunction with subsequent better duodenal levodopa absorption or both.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Levodopa is the most efficacious and best tolerated antiparkinsonian compound. Coadministration of aromatic amino acid decarboxylase inhibitors markedly reduces the peripheral degradation of levodopa [18]. To date, most studies on levodopa plasma levels investigated the pharmacokinetic behaviour of various available standard and sustained release levodopa preparations mostly in healthy volunteers or early patients with Parkinson’s disease (PD) [6, 31]. However, chronic antiparkinsonian drug intake may alter levodopa metabolism and thus appearance in plasma [16, 31]. Further important influencing factors are concomitantly applied: anticholinergic antiparkinsonian drugs, alterations of the large neutral amino acid gastrointestinal transport system and gastric emptying velocity [16, 17, 29, 31]. In contrast to the conventional levodopa preparations, pharmacokinetic behaviour of levodopa of soluble levodopa formulations is more independent of gastric emptying speed. Liquids enable an easier, faster gastrointestinal transit and therefore their absorption is more independent of the gastric emptying interval and other putative impacting variables. Therefore such a trial with liquid levodopa after oral intake may be of interest, since the optimum duodenal delivery of levodopa, the continuous levodopa infusion, contributed to more stable levodopa plasma concentrations and thus reduced motor fluctuations in PD patients [26]. Additionally, new neuropathological findings and a corresponding novel clinical staging of PD put emphasis on reduced gastrointestinal motility as one of the initial and later events in PD [9, 24]. This may also influence duodenal and jejunal resorption and subsequent plasma appearance of levodopa, its metabolism and its efficacy. Moreover it may contribute to onset of dyskinesia in later stages of PD due to more intense fluctuations of levodopa in plasma [22, 26, 29]. The aim of this trial was to compare the pharmacokinetic behaviour of soluble levodopa in PD patients in various stages of the disease under standardised conditions, since an earlier trial reported a reduced absorption of levodopa in previously untreated PD patients [17].
Subjects and Methods
Subjects
We enrolled 50 PD patients in various stages of PD into the study at random. We subdivided them according to their increasing Unified Parkinson’s Disease Rating Scale (UPDRS) total score in four groups with nearly equal numbers for the fundamental statistical analysis ([group] I: [UPDRS] < 35, N [number] = 12; II: 35 – 56, N = 13; III: 57 – 72, N = 12; IV: >72 – 123; N = 13). Clinical characteristics are given in table 1. Age did not significantly differ between groups [3]. They were on an additional stable drug regime with one of the dopamine agonists bromocriptine, pergolide, ropinirole, pramipexole or cabergoline. PD patients with gastric motility influencing drug intake, H. pylori infection and complaints of gastrointestinal symptoms did not participate [23]. All subjects fulfilled clinical diagnostic UK Brain bank criteria for PD [12].
Design
The hospitalised PD patients only received one tablet, which contained 100 mg levodopa and 25 mg benserazide [Madopar LT®], at 6.30 a.m. after an overnight fasting and without additional intake of their regular antiparkinsonian compounds for at least 12 hours [16, 30]. We dissolved this only in Europe available levodopa/benserazide dispersible formulation in 100 ml water immediately before administration [5]. Levodopa plasma levels were measured at fixed time points (0, 15, 30, 45, 60, 90, 120, 150,180 minutes) between 6.30 a.m. to 9.30 a.m. All participants were on identical standardized conditions until 9.30 a.m., they received an identical breakfast (Fresubin Original® (content in 100 ml: protein 3.8 gram [g], carbohydrate 13.8 g, fat 3.8 g, water 84 ml) at 6.00 a.m. and mostly remained in the sitting position. We scored all PD patients at baseline in the off state before levodopa intake with the UPDRS and Hoehn and Yahr Scale in the morning [8, 10, 11].
Assessment of levodopa in plasma
We took 10 ml venous blood samples for levodopa estimation from an antecubital vein through an indwelling cannula kept patent by an infusion of heparin in saline solution (10 U/ml). We performed venous puncture 20 minutes before the baseline investigation, to enable stable conditions. 3 ml of blood was drawn with a separate syringe and discarded before taking each 10 ml specimen. We collected blood samples in EDTA-test tubes containing 100 µl of 0.5% sodium disulfite solution. The plasma obtained from rapid centrifugation was immediately frozen at −80°C until analysis within 14 days. We used reversed-phase high performance liquid chromatography in combination with electrochemical detection for the assessment of levodopa levels in plasma, which we diluted with a factor of 1 : 1.95 before assessment.
Statistics
Data showed a normal distribution according to the Kolmogorow-Smirnow test. As a result, we only performed parametric tests. We calculated the total area under the curve (AUC) values and the necessary interval (Tmax) to reach the maximum concentration (Cmax) of levodopa levels using the linear trapezoidal rule. We used ANCOVA and set duration of PD, body mass index, the daily levodopa dosage as covariates for comparisons between various groups of PD patients (Analysis 1) [17, 19]. Staging of progression of PD into groups of patients is crucial. Group I and II of our PD patients were nearly identical, therefore we also analysed our data by putting PD patients of groups I and II into one group ([term]: A), whereas groups III (B) and IV (C) remained unchanged (AUTHOR PLEASE CHECK THAT MY CHANGE IS CORRECT) identical (Analysis 2). Additionally we performed grouping according to the HYS ranges (Analysis 3) and a subdivision in four groups (1: < 22, 2: 23 – 31, 3: 32 - 45, 4: > 46 [UPDRS III score] (Analysis 4). We employed the Tukeys HSD-test for different numbers for the post hoc analysis. We performed correlation analysis with Spearman rank correlation, since we employed ordinal rating scales. We regarded p < 0.05 as significant.
Ethics
Each subject gave written informed consent. The ethical committee of the university approved this study.
Results
Comparisons
Analysis 1
The computed levodopa AUC values were significantly higher in more advanced PD patients (F(dF 3, dF 43) = 3.43, p = 0.025). The post hoc analysis only showed significant differences between groups I and III (p = 0.04) and I and IV (p = 0.01). No further significant differences were found (Table 1). The Cmax of levodopa in plasma did not significantly (F(dF 3, dF 43) = 2.68, p = 0.058) differ (Table 1).
Analysis 2
ANCOVAS of AUC (F(dF 2, dF 44) = 3.39, p = 0.042) and Cmax (F(dF 2, dF 44) = 3.59, p = 0.036) of levodopa in plasma were significant. There were no significant differences between groups A (N = 25, AUC: 34.67 ± 17.60 [mean ± SD] ng/ml*180 min; Cmax : 338.13 ± 169.51 µg/ml); B (N = 12, AUC: 46.10 ± 14.06 ng/ml*180 min; Cmax : 431.99 ± 167.61 µg/ml) and C (N = 13, AUC: 48.95 ± 17.35 ng/ml*180 min; Cmax : 498.31 ± 203.86 µg/ml) in the post hoc analysis.
Analysis 3
There were significant different differences between computed levodopa AUC outcomes (F(dF 3, dF 43) = 3.82, p = 0.016) and Cmax values (F(dF 3, dF 43) = 4.60, p = 0.007). In the post hoc analysis, only the differences between AUC results of groups HYS I and IV (I: N = 14, 30.60 ± 11.60; II: N = 12, 37.78 ± 20.33; III: N = 15, 46.64 ± 15.23; IV: N = 9, 52.76 ± 17.50 [mean ± SD; ng/ml*180 min]) were significant (p = 0.03). Cmax (I: 282.48 ± 80.46; II: 382.76 ± 203.02; III: 465 ± 197.42; IV: 509.58 ± 186.64 [mean ± SD; µg/ml]) significantly differed between HYS I and III (p = 0.036) and I and IV (p = 0.038).
Analysis 4
Only the ANCOVA of AUC (F(dF 3, dF 46) = 3.10, p = 0.036; (1: N = 11, 29.20 ± 12.47; 2: N = 14, 43.45 ± 20.67; 3: N = 13, 40.55 ± 15.03; 4: N = 12, 49.96 ± 16.37 [mean ± SD; ng/ml*180 min]; post hoc comparison: 1 versus 4: p = 0.027) but not of Cmax (F(dF 3, dF 46) = 1.80, p = 0.16; 1: 306.57 ± 145.73; 2: 415.70 ± 227.93, 3: 384.93 ± 129.70; 4: 493.25 ± 198.77 [mean ± SD; µg/ml]) of levodopa in plasma turned out as significant.
There were no significant variations of the Tmax outcomes. We found no significant differences of AUC outcomes (F(dF 1, dF 45) = 0.15, p = 0.70; patients without previous levodopa intake: 40.47 ± 19.38; 35 levodopa treated patients: 41.40 ± 17.22 [mean ± SD; ng/ml*180 min]) and Cmax results (F(dF 1, dF 45) = 0.02, p = 0.89; patients without previous levodopa intake: 391.27 ± 199.32; levodopa treated patients: 407.03 ± 185.66 [mean ± SD; µg/ml]) between PD patients with and without prior levodopa therapy.
Correlation analysis
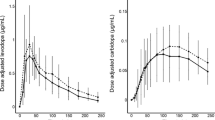
The correlation analysis revealed significant association between the various UPDRS scores and the HYS ranges and both levodopa AUC - and Cmax values (Table 2, Figure 1).
Discussion
We show relationships between intensity of PD and levodopa plasma availability after oral intake of a soluble levodopa formulation. Thus our trial confirms pharmacokinetic investigations in more advanced PD patients with standard levodopa preparations, which report a significant enhancement of levodopa availability [4, 20, 21]. These studies also described an increase of Cmax and variations of Tmax of levodopa plasma levels [20, 21]. In contrast, we found no differences of Cmax dependent on the kind of analysis and Tmax of levodopa. This may hypothetically result from the administration of a soluble levodopa formulation with corresponding faster gastrointestinal absorption in our present study [1, 6, 7, 15, 27]. In contrast to an earlier trial with PD patients in more earlier stages and application of 200 mg and 50 mg benserazide, we now only administered soluble 100 mg levodopa and 25 mg benserazide [17]. This lower dosage of levodopa administration could have caused the missing significant distinct differences of levodopa plasma degradation between PD patients of groups I, II, and III and could explain why the set covariate oral daily levodopa dosage did not significantly influence our outcomes. But nevertheless our present outcomes with a soluble levodopa/benserazide formulation confirm that levodopa availability increases with progression of PD. This effect may result from a deteriorated peripheral activity of levodopa metabolising enzymes due to levodopa long-term intake.
Our study design allows no conclusion on a putative impact of the concomitant chronic treatment with dopamine agonists. However, we tried to reduce this influence of combined medication by taking the PD patients off their additional antiparkinsonian drug regime for at least 12 hours. Moreover they had a prior standardised breakfast with Fresubin Orginal® at 6.00 a.m. Thus we tried to avoid a putative impact of different protein intake. However, we cannot exclude a certain impact of controversially discussed different long term behaviour of protein avoidance in the more advanced PD patients, to ensure a better absorption of levodopa [25, 27]. We did not additionally measure 3-OMD levels, since it is known, that 3-OMD accumulates due to its long plasma half life in relation to dosage and duration of levodopa intake. Moreover this 3-OMD increase did not effect reponse to levodopa and showed no relation to its pharmacokinetics according to our earlier trial [17].
A further still hypothetical explanation of the augmented levodopa bioavailability in more advanced PD patients may be an increase of enteric dysfunction due to the further progression of PD. This may result in better duodenal levodopa absorption due to a reduced duodenal velocity [2, 24, 29]. Thus enteric dysfunction could particularly contribute to increased levodopa absorption in more advanced PD patients without previous levodopa intake. Both hypotheses may also explain a certain trend for the appearance of distinct higher Cmax levodopa levels in more advanced PD patients. This phenomenon is supported by the significant correlations between Cmax levodopa plasma concentrations and the various UPDRS scores by circumstantial evidence. This increase of Cmax levodopa levels may indicate, that fluctuations of levodopa concentrations get more intense with progression of PD. This may result in a more intense, pulsatile levodopa delivery to the brain and contribute to onset of motor complications [13–15, 26, 28]. From this point of view we suggest, that fine tuning of levodopa application is essential in particular in advanced PD patients. Dispersible levodopa formulations or direct duodenal levodopa infusion may be superior to the conventional levodopa preparations, since they circumvent gastroparesis to a certain extent. However, only further necessary future trials will show the relationships between enteric dysfunction, levodopa plasma availability and intensity of PD [6, 14, 15, 17, 26]. An optimum design would be to study the same PD patients in various stages of PD in regular intervals with more frequent sampling over a four hour interval over several years.
Conclusion
We show that levodopa availability improves with progression of PD. This may result from deteriorated peripheral activity of levodopa metabolising enzymes or an increasing enteric dysfunction with subsequent better levodopa absorption or both.
References
Arabia G, Zappia M, Bosco D, Crescibene L, Bagala A, Bastone L, et al. (2002) Body weight, levodopa pharmacokinetics and dyskinesia in Parkinson’s disease. Neurol Sci 23(Suppl 2):S53–S54
Braak H, Rub U, Gai WP, Del Tredici K (2003) Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm 110:517–536
Contin M, Riva R, Martinelli P, Albani F, Baruzzi A (1991) Effect of age on the pharmacokinetics of oral levodopa in patients with Parkinson’s disease. Eur J Clin Pharmacol 41:463–466
Contin M, Riva R, Martinelli P, Cortelli P, Albani F, Baruzzi A (1998) A levodopa kinetic-dynamic study of the rate of progression in Parkinson’s disease. Neurology 51:1075–1080
Contin M, Riva R, Martinelli P, Cortelli P, Albani F, Baruzzi A (1999) Concentration-effect relationship of levodopa-benserazide dispersible formulation versus standard form in the treatment of complicated motor response fluctuations in Parkinson’s disease. Clin Neuropharmacol 22:351–355
Djaldetti R, Rosmarin V, Ziv I, Melamed E (2001) The pharmacokinetic profile of the “first ever” oral dose of levodopa in de novo patients with Parkinson’s disease. Clin Neuropharmacol 24:95–98
Djaldetti R, Ziv I, Melamed E (1996) Impaired absorption of oral levodopa: a major cause for response fluctuations in Parkinson’s disease. Isr J Med Sci 32:1224–1227
Fahn S, Elton R, Members of the UPDRS Development Committee (1987) Unified Parkinson’s Disease Rating Scale. Fahn S, Marsden CD, Goldstein M, et al. (eds) Recent developments in Parkinson’s disease II. 153–163 New York, Macmillan
Goetze O, Wieczorek J, Mueller T, Przuntek H, Schmidt WE, Woitalla D (2005) Impaired gastric emptying of a solid test meal in patients with Parkinson’s disease using 13C-sodium octanoate breath test. Neurosci Lett 375:170–173
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17:427–442
Hogl B, Peralta C, Wetter TC, Gershanik O, Trenkwalder C (2001) Effect of sleep deprivation on motor performance in patients with Parkinson’s disease. Mov Disord 16:616–621
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Kurlan R, Rothfield KP, Woodward WR, Nutt JG, Miller C, Lichter D, et al. (1988) Erratic gastric emptying of levodopa may cause “random” fluctuations of parkinsonian mobility. Neurology 38:419–421
Kurlan R, Rubin AJ, Miller C, Rivera-Calimlim L, Clarke A, Shoulson I (1986) Duodenal delivery of levodopa for on-off fluctuations in parkinsonism: preliminary observations. Ann Neurol 20:262–265
LeWitt PA, Nyholm D (2004) New developments in levodopa therapy. Neurology 62:S9–S16
Minea D, Varga I, Falup-Pecurariu C, de Mey C, Retzow A, Althaus M (2001) Influence of the dopamine agonist alpha-dihydroergocryptine on the pharmacokinetics of levodopa in patients with Parkinson’s disease. Clin Neuropharmacol 24:235–238
Muhlack S, Woitalla D, Welnic J, Twiehaus S, Przuntek H, Müller T (2004) Chronic levodopa intake increases levodopa plasma bioavailability in patients with Parkinson’s disease. Neurosci Lett 363:284–287
Müller T (2002) Dopaminergic substitution in Parkinson’s disease. Expert Opin Pharmacother 3:1393–1403
Müller T, Woitalla D, Saft C, Kuhn W (2000) Levodopa in plasma correlates with body weight of parkinsonian patients. Parkinsonism Relat Disord 6:171–173
Murata M, Kanazawa I (1997) Effects of chronic levodopa therapy on dopa pharmacokinetics. Eur Neurol 38(Suppl 2):50–55
Murata M, Mizusawa H, Yamanouchi H, Kanazawa I (1996) Chronic levodopa therapy enhances dopa absorption: contribution to wearing-off. J Neural Transm 103:1177–1185
Pfeiffer RF (2003) Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol 2:107–116
Pierantozzi M, Pietroiusti A, Galante A, Sancesario G, Lunardi G, Fedele E, et al. (2001) Helicobacter pylori-induced reduction of acute levodopa absorption in Parkinson’s disease patients. Ann Neurol 50:686–687
Przuntek H, Müller T, Riederer P (2004) Diagnostic staging of Parkinson’s disease: conceptual aspects. J Neural Transm 111:201–216
Robertson DR, Higginson I, Macklin BS, Renwick AG, Waller DG, George CF (1991) The influence of protein containing meals on the pharmacokinetics of levodopa in healthy volunteers. Br J Clin Pharmacol 31:413–417
Sage JI, Schuh L, Heikkila RE, Duvoisin RC (1988) Continuous duodenal infusions of levodopa: plasma concentrations and motor fluctuations in Parkinson’s disease. Clin Neuropharmacol 11:36–44
Simon N, Gantcheva R, Bruguerolle B, Viallet F (2004) The effects of a normal protein diet on LevoDOPA plasma kinetics in advanced Parkinson’s disease. Parkinsonism Relat Disord 10:137–142
Smith LA, Jackson MJ, Hansard MJ, Maratos E, Jenner P (2003) Effect of pulsatile administration of levodopa on dyskinesia induction in drug-naive MPTP-treated common marmosets: Effect of dose, frequency of administration, and brain exposure. Mov Disord 18:487–495
Soykan I, Lin Z, Bennett JP, McCallum RW (1999) Gastric myoelectrical activity in patients with Parkinson’s disease: evidence of a primary gastric abnormality. Dig Dis Sci 44:927–931
Soykan I, Sarosiek I, Shifflett J, Wooten GF, McCallum RW (1997) Effect of chronic oral domperidone therapy on gastrointestinal symptoms and gastric emptying in patients with Parkinson’s disease. Mov Disord 12:952–957
van de Vijver DA, Roos RA, Jansen PA, Porsius AJ, de Boer A (2002) Influence of benzodiazepines on antiparkinsonian drug treatment in levodopa users. Acta Neurol Scand 105:8–12
Author information
Authors and Affiliations
Corresponding author
Additional information
Received in revised form: 12 February 2005
Rights and permissions
About this article
Cite this article
Woitalla, D., Goetze, O., Kim, J.I. et al. Levodopa availability improves with progression of Parkinson’s disease. J Neurol 253, 1221–1226 (2006). https://doi.org/10.1007/s00415-006-0207-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-006-0207-y