Abstract
Objective
The diagnosis of leptomeningeal metastases (LM) is based on clinical symptoms, magnetic resonance imaging (MRI) of brain and spine and cytological analysis of cerebrospinal fluid (CSF). The clinical picture of LM is highly variable and both cytological CSF analysis and contrast-enhanced MRI are limited in sensitivity. More sensitive tools are needed to diagnose LM. We measured a profile of proteins involved in adhesion and inflammation in the CSF of LM and control patients and determined their potential diagnostic value for LM.
Patients and methods
Using Multiplex Immuno-Assay (MIA), the CSF concentrations of nine soluble adhesion molecules, cyto- and chemokines were measured in patients with cytologically proven LM (n=57) and control patients with a systemic malignancy (n=20), aseptic/viral meningitis (n=11) or other (non-)neurological diseases (n=19).
Results
We found high CSF levels of soluble Vascular Cell Adhesion Molecule-1 (sVCAM-1), soluble Intercellular Adhesion Molecule-1 (sICAM-1), Interleukin-8 (IL-8), Pulmonary and Activation Regulated Chemokine (PARC), Interleukin-18 (IL-18) and Interferon-γ inducible protein (IP-10) in patients with LM. The CSF protein profile in LM patients differed significantly from the profile found in control patients. Multivariate logistic regression and ROC analysis showed that the MIA-measured CSF protein profile has an additive discriminating value for LM above standard CSF parameters. A combination of total protein, glucose, IL-8, PARC and IP-10 CSF levels proved to be most discriminative between LM and non-LM patients.
Conclusion
Our results warrant a prospective study to determine whether a CSF protein profile, including IL-8, PARC and IP-10 has diagnostic value compared with CSF cytology, the golden standard for LM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leptomeningeal metastases (LM) are an increasingly important complication of systemic cancer. Cancer patients survive longer and more frequently develop metastases in the central nervous system, as chemotherapy often fails to penetrate the blood- brain barrier [5]. The diagnosis of LM is based on clinical symptoms, contrast-enhanced Magnetic Resonance Imaging (MRI) of brain and spine and cytology of cerebrospinal fluid (CSF). Diagnosing LM is a significant challenge since the clinical picture is highly variable and both MRI and CSF cytology are limited in sensitivity. MRI of brain or spine shows distinct contrast enhancement of the leptomeninges in only 60% of patients with LM, while enhancement may be due to other (inflammatory) causes [7, 21]. CSF cytology, the golden standard for LM, is positive in only 55% of patients with LM derived from solid tumours on the initial CSF examination, whereas its sensitivity increases to 80–90% on repeated CSF analyses [26, 27]. Results from CSF cytology in haematological patients with LM are more favourable: 71% of patients have positive results on the initial lumbar puncture, which increases to 92% at the second CSF analysis [13]. Polymerase chain reaction (PCR) and flow cytometry result into a further increase of the sensitivity for the diagnosis LM in patients with haematological malignancies [20, 23].
To further improve the diagnostic yield of CSF analysis especially in patients with solid tumours, biochemical markers in the CSF have been studied. Standard parameters (number of leucocytes, total protein and glucose concentration) were virtually always abnormal in patients with LM, but in a non-specific way [27]. Elevated CSF concentrations of β-glucuronidase, lactate dehydrogenase (LDH), β2-microglobulin and carcino-embryogenic antigen (CEA) were found in LM patients but they either lacked specificity (β-glucuronidase, LDH, β2-microglobulin) or sensitivity (CEA) [24, 25, 27]. Instead of determining a single biomarker with traditional assays like Enzyme Linked Immunosorbent Assay (ELISA) or PCR, new technologies like Multiplex Immuno-Assay (MIA) and protein arrays are emerging, which are used to analyse protein profiles in body fluids [4, 6, 14, 18].
In previous studies on the pathofysiological processes involved in LM, we obtained evidence that tumour cell adhesion to the leptomeninges is crucial for in vivo LM progression in mice (Brandsma et al., neuro-oncology, in press). We showed that in vitro cultured mouse leptomeningeal cells express intercellular adhesion molecule-1 (ICAM-1, CD54) and vascular cell adhesion molecule-1 (VCAM-1, CD106), the latter being important for initial tumour cell adhesion to the leptomeninges [2]. Furthermore, leptomeningeal tumour growth elicits an intrathecal inflammatory response in the CSF, which is reflected by the influx of leukocytes and elevated IgG and IgM indices [29]. Inflammatory processes are mediated by cytokines, (e.g., interleukins, interferons and tumour necrosis factor-α (TNF-α)), proteins that function as chemical communicators between cells during inflammatory responses [1, 16, 17]. A subgroup of small cytokines, chemokines, can also chemo-attract leukocytes and include Interleukin-8 (CXCL8; IL-8), Interferon-γ inducible protein (CXCL10, IP-10) and Pulmonary and Activation Regulated Chemokine (PARC; CCL18) [8, 12, 15, 30].
As both tumour cell adhesion to the leptomeninges and an intrathecal inflammatory response occur in LM, we hypothesize that (I) CSF levels of soluble adhesion molecules, cyto- and chemokines are increased in patients with LM as compared with non-LM patients and (II) these CSF protein levels have diagnostic value for LM. In the underlying study we measured the CSF levels of nine soluble adhesion molecules, cyto- and chemokines in LM and non-LM patients with Multiplex Immuno-assay and determined their potential diagnostic value for LM.
Patients and methods
Patients
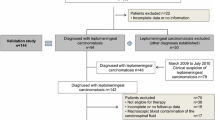
We studied consecutive patients with LM (group I), patients with a systemic malignancy but no LM (group II) and patients with other (non-) neurological diseases (group IV) out of a CSF bank of 573 patients, who visited the outpatient’s clinic or were admitted to the Departments of Neurology and Medical Oncology at the University Medical Center Utrecht (UMCU) or the Antoni van Leeuwenhoek Hospital (AVL), Amsterdam from 1994 until 2002. As the clinical signs and symptoms of patients with aseptic or viral meningitis can resemble those seen in patients with LM, we also selected a group of patients with aseptic or viral meningitis (group III) out of a CSF bank of 2500 patients, who visited the University Medical Center Nijmegen (UMCN) from 1997 to 2002. A lumbar puncture was indicated in all patients because of neurological signs and/or symptoms. The puncture was performed before any treatment was started.
Patients were included in group I when they had a systemic malignancy and cytologically proven LM. Group II consisted of patients with a systemic malignancy, who presented with neurological symptoms, but proved to have no LM. The absence of LM was based on the following criteria: (1) no malignant cells on CSF cytology, (2) normal standard CSF parameters (defined as number of leucocytes ≤ 3 * 106/l, total protein content ≤ 0.6 g/l and glucose levels ≥ 2.5 mmol/l), (3) no progression of neurological symptoms and (4) survival of at least 6 months after the diagnostic lumbar puncture. Group III was composed of patients with either aseptic or viral meningitis in the absence of cancer. The diagnosis of aseptic or viral meningitis was based on clinical symptoms (fever, meningeal irritation) and an elevated number of leukocytes in the CSF (>10 * 106 leukocytes/l), predominantly lymphocytes. Group IV consisted of patients without cancer in their medical history, who underwent a lumbar puncture because of neurological signs and/or symptoms (e.g., thunderclap headache, sensory symptoms of lower extremities). The patients either proved to have no neurological disease or had a non-inflammatory and non-malignant neurological disease.
CSF samples
CSF samples were drawn by a lumbar puncture. Samples were centrifuged during 10 minutes at 1500 rpm and supernatants were aliquotted and stored at −80 °C until measurements.
Multiplex Immuno-assay
CSF levels of soluble Vascular Cell Adhesion Molecule-1 (sVCAM-1; CD106), soluble Intercellular Adhesion Molecule-1 (sICAM-1; CD54), Interleukin-8 (IL-8; CXCL8), Pulmonary and Activation Regulated Chemokine (PARC; CCL18), Interleukin-18 (IL-18), Interferon-γ inducible protein (IP-10, CXCL10), Interferon-γ (IFN-γ), Tumor Necrosis Factor-α (TNF-α) and Interleukin-1β (IL-1β) were measured with the Multiplex immuno-assay (MIA). Antibody pairs used for the Multiplex Immuno-assay were purchased from different commercial sources and coupled as described previously [4]. The capture antibody for sVCAM-1/CD106 (clone sVCAM1–2) and its detection antibody (clone B-N8) were purchased from BenderMedSystems (Vienna, Austria). The capture antibody for sICAM-1/CD54 (clone 14C11) and its polyclonal sheep detection antibody were obtained from R&D Systems (Abingdon, United Kingdom). Calibration curves from recombinant protein standards were prepared using two-fold dilution steps in diluent buffer (R&D Systems, Abingdon, United Kingdom). Recombinant proteins were reconstituted in PBS, pH 7.4, containing 0.5% bovine serum albumin (BSA; Sigma-Aldrich, Zwijndrecht, The Netherlands) to a concentration of 5 µg/ml. Samples with recombinant protein standards and CSF samples were measured in duplicate and blank values were subtracted from all readings. All CSF samples were measured blinded to the clinical status of the patient. To inhibit non-specific binding from the matrix, normal rat and mouse serum (5% each; Rockland, Gilbertsville PA, USA) were added. All samples were run undiluted and diluted 1:50 with High Performance ELISA buffer (HPE-buffer; Sanquin, Amsterdam, the Netherlands). All assays were carried out directly in a 96 well 1.2 µm filter plate (Millipore, Amsterdam, the Netherlands) at room temperature and protected from light. A mix containing 1000 microspheres per mediator (total volume: 10 µl/well) was incubated together with 50 µl standard, sample or blank for 60 minutes. Next, 10 µl of a cocktail of biotinylated antibodies (16.5 µg/ml each) was added to each well and incubated for an additional 60 minutes. Beads were washed with PBS-1% BSA-0.5%-Tween 20 pH 7.4 in order to remove the sample and unbound antibodies. After incubation of 10 minutes with 50 ng/well streptavidin R-phycoerythrin (BD Biosciences, San Diego CA, USA) and washing twice with PBS-1% BSA-0.5%-Tween 20 pH 7.4, the fluorescence intensity of the beads was measured in a final volume of 100 µl HPE-buffer. Measurements and data analysis of all assays were performed using the Bio-Plex system in combination with the Bio-Plex Manager software version 3.0 using five parametric curve fitting (Bio-Rad Laboratories, Hercules CA, USA). Detection limits were 3.0 pg/ml for sVCAM-1 and sICAM-1, 1.2 pg/ml for PARC and 0.3 pg/ml for the other cyto- and chemokines. Intra- and inter-assay variance, expressed as coefficient of variation (CV) was calculated using five internal control samples. Intra-assay variance, measured in quadruplicate at two different time-points had a coefficient of variation of 8.1%. Inter-assay variation was evaluated by testing the internal control samples at 7 different time points. This variability ranged between 6.5% and 16.2% with an average of 11.4% [3].
Data analysis
The concentrations of the MIA-measured proteins in the patient groups were compared using the non-parametric Mann-Whitney U test. A p-value <0.05 was considered statistically significant. To determine the potential diagnostic value of the MIA-measured protein profile, multivariate models were made using logistic regression analysis, yielding odds-ratios (OR) with a confidence interval (CI). A backward step-wise multivariate logistic regression model was used to find the parameters with the highest diagnostic value for LM. Variables with a p-value >0.10 were step-wise removed from the model. Receiver-operator characteristic (ROC) curves were drawn and areas under the curves (AUC) were calculated [10].
Results
Patients’ and CSF characteristics
Fifty-seven patients with cytologically proven LM (group I), 20 patients with a systemic malignancy without LM (group II), 11 patients with aseptic or viral meningitis (group III) and 19 patients with either no neurological disease or a non-malignant and non-inflammatory neurological disease (group IV) were included in the study. Patients’ and CSF characteristics of the groups are shown in Table 1 and 2, respectively.
High sVCAM-1, sICAM-1, IL-8, PARC, IL-18 and IP-10 CSF levels in LM patients
Patients with cytologically proven LM (group I; n=57) showed high median CSF levels of sVCAM-1, sICAM-1, IL-8, PARC, IL-18 and IP-10 (Figure 1: panels A-F). These protein levels differed significantly from the CSF levels found in control patients (group II-IV; n=50, p-value <0.001, Table 3). Elevated IFN-γ CSF levels were found in only one LM patient, as compared with 6 of 11 patients with aseptic/viral meningitis (Figure 1: panel G). IL-1β was not detectable in LM patients, but low IL-1β CSF levels were found in 4 of 11 patients with aseptic/viral meningitis (Figure 1: panel H). TNF-α was not detectable in the CSF of any of the four patient groups (data not shown). In a more detailed comparison with the control groups, patients with LM showed significantly higher sVCAM-1, sICAM-1, IL-8, PARC, IL-18 and IP-10 levels as compared to cancer patients without LM (group II) or other (non-) neurological diseases (group IV) (both comparisons: p ≤ 0.001). High median CSF levels of sVCAM-1, sICAM-1, IL-8, IL-18, IP-10 and IFN-γ were found in patients with aseptic/viral meningitis as compared with cancer patients without LM (group II) and patients with other (non-) neurological diseases (group IV) (both comparisons: p < 0.001). Of note, patients with aseptic/viral meningitis showed significantly higher sVCAM-1, IL-18 and IP-10 and IFNγ CSF levels than patients with LM (p < 0.05), whereas PARC levels were significantly lower in patients with aseptic/viral meningitis than in patients with LM (p<0.001).
Dot plots of the CSF levels of soluble adhesion molecules, cyto- and chemokine in four patient groups as measured by Multiplex Immuno-assay. Group I: cytologically proven LM (n=57), group II: systemic malignancy without LM (n=20), group III: aseptic/viral meningitis (n=11) and group IV: other (non-) neurological diseases (n=19). sVCAM-1 (CD106) = soluble Vascular Cell Adhesion Molecule-1; sICAM-1 (CD54) = soluble Intercellular Adhesion Molecule-1; IL-8 (CXCL8) = Interleukin-8; PARC (CCL18) = Pulmonary and Activation Regulated Chemokine; IL-18 = Interleukin-18; IP-10 (CXCL10) = Interferon-γ inducible protein; TNF-α = Tumour Necrosis Factor-α; IL-1β = Interleukin-1β and IFN-γ = Interferon-γ. Median CSF protein concentrations are indicated
Potential diagnostic value of the MIA-measured CSF protein profile for LM
Multivariate logistic regression and ROC analysis were performed to determine whether the MIA-measured CSF protein profile has potential diagnostic value for LM. As we selected the LM patients on positive CSF cytology, we can not compare the diagnostic value of the CSF protein profile with CSF cytology. Therefore, we determined whether the protein profile has additional discriminating value for LM above the standard CSF parameters. The left column of Table 4 shows the multivariate logistic model based on the standard CSF parameters for group I versus group II-IV (model M1). Because of the CSF selection criteria of group II and IV, the area under the curve of the ROC was high (AUC = 0.89; CI 0.82–0.95). However, inclusion of the MIA-measured protein profile in the multivariate model of the standard CSF parameters (model M3), resulted in an AUC increase to 0.95 (CI: 0.91 – 1.00), similar to that of the MIA-measured protein profile alone (model M2). Using backward step-wise multivariate regression analysis, a combination of total protein, glucose, IL-8, PARC and IP-10 CSF levels (model M4) showed a comparably high AUC (0.95; CI: 0.90 - 0.99), as was found for the standard CSF parameters and the full MIA-measured protein profile (Table 4 and Fig. 2). The results of the ROC analyses remained essentially the same after exclusion of the five LM patients with a haematological malignancy.
Receiver-operator characteristic (ROC) curves of the standard CSF parameters (dotted line, table 4, model M1), the standard CSF parameters and the CSF protein profile measured by Multiplex Immuno-assay (solid line, table 4, model M3) and the variables selected by backward step-wise multivariate logistic regression (total protein, glucose, Interleukin-8 (IL-8), Pulmonary Activated Receptor Chemokine (PARC) and Interferon-γ inducible protein (IP-10)) (dashed line, table 4, model M4) for the diagnosis LM
Discussion
False negative results on (initial) CSF cytological examination are frequently observed in cancer patients with proven LM on follow-up. These patients run the risk of postponed treatment for LM which may adversely affect both the duration and quality of survival. In the underlying study, we investigated the diagnostic potential of a protein profiling technique, the Multiplex Immuno-Assay, for the diagnosis LM. Using this technique, we measured in a single volume of 50 µl CSF, nine proteins involved in two pathophysiological processes of LM: adhesion of tumour cells to the leptomeninges and the intrathecal inflammatory reaction. We showed that LM patients have high CSF levels of the soluble forms of VCAM-1, and ICAM-1 and the cytokines IL-8, PARC, IL-18 and IP-10 as compared with cancer patients without LM and patients with other neurological (non-inflammatory) diseases. Detectable IFN-γ CSF levels were found in one patient with LM , whereas IL-1β and TNF-α CSF levels were undetectable in all LM patients, including the five patients with melanoma. These latter results are in contrast with the findings by Weller et al. (1992), who showed high CSF levels in three of four melanoma patients with LM [29]. This may be due to the small sample size but needs to be addressed in larger prospective studies. These studies should also include serum levels of cytokines to monitor passive influx from the circulation into the CSF as a potential confounder.
The CSF proteins measured in this study are involved in adhesion and inflammation and are therefore not likely to be tumour specific. To determine whether soluble forms of VCAM-1, and ICAM-1 and the cytokines IL-8, PARC, IL-18 and IP-10 are also elevated in the CSF of patients with inflammatory CNS diseases, we measured the CSF protein profile in patients with aseptic/viral meningitis. We found similarities between patients with LM and patients with aseptic/viral meningitis, as high CSF levels of sVCAM-1, sICAM-1, IL-8, IL-18, IP-10 were shown in both patient groups. However, there were also differences: patients with LM had significantly lower sVCAM-1, IL-18 and IP-10 and IFNγ CSF levels and higher PARC levels as compared with patients with aseptic/viral meningitis.
Based on the data obtained in this study, we can not determine the diagnostic potential of the MIA-measured CSF protein profile in LM patients over CSF cytology, as we selected the LM patient group by positive CSF cytology. Alternatively, we compared the discriminating value of the CSF protein profile with standard CSF parameters using multivariate regression and ROC analysis. As expected, a high AUC-ROC for LM was found for the CSF standard parameters, since patients in the control group (group II and IV) were selected on normal CSF standard parameters. Nevertheless, addition of the MIA-measured CSF protein profile resulted in a significant increase of the AUC-ROC, indicating the potential diagnostic value of CSF protein profile for LM. A combination of total protein, glucose, IL-8, PARC and IP-10 CSF levels proved to be most discriminative for LM versus non-LM patients.
So far, research on the diagnostic potential of single biochemical markers has been disappointing [24, 25, 28]. The potential advantage of CSF protein profiling is that multiple biomarkers may more accurately reflect pathophysiological processes (e.g. cancer) than one biomarker, resulting into an increase of both specificity and sensitivity [9].
Future studies on CSF protein profiling may include not only adhesion and inflammation related proteins, but also proteins involved in more tumour specific processes like cell dedifferentiation, malignant cell proliferation and tumour angiogenesis. A candidate protein in this respect is vascular endothelial growth factor (VEGF), as this angiogenic protein was found to be highly elevated in the CSF of LM patients as compared with patients with infectious meningitis [11, 19, 22]. Furthermore, dedifferentiation markers like CEA (or CA 15–3), although showing a low sensitivity for LM when measured as a single biomarker (31%), may contribute to the specificity of a CSF protein profile in LM patients [25].
In conclusion, our data indicate that a CSF profile, focused on proteins involved in adhesion and inflammation, has potential diagnostic value for LM. Future prospective studies should determine whether such CSF protein profiles can prove or rule out LM in patients with negative CSF cytology.
References
Aggarwal BB, Vilcek J (1991) Tumor Necrosis Factors: structure, function and mechanism of action. Dekker New York
Brandsma D, Reijneveld JC, Taphoorn MJ, de Boer HC, Gebbink MF, Ulfman LH, Zwaginga JJ, Voest EE (2002) Vascular cell adhesion molecule-1 is a key adhesion molecule in melanoma cell adhesion to the leptomeninges. Lab Invest 82:1493–1502
de Jager W, Prakken BJ, Bijlsma JW, Kuis W, Rijkers GT (2005) Improved multiplex immunoassay performance in human plasma and synovial fluid following removal of interfering heterophilic antibodies. J Immunol Methods 300:124–135
de Jager W, te Velthuis H, Prakken BJ, Kuis W, Rijkers GT (2003) Simultaneous detection of 15 human cytokines in a single sample of stimulated peripheral blood mononuclear cells. Clin Diagn Lab Immunol 10:133–139
Demopoulos A (2004) Leptomeningeal metastases. Curr Neurol Neurosci Rep 4:196–204
Fitzgerald SP, Lamont JV, McConnell RI, Benchikh el O (2005) Development of a high-throughput automated analyzer using biochip array technology. Clin Chem 51:1165–1176
Freilich RJ, Krol G, DeAngelis LM (1995) Neuroimaging and cerebrospinal fluid cytology in the diagnosis of leptomeningeal metastasis. Ann Neurol 38:51–57
Gerard C, Rollins BJ (2001) Chemokines and disease. Nat Immunol 2:108–115
Gilburd B, Abu-Shakra M, Shoenfeld Y, Giordano A, Bocci EB, delle Monache F, Gerli R (2004) Autoantibodies profile in the sera of patients with Sjogren’s syndrome: the ANA evaluation–a homogeneous, multiplexed system. Clin Dev Immunol 11:53–56
Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29–36
Herrlinger U, Wiendl H, Renninger M, Forschler H, Dichgans J, Weller M (2004) Vascular endothelial growth factor (VEGF) in leptomeningeal metastasis: diagnostic and prognostic value. Br J Cancer 91:19–224
Hieshima K., Imai T, Baba M, Shoudai K, Ishizuka K, Nakagawa T, Tsuruta J, Takeya M, Sakaki Y, Takatsuki K, Miura R, Opdenakker G, Van Damme J, Yoshie O, Nomiyama H (1997) A novel human CC chemokine PARC that is most homologous to macrophage-inflammatory protein-1 alpha/LD78 alpha and chemotactic for T lymphocytes, but not for m-onocytes. J Immunol 159:1140–1149
Kaplan JG, DeSouza TG, Farkash A, Shafran B, Pack D, Rehman F, Fuks J, Portenoy R (1990) Leptomeningeal metastases: comparison of clinical features and laboratory data of solid tumors, lymphomas and leukemias. J Neurooncol 9:225–229
Martin DB, Nelson PS (2001) From genomics to proteomics: techniques and applications in cancer research. Trends Cell Biol 11:S60–S65
Neville LF, Mathiak G, Bagasra O (1997) The immunobiology of interferon-gamma inducible protein 10 kD (IP-10): a novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev 8:207–219
Oppenheim JJ, Zachariae CO, Mukaida N, Matsushima K (1991) Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu Rev Immunol 9:617–648
Pestka S, Langer JA, Zoon KC, Samuel CE (1987) Interferons and their actions. Annu Rev Biochem 56:727–777
Prabhakar U, Eirikis E, Davis HM (2002) Simultaneous quantification of proinflammatory cytokines in human plasma using the LabMAP assay. J Immunol Methods 260:207–218
Reijneveld JC, Brandsma D, Boogerd W, Bonfrer JGM, Kalmijn S, Voest EE, Geurts-Moespot A, Visser MC, Taphoorn MJ (2005) CSF levels of angiogenesis related proteins in patients with leptomeningeal metastases. Neurology 65:1120–1122
Scrideli CA, Queiroz RP, Takayanagui OM, Bernardes JE, Tone LG (2003) Polymerase chain reaction on cerebrospinal fluid cells in suspected leptomeningeal involvement in childhood acute lymphoblastic leukemia: comparison to cytomorphological analysis. Diagn Mol Pathol 12:124–127
Singh SK, Leeds NE, Ginsberg LE (2002) MR imaging of leptomeningeal metastases: comparison of three sequences. Am J Neuroradiol 23:817–821
Stockhammer G, Poewe W, Burgstaller S, Deisenhammer F, Muigg A, Kiechl S, Schmutzhard E, Maier H, Felber S, Schumacher P, Gunsilius E, Gastl G (2000) Vascular endothelial growth factor in CSF: a biological marker for carcinomatous meningitis. Neurology 54:1670–1676
Subira D, Castanon S, Roman A, Aceituno E, Jimenez-Garofano C, Jimenez A, Garcia R, Bernacer M (2001) Flow cytometry and the study of central nervous disease in patients with acute leukaemia. Br J Haematol 112:381–384
Tallman RD, Kimbrough SM, O’Brien JF, Goellner JR, Yanagihara T (1985) Assay for beta-glucuronidase in cerebrospinal fluid: usefulness for the detection of neoplastic meningitis. Mayo Clin Proc 60:293–298
Twijnstra A, Nooyen WJ, van Zanten AP, Hart AA, Ongerboer de V (1986) Cerebrospinal fluid carcinoembryonic antigen in patients with metastatic and nonmetastatic neurological diseases. Arch Neurol 43:269–272
van Oostenbrugge RJ, Twijnstra A (1999) Presenting features and value of diagnostic procedures in leptomeningeal metastases. Neurology 53:382–385
Wasserstrom WR, Glass JP, Posner JB (1982) Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer 49:759–772
Wasserstrom WR, Schwartz MK, Fleisher M, Posner JB (1981) Cerebrospinal fluid biochemical markers in central nervous system tumors: a review. Ann Clin Lab Sci 11:239–251
Weller M, Stevens A, Sommer N, Schabet M, Wietholter H (1992) Tumor cell dissemination triggers an intrathecal immune response in neoplastic meningitis. Cancer 69:1475–1480
Xie K (2001) Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev 12:375–391
Author information
Authors and Affiliations
Corresponding author
Additional information
Received in revised form: 2 November 2005
Rights and permissions
About this article
Cite this article
Brandsma, D., Voest, E.E., de Jager, W. et al. CSF protein profiling using Multiplex Immuno-assay. J Neurol 253, 1177–1184 (2006). https://doi.org/10.1007/s00415-006-0187-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-006-0187-y