Abstract
Objectives
Subarachnoid hemorrhage (SAH) is a common cause of chronic hydrocephalus. Blood in the subarachnoid space is intracranially metabolized to bilirubin and iron, and free iron is thereafter detoxified by ferritin. However, no studies have reported the relationship between intracranial heme metabolism and chronic hydrocephalus after SAH. The goal of this prospective study was to clarify the relationship between intracranial heme metabolism and chronic hydrocephalus after SAH.
Methods
The authors measured the levels of bilirubin, iron and ferritin in the cerebrospinal fluid (CSF) of 70 consecutive patients with aneurysmal SAH of Fisher computed tomography Group III, and determined the relationship between these substances’ levels and hydrocephalus requiring ventriculoperitoneal shunting.
Results
The CSF concentrations of ferritin and inflammatory cells were significantly higher in shunted patients (n = 27) than in non-shunted patients (n = 43) on Days 3 and 4 (p<0.05 in ferritin and p<0.01 in inflammatory cells) and 11 to 14 (p<0.005 in ferritin) post-SAH. These results were independent of other clinical factors. The occurrence of chronic hydrocephalus was not affected by the extent of the intracranial heme metabolism in terms of the bilirubin and iron levels.
Conclusions
This is the first study to show that patients who subsequently had chronic hydrocephalus requiring CSF shunting were associated with higher CSF levels of ferritin in the acute stage of SAH. Higher CSF ferritin levels may not reflect the amount of blood in the subarachnoid space that was intracranially metabolized, but rather more intense subarachnoid inflammatory reactions which may cause chronic hydrocephalus after SAH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic hydrocephalus that requires cerebrospinal fluid (CSF) shunting commonly occurs after aneurysmal subarachnoid hemorrhage (SAH) and is frequently associated with poor neurological outcomes and cognitive deficits [23]. Previous studies reported numerous risk factors potentially associated with the occurrence of chronic hydrocephalus after SAH. These factors include advanced age, female sex, preexisting hypertension, alcoholism, neurological grade severity, amount of blood in the subarachnoid space visualized on computed tomography (CT), intraventricular hemorrhage, increased ventricular size at admission, acute hydrocephalus requiring external ventricular drainage, repeat SAH, aneurysm location, increased aneurysm size, endovascular treatment, longer external drainage with a high amount of daily drained CSF, symptomatic vasospasm, Glasgow Outcome Scale (GOS) score, pneumonia, and meningitis [5, 9, 22, 23, 30]. These studies suggested that a larger amount of blood in the subarachnoid space is associated with an increased risk of chronic hydrocephalus, although some studies of these factors have reported conflicting results.
Recent experimental and clinical studies have shown that blood in the subarachnoid space was intracranially metabolized, and that a greater increase in intracranial heme metabolism was associated with a lower risk of cerebral vasospasm [19, 24, 25]. The greater increase in intracranial heme metabolism may also contribute to a lower risk of chronic hydrocephalus because it is expected to reduce the amount of blood in the subarachnoid space. However, to date no study has reported the relationship between intracranial heme metabolism and chronic hydrocephalus after SAH. Since intracranial heme metabolism is associated with increases in iron, bilirubin and ferritin in the CSF [25], we measured these substances in the CSF obtained from SAH patients and determined the relationship between intracranial heme metabolism and chronic hydrocephalus after SAH.
Methods
The protocol was approved by the Ethics Committee of our hospitals, and the study was carried out according to the principles of the Declaration of Helsinki. Appropriate informed consent was obtained from patients or their relatives.
Patients
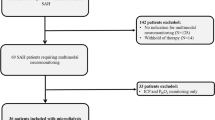
This prospective study included 70 consecutive patients (34 men and 36 women), 35 to 90 years of age (mean 61.8), all with aneurysmal SAH of Fisher CT Group III [8], who were admitted to our 3 hospitals between 1 June 1999, and 30 September 2004. Excluded from the study were patients who suffered from any angiographic or surgical complications and patients who had inflammatory, blood, malignant, or other diseases that can affect iron metabolism. The World Federation of Neurosurgical Societies (WFNS) SAH scores at admission included 18 patients of grade I, 18 of grade II, 11 of grade III, 12 of grade IV, and 11 of grade V [6]. The ruptured aneurysm location was the anterior communicating artery in 23 patients, middle cerebral artery in 18, posterior communicating artery in 15, basilar tip in 3, superior cerebellar artery in 3, posterior inferior cerebellar artery in 3, anterior choroidal artery in 2, anterior cerebral artery in 2, and ophthalmic artery in one.
After angiographic confirmation of the aneurysm, surgical clipping (59 patients) or endovascular coiling (11 patients) of the lesion was performed within 48 hours of initial onset. Cisternal drainage (19 patients) was placed in the basal cistern after surgical clipping, and lumbar spinal drainage (10 patients) was placed after endovascular coiling in all patients treated at two hospitals, but in no patients treated at the other hospital (41 patients), according to each hospital’s protocol. The drainage was continued for 7 to 14 days, and the volume of drained CSF was maintained at 150 to 250 ml per day by changing the height of the drainage siphon. Of 41 patients without cisternal or spinal drainage, a ventricular catheter was placed in 5 patients with ventriculomegaly and a decreased level of consciousness that could not be attributed to causes other than acute hydrocephalus. All patients received intravenous fasudil hydrochloride from one day post-surgery to Day 14 post-hemorrhage. Additional treatment was administered to maintain normovolemia, prevent meningitis, pneumonia and hypoxia, and correct anemia and hypoproteinemia.
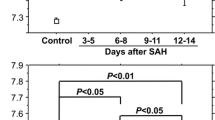
All patients with symptomatic vasospasm were treated with hypertensive hypervolemic therapy. Symptomatic vasospasm was defined as otherwise unexplained clinical deterioration (i.e., a new focal deficit, decrease in the level of consciousness, or both) or a new infarct on CT that was not visible on admission or immediate postoperative scans, or both. Other potential causes of clinical deterioration, such as hydrocephalus, rebleeding, or seizures, were rigorously excluded. Chronic hydrocephalus was diagnosed when a clinical deterioration with no detectable cause other than hydrocephalus occurred after Day 14 post- hemorrhage, and when the ventricular size progressively increased and the Evans index became greater than 0.30 (Fig. 1) [26]. Chronic hydrocephalus was treated with ventriculoperitoneal shunting. The clinical outcome was evaluated using the GOS at 3 months after onset [13].
CSF analysis
A total of 136 CSF samples was obtained from cisternal (43 samples), spinal (25 samples) or ventricular (11 samples) drainage or via a lumbar tap (57 samples) on Days 3 to 14. For the analyses, 4 CSF sampling groups were chosen according to the time points on which the samples had been obtained: Days 3 through 4 (17 samples), 5 through 7 (46 samples), 8 through 10 (40 samples), and 11 through 14 (33 samples) after onset. Two to 4 CSF samples falling into different time groups were obtained from 42 patients. In patients with CSF drainage, CSF samples were obtained only from the CSF drainage, and this was removed after the last CSF sampling. Control CSF samples were obtained from 10 patients with minimal cervical or lumbar spondylosis without myelopathy or radiculopathy on CT myelography, all of whom gave informed consent to participate in this study. The concentrations of total hemoglobin, proteins and inflammatory cells in the CSF were determined with an automatic chemistry analyzer. CSF samples were analyzed at an outside laboratory (BML, Inc., Tokyo) for the levels of iron (non-heme iron), bilirubin and ferritin, which were determined using quick- auto-neo-Fe (K) (Shino-Test Corp., Tokyo), total bilirubin E-HR-Wako (Wako Pure Chemicals Industries, Ltd., Osaka) and Immunoticles Auto-ferritin 2 (A & T Corp., Yokohama), respectively.
Statistical analysis
All values were expressed as means (the standard error of the mean). Comparisons between the two groups were made using unpaired t tests, chi-square tests or Fisher’s exact test, as appropriate. Intergroup comparisons among three or more groups were determined by one-way analysis of variance and then the Tukey-Kramer multiple comparison procedure (95% lower and upper confidence interval) if significant variance was found. Spearman’s rank correlation coefficient was used to assess the correlation between each continuous variable. The sensitivity and specificity were estimated for each different set of cut-off values. To minimize the potential bias introduced by choosing a single cut-off value for positivity, a receiver operating characteristic curve [10] was constructed. A probability value of less than 0.05 was considered statistically significant.
Results
Incidence and clinical features of chronic hydrocephalus after SAH
Twenty-seven of 70 patients (38.6%) underwent ventriculoperitoneal shunting for chronic hydrocephalus. Shunting was performed from 20 to 77 days (mean, 35.4 days) after SAH. Patients with chronic hydrocephalus were associated with worse WFNS grades at admission, more frequent symptomatic vasospasm and worse GOS scales at 3 months after the onset of SAH in comparison with patients without hydrocephalus (Table 1). Among the measured CSF levels of heme metabolites and related substances, only the ferritin levels were significantly higher in patients with, rather than without, hydrocephalus (Table 2).
Ferritin concentrations in the CSF
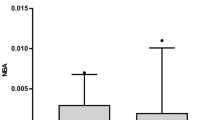
The CSF levels of ferritin in patients with SAH [1,327.9 (139.3) ng/mL] were more than 200 times higher than in control patients [6.0 (0.9); p < 0.025]. The ferritin levels peaked on Days 8 through 10 after SAH, but significant differences between patients with and without hydrocephalus were observed on Days 3 through 4 (p < 0.05), and 11 through 14 (p < 0.005; Fig. 2). The ferritin levels in the CSF obtained via a lumbar tap or from cisternal, spinal or ventricular drainage were not significantly different.
Chronic hydrocephalus and ferritin concentrations in the CSF of SAH patients. Bars represent means (the standard error of the mean). Ο, patients with chronic hydrocephalus requiring ventriculoperitoneal shunting; □, patients without chronic hydrocephalus. Significantly different from the values in patients without chronic hydrocephalus on Days 3 through 4 (*p < 0.05) and Days 11 through 14 (†p < 0.005)
Concentrations of heme metabolites and related substances other than ferritin in the CSF
Considering the CSF levels of heme metabolites and related substances other than ferritin at each CSF sampling time, the inflammatory cell concentrations in patients with hydrocephalus were significantly higher than in patients without hydrocephalus on Days 3 through 4 (p < 0.01; Fig. 3). The inflammatory cell concentrations were also significantly higher in endovascularly treated patients than in surgically treated patients [3,809.9 (1453.4) vs. 1,208.8 (228.0)/mm3; p < 0.0025], but were significantly higher in patients with than without hydrocephalus, irrespective of treatment modalities (p < 0.0001 in endovascularly treated patients; p < 0.05 in surgically treated patients). The inflammatory cell concentrations were significantly correlated with the CSF ferritin levels (p < 0.0001), although their peak times were different. Other substances were not significantly different between patients with and without hydrocephalus at any sampling point.
Chronic hydrocephalus and inflammatory cell concentrations in the CSF of SAH patients. Bars represent means (the standard error of the mean). Ο, patients with chronic hydrocephalus requiring ventriculoperitoneal shunting; □, patients without chronic hydrocephalus. Significantly different from the values in patients without chronic hydrocephalus on Days 3 through 4 (*p < 0.01)
Ferritin concentrations in the CSF and clinical features
There were no significant differences in the CSF ferritin levels between the WFNS grades I – III and IV – V at admission [1,221.6 (184.6) vs. 1,550.3 (189.4) ng/mL], or between the presence and absence of symptomatic vasospasm [1,359.7 (254.7) vs. 1,314.2 (167.3) ng/mL]. The ferritin levels were higher in patients with, rather than without, hydrocephalus irrespective of the WFNS grade at admission [1,885.8 (419.3) vs. 867.4 (158.7) ng/mL in the WFNS grades I – III, p < 0.005; 1,845.8 (264.9) vs. 1,226.6 (259.1) ng/mL in the WFNS grades IV – V, p = 0.10] or the presence or absence of symptomatic vasospasm [1,563.6 (323.1) vs. 727.6 (211.1) ng/mL in the presence of vasospasm, p = 0.16; 2,263.7 (438.7) vs. 986.8 (154.0) ng/mL in the absence of vasospasm, p < 0.001]. Although the ferritin levels in patients with good outcome (good recovery and moderate disability) were significantly lower than in patients with poor outcome [severe disability and a persistent vegetative state; 1,103.6 (147.5) vs. 2,028.0 (317.3) ng/mL; p < 0.005], this was attributed to the fact that all patients with poor outcome had chronic hydrocephalus. The ferritin levels were also higher in patients with, rather than without, hydrocephalus for a good outcome [1,630.7 (468.9) vs. 960.5 (135.7) ng/mL; p = 0.06], and the ferritin levels in patients with chronic hydrocephalus were not different between good and poor outcomes [1,630.7 (468.9) vs. 2028.0 (317.3) ng/mL; p = 0.5].
Prediction of chronic hydrocephalus occurrence
To predict onset of chronic hydrocephalus, the appropriate cut-off value of CSF ferritin was 300 ng/mL on Days 3 through 4 (a sensitivity of 62.5% and a specificity of 100%), and 800 ng/mL on Days 11 through 14 (a sensitivity of 73.3% and a specificity of 72.2%). For inflammatory cells in the CSF, 3,000/mm3 was considered to be the appropriate cut-off value with a sensitivity of 83.3% and a specificity of 100% on Days 3 through 4. On Days 3 through 4, elevated CSF ferritin levels of more than 300 ng/mL or inflammatory cell levels of more than 3,000 /mm3 exhibited a positive predictive value of 100%.
Discussion
The major findings of the present study were that patients who subsequently had chronic hydrocephalus requiring CSF shunting were associated with higher CSF levels of ferritin in the acute stage of SAH, when the volume of blood in the subarachnoid space was roughly estimated to be the same using CT. The CSF ferritin levels were significantly correlated with the CSF inflammatory cell levels. This study tested some of the reported risk factors associated with chronic hydrocephalus after SAH, and reconfirmed that chronic hydrocephalus occurred more frequently in patients with worse WFNS grades at admission, symptomatic vasospasm and worse GOS scales at 3 months after SAH. Irrespective of these factors, however, the CSF levels of ferritin were higher in patients with, rather than without, hydrocephalus. This is the first study to show that chronic hydrocephalus after SAH is associated with higher CSF levels of ferritin.
Ferritin, a naturally occurring iron-binding protein, is involved in maintaining intracranial iron homeostasis, and various intracranial cells can produce ferritin [11, 17, 19, 31, 32]. Iron is a potentially toxic and pro-oxidant molecule [7], but is detoxified by its binding to ferritin [2]. Ferritin is primarily localized intracellularly [2], and its concentration in CSF is very low under normal conditions [32], although a relatively high amount of iron can be bound to and delivered via ferritin in CSF [11]. Ferritin in CSF has been reported to increase in some pathological conditions [4, 15, 17, 25, 27, 32]. However, elevated CSF ferritin may either mitigate [15, 17, 25] or aggravate iron-mediated brain disease or damage [4], as ferritin is a potential source of catalytic iron, depending on the condition [20].
Some explanations for the elevated CSF ferritin levels in this study are possible. Firstly, the elevated CSF ferritin might be derived from transfer of serum ferritin at the rupture of an aneurysm or across the impaired blood brain barrier after SAH [32]. However, this hypothesis is in disagreement with the findings that the CSF ferritin levels increased in a delayed fashion and peaked on Days 8 through 10 after SAH, and that they were not correlated with the total protein concentration. Secondly, the elevated CSF ferritin concentrations might be the result of release from damaged cells in the brain, meninges or within the CSF due to SAH, vasospasm and/or acute hydrocephalus [32]. However, elevated CSF ferritin was related to chronic hydrocephalus, rather than a worse WFNS grade at admission, symptomatic vasospasm or a worse GOS scale at 3 months after SAH in this study. Thirdly, the elevated CSF ferritin concentrations might merely be the result of impaired clearance via the arachnoid villi and other routes associated with impaired CSF absorption [32]. However, this hypothesis is also inconsistent with the findings that the elevated CSF ferritin was not associated with the elevated total hemoglobin and protein concentrations. Fourthly, SAH might increase both the intracranial ferritin synthesis and secretion into the CSF [17, 25]. There are some studies reporting increased intracranial ferritin synthesis after SAH; iron, hemin, oxidative stress or inflammatory cytokines may stimulate ferritin synthesis [19, 20, 25]. In this study, the occurrence of chronic hydrocephalus was not related with intracranial heme metabolism in terms of hemoglobin and heme metabolites, bilirubin and iron measurements, but was associated with increased levels of ferritin and inflammatory cells in the CSF. The CSF levels of ferritin and inflammatory cells were significantly correlated. These findings suggest that the CSF ferritin level was not simply an expression of the amount of blood in the subarachnoid space that had been intracranially metabolized. Rather, more CSF ferritin might be induced by more intense inflammatory reactions in the CSF in patients with chronic hydrocephalus.
The etiology of chronic hydrocephalus after SAH remains unknown. Previous studies suggested that leptomeningeal fibrosis and/or proliferation of arachnoid cells, triggered by an inflammatory reaction or blood clotting products, may impair CSF flow in the subarachnoid space and/or CSF absorption at the arachnoid villi, generating a mild pressure gradient which ultimately leads to the development of slowly progressive ventricular dilatation [14, 18, 21, 28]. This suggests that more severe SAH may be more frequently associated with chronic hydrocephalus because it may cause a more severe inflammatory reaction and more blood clotting products. Since blood in the subarachnoid space is removed via the arachnoid villi or intracranially metabolized by inflammatory, pial membrane, brain glial and vascular cells [1, 12, 19, 24, 25, 29], we tested the hypothesis that increased intracranial heme metabolism might result in lower levels of blood in the subarachnoid space, and therefore decrease the risk of chronic hydrocephalus occurrence. However, this study showed that the extent of intracranial heme metabolism was not related to the occurrence of chronic hydrocephalus. On the other hand, ferritin (Days 3 through 4 and 11 through 14) and inflammatory cells (Days 3 through 4) in the CSF increased more in patients with chronic hydrocephalus, although the volume of blood in the subarachnoid space on the pre-treatment CT scans and CSF hemoglobin concentrations were not significantly different between patients with and without chronic hydrocephalus. Surgery to some extent removed the subarachnoid blood, and therefore significantly reduced the number of CSF inflammatory cells. However, chronic hydrocephalus patients had significantly increased CSF inflammatory cell concentrations irrespective of treatment modalities. Thus, chronic hydrocephalus after SAH might be caused by more intense subarachnoid inflammatory reactions, which might also increase CSF ferritin levels.
Increased CSF ferritin levels were also reported in meningitis, a representative inflammatory disorder in CSF [15, 27]. More severe meningitis is associated with more increased CSF ferritin levels, which is considered to prevent iron usage by bacteria and play a protective role against meningitis [15]. In SAH, induced ferritin, associated with a greater increase in intracranial heme metabolism, was reported to act against cerebral vasospasm by detoxifying free iron in the CSF [17, 25]. In this study, higher CSF levels of ferritin were observed in patients with chronic hydrocephalus, although the occurrence of chronic hydrocephalus was not associated with a greater increase in intracranial heme metabolism. Both the volume of blood in the subarachnoid space, which was roughly estimated using CT scans, and the CSF levels of iron and hemoglobin were not significantly different between the patients with and without chronic hydrocephalus. Therefore, the redox-active iron in patients with chronic hydrocephalus might be lower due to ferritin-mediated iron detoxification; the measured iron in the present study included free and protein-bound iron other than heme-bound iron, and the present method of measuring iron did not allow for the differentiation of ferritin-bound iron from other iron. In chronic hydrocephalus, iron is expected to be retained longer in the CSF because most iron can leave CSF by bulk drainage of CSF via the arachnoid villi and other routes [3]. Elevated CSF ferritin may act protectively against prolonged iron-mediated brain damage associated with impaired CSF absorption.
Global cerebral edema, which may be a manifestation of transient global ischemia related to elevated intracranial pressure at the time of bleeding, has been reported to be an important risk factor for cognitive dysfunction after SAH, as well as thick SAH [16]. However, the relationship between global cerebral edema or transient global ischemia and chronic hydrocephalus remains unknown. The deleterious effects of slowly progressive ventriculomegaly after SAH may be augmented by increased vulnerability of the brain, initiated by transient global ischemia at onset, and subsequently presenting as chronic hydrocephalus.
In conclusion, this study showed that higher CSF levels of ferritin and inflammatory cells at the acute stage might be an independent predictive factor associated with chronic hydrocephalus after SAH. This knowledge will help to predict the occurrence of chronic hydrocephalus after SAH and guide neurologists and neurosurgeons in the long-term care of patients who have experienced SAH.
References
Alksne JF, Lovings E (1972) The role of the arachnoid villi in the removal of red blood cells from the subarachnoid space: an electron microscopic study in the dog. J Neurosurg 36:192–200
Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, Vercellotti GM (1992) Ferritin: a cytoprotective antioxidant stratagem of endothelium. J Biol Chem 267:18148–18153
Bradbury MWB (1997) Transport of iron in the blood-brain-cerebrospinal fluid system. J Neurochem 69:443–454
Davalos A, Castillo J, Marrugat J, Fernandez-Real JM, Armengou A, Cacabelos P, Rama R (2000) Body iron stores and early neurologic deterioration in acute cerebral infarction. Neurology 54:1568–1574
Dorai Z, Hynan LS, Kopitnik TA, Samson D (2003) Factors related to hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery 52:763–771
Drake CG (1988) Report of World Federation of Neurological Surgeons Committee on a universal subarachnoid hemorrhage grading scale [letter]. J Neurosurg 68:985–986
Everse J, Hsia N (1997) The toxicities of native and modified hemoglobins. Free Radic Biol Med 22:1075–1099
Fisher CM, Kistler JP, Davis JM (1980) Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 6:1–9
Gruber A, Reinprecht A, Bavinzski G, Czech T, Richling B (1999) Chronic shunt-dependent hydrocephalus after early surgical and early endovascular treatment of ruptured intracranial aneurysms. Neurosurgery 44:503–512
Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29–36
Hulet SW, Heyliger SO, Powers S, Connor JR (2000) Oligodendrocyte progenitor cells internalize ferritin via clathrin-dependent receptor mediated endocytosis. J Neurosci Res 61:52–60
Jackowski A, Crockard A, Burnstock G, Russell RR, Kristek F (1990) The time course of intracranial pathophysiological changes following experimental subarachnoid haemorrhage in the rat. J Cereb Blood Flow Metab 10:835–849
Jennett B, Bond M (1975) Assessment of outcome after severe brain damage. Lancet 1:480–484
Kibler RF, Couch RSC, Crompton MR (1961) Hydrocephalus in the adult following spontaneous subarachnoid hemorrhage. Brain 84:45–61
Kim YO, Kang JS, Youm MH, Woo YJ (2003) Diagnostic capability of CSF ferritin in children with meningitis. Pediatr Neurol 28:271–276
Kreiter KT, Copeland D, Bernardini GL, Bates JE, Peery S, Claassen J, Du YE, Stern Y, Connolly ES, Mayer SA (2002) Predictors of cognitive dysfunction after subarachnoid hemorrhage. Stroke 33:200–209
LeVine SM, Lynch SG, Ou CN, Wulser MJ, Tam E, Boo N (1999) Ferritin, transferrin and iron concentrations in the cerebrospinal fluid of multiple sclerosis patients. Brain Res 821:511–515
Massicotte EM, Del Bigio MR (1999) Human arachnoid villi response to subarachnoid hemorrhage: possible relationship to chronic hydrocephalus. J Neurosurg 91:80–84
Ono S, Zhang ZD, Marton LS, Yamini B, Windmeyer E, Johns L, Kowalczuk A, Lin G, Macdonald RL (2000) Heme oxygenase-1 and ferritin are increased in cerebral arteries after subarachnoid hemorrhage in monkeys. J Cereb Blood Flow Metab 20:1066–1076
Ryter SW, Tyrrell RM (2000) The heme synthesis and degradation pathways: role in oxidant sensitivity: heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med 28:289–309
Sajanti J, Heikkinen E, Majamaa K (2000) Transient increase in procollagen propeptides in the CSF after subarachnoid hemorrhage. Neurology 55:359–363
Schmieder K, Koch R, Lucke S, Harders A (1999) Factors influencing shunt dependency after aneurysmal subarachnoid hemorrhage. Zentralbl Neurochir 60:133–140
Sheehan JP, Polin RS, Sheehan JM, Baskaya MK, Kassell NF (1999), Participants Factors associated with hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery 45:1120–1128
Suzuki H, Kanamaru K, Tsunoda H, Inada H, Kuroki M, Sun H, Waga S, Tanaka T (1999) Heme oxygenase-1 gene induction as an intrinsic regulation against delayed cerebral vasospasm in rats. J Clin Invest 104:59–66
Suzuki H, Muramatsu M, Kojima T, Taki W (2003) Intracranial heme metabolism and cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke 34:2796–2800
Synek V, Reuben JR, Du Boulay GH (1976) Comparing Evans’ index and computerized axial tomography in assessing relationship of ventricular size to brain size. Neurology 26:231–233
Takahashi S, Oki J, Miyamoto A, Moriyama T, Asano A, Inyaku F, Okuno A (1999) Beta-2-microglobulin and ferritin in cerebrospinal fluid for evaluation of patients with meningitis of different etiologies. Brain Dev 21:192–199
Takizawa T, Tada T, Kitazawa K, Tanaka Y, Hongo K, Kameko M, Uemura K (2001) Inflammatory cytokine cascade released by leukocytes in cerebrospinal fluid after subarachnoid hemorrhage. Neurol Res 23:724–730
Turner CP, Bergeron M, Matz P, Zegna A, Noble LJ, Panter SS, Sharp FR (1998) Heme oxygenase-1 is induced in glia throughout brain by subarachnoid hemoglobin. J Cereb Blood Flow Metab 18:257–273
Widenka DC, Wolf S, Schurer L, Plev DV, Lumenta CB (2000) Factors leading to hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurol Neurochir Pol 34(6 Suppl):56–60
Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G (2003) Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke 34:2964–2969
Zappone E, Bellotti V, Cazzola M, Ceroni M, Meloni F, Pedrazzoli P, Perfetti V (1986) Cerebrospinal fluid ferritin in human disease. Haematologica 71:103–107
Acknowledgements and Funding
None to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Received in revised form: 19 January 2006
Rights and permissions
About this article
Cite this article
Suzuki, H., Muramatsu, M., Tanaka, K. et al. Cerebrospinal fluid ferritin in chronic hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurol 253, 1170–1176 (2006). https://doi.org/10.1007/s00415-006-0184-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-006-0184-1