Abstract
Forensic entomology is primarily concerned with the estimation of time since death and involves determination of the age of immature insects colonising decomposing remains. Accurate age determination of puparia is usually accomplished by dissection, which means destructive sampling of evidence. As part of improving abilities to correctly identify species and developmental age, it is highly desirable to have available non-destructive methods. In this study, we acquired external hyperspectral imaging (HSI) data (77 spectral bands, 389–892 nm) from the dorsal and ventral sides of individual puparia of two species of blowfly (Diptera: Calliphoridae), Calliphora dubia Macquart 1855 and Chrysomya rufifacies Macquart 1842. Puparia were dissected to determine the presence/absence of eight internal morphological development characteristics (legs, wings, labella, abdominal segments, antennae, thoracic bristles, orbital/facial bristles and eye colour and arista). Based on linear discriminant analysis and independent validation of HSI data, reflectance features from puparia could be used to successfully (1) distinguish the two species (classification accuracy = 92.5 %), (2) differentiate dorsal and ventral sides of puparia (classification accuracy C. dubia = 81.5 %; Ch. rufifacies = 89.2 %) and (3) predict the presence of these morphological characteristics and therefore the developmental stage of puparia (average classification accuracy using dorsal imaging: C. dubia = 90.3 %; Ch. rufifacies = 94.0 %). The analytical approach presented here provides proof of concept for a direct puparial age relationship (i.e. days since the onset of pupation) between external puparial reflectance features and internal morphological development. Furthermore, this approach establishes the potential for further refinement by using a non-invasive technique to determine the age and developmental stage of blowflies of forensic importance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Forensic entomology is a field of research which applies knowledge of insect biology to legal matters and is commonly employed as a means of establishing time since death or minimum post-mortem interval (minPMI) [1]. Typically, blowflies (Diptera: Calliphoridae) are the predominant taxa used to indicate minPMI, as they are cosmopolitan and among the first insects colonising remains after death [2, 3]. Estimates of minPMI are based on determination of the age of immature insects associated with remains and the predictable sequence of insect colonisation of remains for a given set of abiotic and biotic conditions [4–6]. Consequently, accurate species identification is important but morphological approaches are often challenging. Molecular-based identification involves specimen modification and sample preparation that can inhibit additional forms of analysis [7]. The age of the oldest immature specimen collected from decomposing remains provides an indication of the minimum time that the decomposing remains were available for insect colonisation and thus minPMI.
The developmental duration of blowfly offspring and other forensically relevant insects is strongly correlated with temperature [8–12]. Specimen age is determined using growth data detailing temperature-dependent developmental timeframes for specific life stages encompassing egg, larval instars, pupation and eclosion [13–15]. The availability of species-specific and highly detailed development data is essential for the accurate estimation of minPMI.
Current approaches to age estimation of immature blowflies collected from human remains are limited to the timeframes associated with the start and end of the life stage collected, such as the onset and completion of an instar or pupation. The duration of early life stages (i.e. egg, 1st larval instar) is generally short, so estimates of minPMI based on such life stages are relatively accurate [16]. More advanced immature life stages (i.e. puparial stage), however, have a comparatively greater duration accounting for >50 % of total development time, and as such, the generally practiced use of life stage landmarks (i.e. onset of pupation and eclosion) as indicators of specimen age results in wide confidence intervals associated with arising minPMI estimates [16–18]. Several approaches have been proposed that allow more precise estimation of developmental stage including morphological examination of external and internal developmental changes using a conventional light microscope [19], histological staining [20, 21], scanning electron microscopy (SEM) [22, 23], gas chromatography mass spectrometry (GC-MS) [24–26], micro-computed tomography [18] and optical coherence tomography [27]. Advances in gene expression analysis are also proving useful in improving the precision of puparial age estimation [16, 28, 29]. For instance, puparial age can be indicated by differential changes in the up and down regulation of genes throughout metamorphosis [30, 31]. Several issues exist, however, in regard to these methods including evidence modification, destructive analysis and underdeveloped reference data.
Importantly, there exists a preference in many legal systems for non-invasive techniques whereby evidence remains ‘unchanged’ following examination [32]. Morphological assessment methods, including tomography, typically involve evidence modification for specimen preparation such as dissection, slide sectioning or histological staining to provide high contrast images for analysis [18, 21]. Similarly, analysis of the quantitative composition of cuticular hydrocarbons extracted from the blowfly puparium using GC-MS and molecular-based methodologies encompasses destructive sample preparation for analysis [29, 30]. In the case of limited specimen collection, destruction of evidence can greatly inhibit investigative practice. In addition, these techniques are often labour intensive, require expensive analysis equipment and involve a high degree of specialist expertise to interpret relevant morphological changes as these are often based on subjective measures such as colour changes [27, 33]. Furthermore, accurate aging of advanced immature life stages typically requires specially trained technicians, which may not always be available. While current approaches to pupae/puparial aging offer improved accuracy in minPMI estimation, most are associated with disadvantages such as evidence modification, destructive sampling, labour-intensive analysis and/or expensive equipment.
Recently, new developments in proximal hyperspectral imaging (HSI) spectroscopy have given rise to potential applications in forensic science. Forensic applications of HSI have previously encompassed identification of chemical signatures in bioagent materials [34, 35], age estimation of blood stains [36, 37], drug analysis [38], document analysis [39–41], facial recognition [42] and forensic assessment of food quality and safety [43]. However, to our knowledge, HSI has never been applied to issues within the area of forensic entomology.
Proximal HSI consists of the acquisition of detailed reflectance profiles (reflectance data in hundreds of narrow wavelength bands) under controlled laboratory conditions (with constant lighting, spatial resolution and projection angle) and subsequent processing, calibration and classification to separate objects that appear indistinguishable to the human eye [44–46]. Using this technology, it is assumed that different target objects, such as blowfly puparia of different age or species, will reflect light differently based on their difference in physical structure and biochemical composition [47].
Changes in the biochemical profile of the insect cuticle and puparium over time have previously been demonstrated using GC-MS [48–50]. Potentially, corresponding changes in reflectance profiles of the puparium over time may be evident, providing age markers for within-stage estimation of minPMI [48–50]. The insect cuticle is highly dynamic in its composition, particularly once it forms a puparia, playing a critical role in water regulation, thermo-regulation, aspects of communication and defence against pathogens [51]. The various complex roles of the insect cuticle support the view that the composition of the cuticle is tightly associated with complex internal physiological processes and that detectable changes in the biochemical composition of the cuticle of blowfly puparia can aid in differentiation between species and determination of age [25, 26, 52]. Consequently, we predict discernible differences, using HSI, in the reflectance profiles of blowfly puparia of different ages and species.
Additionally, as HSI encompasses a component of the infrared spectrum (>700 nm), which can potentially penetrate several millimetres into tissue, there is the possibility that the epicuticular structure of HSI-imaged insects is not the only determinant of acquired reflectance profiles. Physiological and biochemical composition of internal tissues may also contribute [46]. For instance, internal morphological changes, commonly used as markers for puparial age, may be linked to any changes in the acquired reflectance profile over time. It is therefore proposed that differences in images acquired using HSI of blowfly puparia of different ages and species may be linked to internal morphological changes.
HSI has numerous potential advantages over previously proposed methods providing enhanced discrimination potential that is non-destructive and non-invasive and can be applied to both live and preserved specimens. HSI technologies are relatively inexpensive, the camera system is portable thus having the potential for images to be captured at a crime scene and there is scope for classification automation [47, 53]. Finally, as reflectance profiles provide a non-subjective basis for discrimination, the potential exists for the development of supporting software for image analysis, reducing the level of expertise required for interpretation.
As proof of concept, this study aimed to demonstrate that HSI can be used to discriminate between puparia of different ages in relation to daily changes in puparial development. Two blowfly species (Diptera: Calliphoridae), Calliphora dubia Macquart 1855 and Chrysomya rufifacies Macquart 1842, were reared at two developmental temperatures (24 and 30 °C), and hyperspectral images were acquired daily until adult eclosion. The presence/absence of eight morphological characteristics was assessed through daily dissection of sub-samples of puparia reared at the two developmental temperatures to establish day/age indicators for puparial development of both species under these conditions [27]. Data were then compared to daily reflectance profiles of corresponding puparial age using HSI technology to determine proof of concept for discrimination of puparial age based on reflectance data. Reflectance of both dorsal and ventral surfaces of puparia was assessed to determine if reflectance profiles varied with puparial orientation during imaging. Additionally, the potential to distinguish between puparia of the two species using HSI was assessed.
Materials and methods
Insect cultures
C. dubia and Ch. rufifacies colonies were established between November and December 2013 from specimens collected from the Jandakot wildlife reserve (32° 10′ S, 115° 50′ E), located ∼23 km south of Perth, Western Australia. Adult flies were maintained in screened cages and supplied with sugar and water ad libitum. Laboratory conditions (constant temperature room) were maintained at 24 ± 1 °C, 60–70 % relative humidity (RH) and photoperiod of 12L:12D. Bovine liver was used for oviposition/larviposition [54]. Deposited eggs and larvae were transferred to a meatmeal (Mirco Bros, WA, Australia) food substrate (slaughterhouse by-product consisting of rendered mixed animal offal) and placed in a rearing cabinet within the laboratory until emergence. Field-collected individuals were regularly introduced into the established colonies. Experimental females were exposed to bovine liver once per week for 1 h prior to experiments to obtain protein for egg development [15].
In all treatment groups, 3-week-old, sugar-fed, mated female blowflies were used. At the start of each trial, females were exposed to bovine liver for 1 h. Resulting eggs (Ch. rufifacies) and larvae (C. dubia) were subsequently set up on pre-prepared trays of meatmeal in batches of 200 specimens, placed within meshed rearing containers above a layer of sterilised sand (required for pupation) and transferred to environmental chambers (Thermoline Scientific Pty. Ltd., NSW, Australia) for development. Replicates were placed in one of two environmental chambers set at either 24 or 30 °C, a relative humidity of 65 ± 10 % and a photoperiod of 12L:12D. Average environmental chamber temperature was measured using three data loggers (Tinytag Plus®, Hastings Data Loggers, NSW, Australia) distributed within each cabinet on the top, middle and bottom shelf. The resulting means differed from chamber set values by no more than ±0.25 °C. Rearing containers for both study species were assessed twice a day (0800 and 1700 hours) for the onset of puparial development. Specimens were considered to have entered the puparial stage at the point where movement had ceased and the external puparium (white) was evident [55]. Two study species (C. dubia and Ch. rufifacies) and two constant rearing temperatures (24 and 30 °C) were assessed to allow comparison of puparial surface structure, size, associated reflectance profiles and internal morphology over time.
Sampling protocol
At the onset of pupation, samples of 18 puparia were removed from the rearing containers of each of the four treatment groups (2 species × 2 rearing temperatures). For each treatment group, puparia were affixed to a paper card within a 2 × 9 sample grid using the natural adhesive, gum arabic (Henrietta’s Beading Glue, CA, USA). To determine an optimal sampling protocol, the ventral and dorsal surfaces of puparia were imaged for comparison. Within each treatment grid, nine puparia were affixed ventrally along the upper row (dorsal surface imaged) and dorsally along the lower row (ventral surface imaged) (Fig. 1). Orientation was determined by visual assessment of the location of the posterior spiracles and/or dorsal spines [56, 57].
Representative image of a single treatment grid of nine replicate puparia prepared for daily hyperspectral imaging. Puparia are affixed to the grid either dorsal side (Dors) or ventral (Vent) side up. In this instance, 4-day-old Calliphora dubia (Diptera: Calliphoridae) puparia are shown following rearing at 24 ± 0.5 °C, relative humidity of 65 ± 10 % and a photoperiod of 12L:12D
Puparia affixed to each of the four grids (N = 9 replicate puparia × 2 orientations/grid) were subsequently imaged and returned to allocated treatment rearing conditions for continued development. Thus, the same nine pupae were scanned per treatment until emergence. Hyperspectral images (see below) were captured daily between 0800 and 1000 hours until completion of the puparial stage. During the daily hyperspectral image acquisition, puparia were only removed from the allocated rearing conditions for <15 min. Scanned pupae developed and emerged in the same timeframe as the cohort from which they were sampled, which remained in the allocated environmental chamber. The entire procedure was repeated over time (2 weeks apart), so that HSI data were obtained from a total of 144 puparia image captures (2 species × 2 rearing temperatures × 18 puparia × 2 time series).
Starting on day 1 of puparial formation, five puparia were removed daily from the original rearing containers of each of the four combinations of species and rearing temperature and preserved for morphological analysis. Puparial specimens were pierced twice along the ventral surface (head and abdomen regions), immersed in hot water for 10 s and then preserved in 70 % ethanol according to standard protocol [58]. Original rearing containers were maintained under allocated treatment rearing conditions with morphological samples collected between 0800 and 1000 hours daily until emergence.
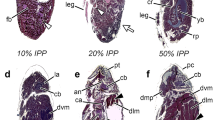
Puparia of preserved specimens where then removed according to Brown et al. [17] and examined under an Olympus Stereo Microscope SZ6 (Olympus Australia Pty. Ltd.). Image records were obtained using an Olympus DP70 digital microscope camera attachment. Based on descriptive and categorical measures [17], presence/absence of eight morphological characteristics of puparia was recorded for each combination of species and rearing temperature: (1) legs, (2) wings, (3) labella, (4) abdominal segments, (5) antennae, (6) thoracic bristles, (7) orbital/facial bristles and eye colour and (8) arista (Figs. 2 and 3).
Development of Calliphora dubia (Diptera: Calliphoridae) at 30 °C, dorsal view (left), ventral view (centre) and lateral view (right), after removal of the puparial case. Day 2 of pupation (a–c); day 7 of pupation (d–f); day 9 of pupation (g–i). Characteristics considered in this study included the following: L = legs, W = wings, La = labella, AS = abdominal segmentation, A = antennae, TB = thoracic bristles, OFB = orbital/facial bristles and eye colour and Ar = arista
Development of Chrysomya rufifacies (Diptera: Calliphoridae) at 30 °C, dorsal view (left), ventral view (centre) and lateral view (right), after removal of the puparial case. Day 2 of pupation (a–c); day 3 of pupation (d–f); day 4 of pupation (g–i). Characteristics considered in this study included the following: L = legs, W = wings, La = labella, AS = abdominal segmentation, A = antennae, TB = thoracic bristles, OFB = orbital/facial bristles and eye colour and Ar = arista
Hyperspectral imaging
The HSI system consisted of a push broom hyperspectral camera (PIKA II, www.resonon.com) which used a Firewire (IEEE 1394b) interface (12-bit digital) with a 7° angular field of view processed 240 spectral bands from 392 to 889 nm with a spectral resolution of 2.07 nm (spectral) by 640 pixels (spatial). The objective lens was optimised for the near-infrared and visible near-infrared spectra and consisted of a 35-mm focal length (maximum aperture of F1.4). HSI data were acquired with the spatial resolution of 5 by 5 pixels per mm2.
Reflectance data were captured under controlled laboratory conditions consisting of 2× 15 W, 12 V LED artificial light bulbs which were mounted on either side of the lens in a room with 24 ± 2 °C temperature and 30–40 % relative humidity. White calibration was achieved using a piece of white Teflon (K-Mac Plastics, USA), and ‘relative reflectance’ refers to proportional reflectance compared to reflectance obtained from Teflon (white = 1) and complete darkness (dark = 0). Additional calibration measures were employed to ensure consistent data acquisition over time. This included the acquisition of daily average reflectance profiles using a green plastic card as a standard. Average reflectance from the green plastic card before and after each daily imaging acquisition event was used to confirm consistency of imaging conditions, and less than 3 % variation within and among days was observed. As such, we were confident that significant changes observed in reflectance profiles could be attributed to changes in puparial reflectance. With hyperspectral images being acquired daily from puparia over 4–11 days (depending on species and rearing temperature), a total of 1224 average reflectance profiles (774 from C. dubia and 450 from Ch. rufifacies) were included in this study.
Data analysis
All data analyses were conducted in PC-SAS 9.4 (SAS Institute, USA) and based on average reflectance profiles from individual puparia. Separate analyses were conducted across temperature regimes for each species. The first 10 spectral bands were omitted from each hyperspectral data file, as these were considered to be associated with stochastic noise. Consequently, 230 spectral bands from 411 to 889 nm were included in the analysis. After acquisition, we conducted 1 × 3 spectral binning (decreased the spectral resolution from 2.1 to 6.3 nm), which resulted in 77 spectral bands being included in the analyses. This pre-processing step of spectral binning was included, as it has been shown to increase the classification accuracy in similar analysis of HSI data [59, 60]. Daily reflectance data for individual puparia were examined visually using non-metric multidimensional scaling ordination (MDS), based on a Euclidean distance matrix in the software package, PRIMER v.6.0 (PRIMER-E Ltd, Plymouth, UK) [61, 62].
The first day of appearance for each of the eight morphological characteristics was determined such that if the formation of legs became noticeable on day 2 and puparial development was completed in 6 days, then the dichotomous values for the variable, legs, would be 0, 1, 1, 1, 1, 1. Furthermore, we compared average reflectance profiles from day 1 with those from days 2 to 6 as part of determining the relative difference between morphological stages with/without appearance of leg development.
In each analysis of the morphological characteristics, linear discriminant analysis was conducted (proc discrim) [63] to differentiate between average reflectance profiles from puparium before (without) and after (with) appearance of the morphological trait. For each classification, stepwise linear discriminant analysis (proc stepwise) was used initially to only select the spectral bands with significant contribution to each classification model. In other words, spectral bands with low or negligible identification contribution were omitted. Subsequently, a linear discriminant classification model was generated on the basis of 75 % of the data (a randomly selected training data set) and the remaining 25 % of the data was used for independent validation. The division of reflectance data into training and validation was repeated five times, and the average classification accuracy was calculated on the basis of the five independent validations of each classification model.
Results
Developmental morphology
Puparial development of C. dubia was completed in 11 days at 24 °C and 9 days at 30 °C (Fig. 4a). Regarding Ch. rufifacies, puparial development at 24 and 30 °C was completed in 5 and 4 days, respectively (Fig. 4b). None of the morphological characteristics assessed through dissection of individual puparia were present until after day 1. Legs and wings appeared on days 2 and 3 in both species. Bristles on thorax and face and presence of arista were the last of the morphological characteristics to appear in the puparial ontogeny.
Timeline of the appearance of key morphological characteristics during puparial development of the blowflies (Diptera: Calliphoridae), Calliphora dubia (a) and Chrysomya rufifacies (b), reared under two different constant temperature treatments, 24 and 30 °C. Morphological characteristics examined included the following: L = legs, W = wings, La = labella, AS = abdominal segmentation, A = antennae, TB = thoracic bristles, OFB = orbital/facial bristles and eye colour and Ar = arista
Reflectance response to ontogeny
Within species, reflectance response of puparia demonstrated a similar trend as a function of time (day). Corresponding to observable colour changes with puparia darkening over time, young puparia typically demonstrated greater reflectance than older puparia at wavelengths >600 nm (the orange and red light spectrum). As puparia aged, relative reflectance of increasing variation was observed (in spectral bands beyond 600 nm) under both temperature treatments. There was negligible effect of time on reflectance values in spectral bands in the blue and green light (Figs. 5, 6, 7 and 8).
Reared at a constant temperature of 24 °C, puparia of C. dubia demonstrated markedly higher reflectance of wavelengths >600 nm on day 1 compared to all other days. Gradual variation was evident in reflectance profiles over day 2 through day 8. Relative reflectance at wavelengths >600 nm was lowest on day 9 before increasing again on days 10 and 11 to levels closer to days 2–8 (Fig. 5). This pattern was similar for C. dubia puparia reared at 30 °C with the highest reflectance of wavelengths over 600 nm occurring on day 1 and the lowest on day 8. On day 9, relative reflectance increased at wavelengths over 600 nm to levels more consistent with earlier days 2 through 6 (Fig. 6).
Spectral reflectance profiles of Ch. rufifacies puparia were uniquely distinct from C. dubia profiles with comparatively lower reflectance of Ch. rufifacies at wavelengths greater than 700 nm (Figs. 7 and 8). Consistent however with C. dubia, the reflectance profiles of Ch. rufifacies puparia reared at 24 and 30 °C again demonstrated markedly higher reflectance of wavelengths >600 nm on day 1 compared to all other days (Figs. 7 and 8). Gradual variation was evident in reflectance profiles over proceeding days with lower reflectance in spectral bands beyond 600 nm. Relative reflectance at wavelengths >600 nm was lowest on the last day prior to emergence of Ch. rufifacies under both temperature conditions (day 5 at 24 °C and day 4 at 30 °C).
Reflectance-based classification
Significant differences in reflectance responses of Ch. rufifacies and C. dubia were evident (Fig. 9). Linear discriminant classification modelling identified that species could be distinguished throughout puparial development based on reflectance response with a classification accuracy of 92.5 %. In the linear discriminant classification of the presence/absence of the eight morphological characteristics, 10–25 of the 77 spectral bands were selected through forward stepwise selection, and based on five independent validations, the presence/absence of all morphological characteristics could be detected with over 82 % accuracy (Fig. 10). The absence of legs and wings was detected with 90–95 % accuracy in both species and would be an indication that a puparium is less than 24 h old. The absence of thoracic bristles was detected with about 85 % accuracy in both species and would be an indication that a C. dubia puparium is 0–6 days old and a Ch. rufifacies puparium, developing at less than 30 °C, is 0–4 days old. As the presence/absence of each morphological characteristic is clearly associated with a combination of temperature and time, we are proposing that reflectance data from puparia can be used to run all eight classifications, and based on the presence/absence of the eight morphological characteristics, it may be possible to apply a logic framework to predict the days of puparial development. For instance, in a logic framework analysis of C. dubia puparia (Fig. 4a), where the analyses suggest the presence of legs, wings, labella and abdominal segments and the absence of all other characteristics, and it is known that ambient temperatures have been around 24 °C, then the puparium is likely 3–4 days old.
nMDS (non-metric multidimensional scaling) ordination of average daily reflectance profiles from puparia of two blowfly species (Diptera: Calliphoridae), Calliphora dubia and Chrysomya rufifacies, reared under two different constant temperature treatments, 24 and 30 °C. For ease of interpretation, the daily average of the reflectance profiles of individual puparia within each treatment is presented. Data were normalised prior to ordination. The relative distances apart of the points are the same rank order as the relative dissimilarities of the reflectance profiles. Thus, points that are close together represent puparial reflectance profiles that are similar, while points that are further apart are more dissimilar
Linear discriminant classification accuracy (%) for the detection of the presence/absence of eight morphological characters throughout puparial development based on daily reflectance profiles from puparia of two blowfly species (Diptera: Calliphoridae), Calliphora dubia and Chrysomya rufifacies, reared under two different constant temperature treatments, 24 and 30 °C. Morphological characteristics examined included the following: L = legs, W = wings, La = labella, AS = abdominal segmentation, A = antennae, TB = thoracic bristles, OFB = orbital/facial bristles and eye colour and Ar = arista
Within species, differences were evident in reflectance response of dorsal and ventral puparial orientation. Puparial orientation could be distinguished with 81.5 % accuracy for C. dubia and 89.2 % accuracy for Ch. rufifacies based on corresponding reflectance profiles. The classification accuracy of individual traits with corresponding reflectance response of either dorsal or ventral orientated puparia was consistently above 80 % for both species (Table 1). For all morphological traits, either dorsal or ventral imaging performed better than data combining reflectance response of both orientations (Table 1). On average, imaging the dorsal surface of puparia outperformed ventral imaging but not for all combinations of species and traits (Table 1).
Discussion
Determination of the age of developing insects collected from decomposing remains is an important indicator of how long the remains have been colonised by insects and consequently minPMI [64]. Issues currently exist in respect to aging specimens within the lengthy puparial stage, as only the start and end of the life stage is used as a time marker with any certainty [27]. The use of HSI to age insect specimens within life stages for use in forensic applications offers many advantages over currently proposed methods including rapid, non-destructive, in situ analysis with enhanced discrimination potential [43, 53]. Additionally, as reflectance data provides a non-subjective basis for discrimination and can be incorporated into supporting software for image analysis, refinement of the technique can ultimately reduce the level of expertise required for interpretation.
This work aimed to demonstrate the potential of HSI technology as a tool to age blowfly puparia developing under different temperature conditions. Acquisition of daily reflectance profiles of the two blowfly species investigated yielded a wealth of robust data allowing for confidence in predictive modelling. Subtle differences in daily reflectance profiles of puparia were evident and strongly associated with puparial age. Daily differences in reflectance generally corresponded to the puparia darkening over time; however, relative reflectance increased in the days prior to eclosion indicating additional contributing factors to changes in reflectance. Reflectance profiles of 1-day-old puparia, corresponding with undifferentiated tissue upon dissection, were markedly different to profiles of older puparia where the appearance of key internal morphological characters, such as legs and wings, occurred. As internal morphological changes correspond to changes in external reflectance, it is likely that these key morphological changes are linked to associated changes in the external puparium. Such gradual changes over time are thus evident in the reflectance profile of the puparia and can indicate age.
Using an independent validation of classifications models, we demonstrate that the presence/absence of all eight established internal morphological characteristics could be determined with >82 % accuracy. As such, the use of HSI technology has great potential in the application of the development of temperature-dependent predictive models for determining puparial age in forensically relevant species. Notably, within days, differences in ventral and dorsal reflectance profiles were evident and thus orientation is important during image capture and needs to be considered. Overall, however, these findings highlight the potential of the technique in forensic entomological applications and strongly support the value of further research and development of the approach.
On the basis of this work, it is evident that reflectance profiles of the puparium change over time in synchrony with internal developmental changes; however, as reflectance is also externally driven, the cause itself requires further investigation. In refining the technique, future directions should address not only the comprehensive acquisition of HSI data for predicting temperature-dependent development of forensically relevant species but also the cause of the observed relationship. The biochemical profile of the puparium is known to change over time as the insect develops in sync with internal physiological processes and likely morphological changes [25, 26, 48]. This has been demonstrated in respect to the success of hydrocarbon profile analysis as a tool in discriminating blowfly puparial age. Thus, it is hypothesised that differences in biochemical composition of the cuticle, as previously demonstrated in regard to hydrocarbon composition [25, 26, 52], will equate to differences in reflectance profiles of blowfly puparia of different ages and species. In regard to this, there is a need to establish the relationship between hydrocarbons and reflectance profiles to improve our understanding of the cause of external developmental changes. Refinement of HSI techniques in predictive models of blowfly development will further establish associated error rates.
Of the two approaches, HSI and hydrocarbon analysis, both require further development, but hydrocarbon analysis is also destructive in respect to evidence processing. Further refinement efforts would therefore best be served by focussing on HSI techniques for the non-destructive analysis of living specimens for age estimation as this is highly preferred as it accommodates additional forms of analysis and/or preservation for later evidence review [18, 58]. Additionally, although the main aim of this study was to investigate the potential of HSI as a discriminative tool in respect to puparial age, the technique also has applications in regard to species identification. Based on the obtained reflectance profiles, there was a clear indication of discrimination between the two species investigated with >92 % accuracy. As predicative models for aging entomological specimens are species specific, accurate species identification is paramount to the resulting accuracy of associated age-determined minPMI estimation [65]. As the majority of forensic entomologists currently practicing are not taxonomic specialists, the advantages of using HSI to both identify and age specimens collected from decomposing remains are high. Much like gene expression analysis and hydrocarbon analysis for aging pupae/puparia, DNA-based species identification is time consuming, destructive and cost prohibitive compared to a refined and further developed HSI approach.
In respect to further development of the technique, reflectance of both dorsal and ventral surfaces of puparia indicated that greater resolution of species and age difference could be achieved by imaging dorsally oriented puparia. Further, the development of any predictive model requires validation under field conditions along with associated error rates prior to adoption in mainstream forensic practice [3]. Blind test validation in field research and case work is needed to comprehensively establish the technique as reputable in regard to delivery during expert testimony in court proceedings. Results presented in this paper were executed in a controlled laboratory environment under two different temperature regimes with successful age-based identification achieved with incorporation of temperature in the predictive model. Further investigation of puparial age-linked reflectance profiles under more variable field environmental conditions is however required to establish the validity of the proposed method and any associated error [3, 5]. Ongoing development of the technique will test if the practical implications discussed will be an issue for application of HSI analysis under field conditions.
Conclusion
This study has demonstrated the potential of HSI to discriminate between puparia of different ages that appear similar to the naked eye. Although factors other than age may influence reflectance profiles of developing puparia, there is still great potential for determining minPMI using daily changes in reflectance profiles of puparia as a basis for discrimination. This successful proof-of-concept study identifies the value of further refinement of the technique in forensic applications involving entomological specimens and identifies the considerable potential of HSI in forensic practice.
References
Amendt J, Richards CS, Campobasso CP, Zehner R, Hall MRJ (2011) Forensic entomology: applications and limitations. Forensic Sci Med Pathol 7:379–92
Matuszewski S, Bajerlein D, Konwerski S, Szpila K (2010) Insect succession and carrion decomposition in selected forests of central Europe. Part 1: pattern and rate of decomposition. Forensic Sci Int 194:85–93
Baque M, Amendt J (2013) Strengthen forensic entomology in court—the need for data exploration and the validation of a generalised additive mixed model. Int J Legal Med 127:213–23
Matuszewski S, Madra A (2015) Factors affecting quality of temperature models for the pre-appearance interval of forensically useful insects. Forensic Sci Int 247:28–35
Tomberlin JK, Mohr R, Benbow ME, Tarone AM, VanLaerhoven SL (2011) A road map for bridging basic and applied research in forensic entomology. Annu Rev Entomol 56:401–21
Voss SC, Spafford H, Dadour IR (2010) Temperature-dependant development of Tachinaephagus zealandicus Ashmead (Hymenoptera: Encyrtidae), on five forensically important carrion fly species. Med Vet Entomol 24:189–98
Harvey ML, Dadour IR, Gaudieri S (2003) Mitochondrial DNA cytochrome oxidase I gene: potential for distinction between immature stages of some forensically important fly species (Diptera) in Western Australia. Forensic Sci Int 131:134–9
Warren JA, Anderson GS (2013) Effect of fluctuating temperatures on the development of a forensically important blow fly, Protophormia terraenovae (Diptera: Calliphoridae). Environ Entomol 42:167–72
Ikemoto T, Takai K (2000) A new linearized formula for the law of total effective temperature and the evaluation of line-fitting methods with both variables subject to error. Environ Entomol 29:671–82
Campbell A, Frazer BD, Gilbert N, Gutierrez AP, Mackauer M (1974) Temperature requirements of some aphids and their parasites. J Appl Ecol 11:431–8
Davidson J (1944) On the relationship between temperature and rate of development of insects at constant temperatures. J Anim Ecol 13:26–38
Damos P, Savopoulou-Soultani M (2012) Temperature-driven models for insect development and vital thermal requirements. Psyche 2012:1–13
Nabity P, Higley L, Heng-Moss T (2006) Effects of temperature on development of Phormia regina (Diptera: Calliphoridae) and use of developmental data in determining time intervals in forensic entomology. J Med Entomol 43:1276–86
Richards CS, Villet MH (2009) Data quality in thermal summation development models for forensically important blowflies. Med Vet Entomol 23:269–76
Voss SC, Cook DF, Wei-Feng H, Dadour IR (2014) Survival and development of the forensically important blow fly, Calliphora varifrons (Diptera: Calliphoridae) at constant temperatures. Forensic Sci Int 10:314–21
Tarone AM, Foran DR (2011) Gene expression during blow fly development: improving the precision of age estimates in forensic entomology. J Forensic Sci 56:S112–22
Brown K, Thorne A, Harvey M (2015) Calliphora vicina (Diptera: Calliphoridae) pupae: a timeline of external morphological development and a new age and PMI estimation tool. Int J Legal Med 129:835–50
Richards CS, Simonsen TJ, Abel RL, Hall MJR, Schwyn DA, Wicklein M (2012) Virtual forensic entomology: improving estimates of minimum post-mortem interval with 3D micro-computed tomography. Forensic Sci Int (Online) 220:251–64
Greenberg B (1991) Flies as forensic indicators. J Med Entomol 28:565–77
Barritt LC, Birt LM (1971) Development of Lucilia cuprina: correlation of biochemical and morphological events. J Insect Physiol 17:1169–83
Davies K, Harvey ML (2012) Internal morphological analysis for age estimation of blow fly pupae (Diptera: Calliphoridae) in postmortem interval estimation. J Forensic Sci 58:79–84
Sukontason KL, Kanchai C, Piangjai S et al (2006) Morphological observation of puparia of Chrysomya nigripes (Diptera: Calliphoridae) from human corpse. Forensic Sci Int (Online) 161:15–9
Sukontason KL, Narongchai P, Kanchai C et al (2006) Morphological comparison between Chrysomya rufifacies (Macquart) and Chrysomya villeneuvi Patton (Diptera: Calliphoridae) puparia, forensically important blow flies. Forensic Sci Int (Online) 164:230–4
Feng D-X, Liu G-C (2013) Pupal age estimation of forensically important Megaselia spiracularis Schmitz (Diptera: Phoridae). Forensic Sci Int 231:199–203
Moore HE, Adam CD, Drijfhout FP (2013) Potential use of hydrocarbons for aging Lucilia sericata blowfly larvae to establish the postmortem interval. J Forensic Sci 58:404–12
Xu H, Ye G-Y, Xu Y, Hu C, Zhu G-H (2014) Age-dependent changes in cuticular hydrocarbons of larvae in Aldrichina grahami (Aldrich) (Diptera: Calliphoridae). Forensic Sci Int 242:236–41
Brown K, Harvey M (2014) Optical coherence tomography: age estimation of Calliphora vicina pupae in vivo? Forensic Sci Int 242:157–61
Ames C, Turner B, Daniel B (2006) Estimating the post-mortem interval (II): the use of differential temporal gene expression to determine the age of blowfly pupae. Int Congr Ser 1288:861–3
Zajac BK, Amendt J, Horres R, Verhoff MA, Zehner R (2015) De novo transcriptome analysis and highly sensitive digital gene expression profiling of Calliphora vicina (Diptera: Calliphoridae) pupae using MACE (Massive Analysis of cDNA Ends). Forensic Sci Int Genet 15:137–46
Boehme P, Spahn P, Amendt J, Zehner R (2014) The analysis of temporal gene expression to estimate the age of forensically important blow fly pupae: results from three blind studies. Int J Legal Med 128:565–73
Zehner R, Amendt J, Boehme P (2009) Gene expression analysis as a tool for age estimation of blowfly pupae. Forensic Sc Int Genet Suppl Ser 2:292–3
Morris B, Dadour I (2005) Forensic entomology: the use of insects in legal cases. In: Freckelton I, Selby H (eds) Expert evidence. Law Book Company, Sydney
Proença B, Ribeiro AC, Luz RT, Aguiar VM, Maia VC, Couri MS (2014) Intrapuparial development of Chrysomya putoria (Diptera: Calliphoridae). J Med Entomol 51:908–14
Edelman GJ, Gaston E, van Leeuwen TG, Cullen PJ, Aalders MCG (2012) Hyperspectral imaging for non-contact analysis of forensic traces. Forensic Sci Int 223:28–39
Brewer LN, Ohlhausen JA, Kotula PG, Michael JR (2008) Forensic analysis of bioagents by X-ray and TOF-SIMS hyperspectral imaging. Forensic Sci Int 179:98–106
Edelman G, van Leeuwen TG, Aalders MCG (2012) Hyperspectral imaging for the age estimation of blood stains at the crime scene. Forensic Sci Int 223:72–7
Li B, Beveridge P, O’Hare WT, Islam M (2013) The age estimation of blood stains up to 30 days old using visible wavelength hyperspectral image analysis and linear discriminant analysis. Sci Justice 53:270–7
Edelman G, Lopatka M, Aalders MCG (2013) Objective color classification of ecstasy tablets by hyperspectral imaging. J Forensic Sci 58:881–6
Reed G, Savage K, Edwards D, Daeid NN (2014) Hyperspectral imaging of gel pen inks: an emerging tool in document analysis. Sci Justice 54:71–80
Malik MI, Ahmed S, Shafait F et al (2015) Hyper-spectral analysis for automatic signature extraction. 17th biennial conference of the International Graphonomics Society
Rémi C, Prévost L, Anquetil E (2015) Drawing, handwriting processing analysis: new advances and challenges. In 17th biennial conference of the International Graphonomics Society
Uzair M, Mahmood A, Shafait F, Nansen C, Mian AS (2015) Is spectral reflectance of the face a reliable biometric? Opt Express 23:15160–73
Wu D, Sun D-W (2013) Advanced applications of hyperspectral imaging technology for food quality and safety analysis and assessment: a review—part II: applications. Innovative Food Sci Emerg Technol 19:15–28
Nansen C (2016) The potential and prospects of proximal remote sensing of arthropod pests. Pest Manag Sci 72:653–9
Nansen C, Macedo T, Swanson R, Weaver DK (2009) Use of spatial structure analysis of hyperspectral data cubes for detection of insect-induced stress in wheat plants. Int J Remote Sens 30:2447–64
Christian N, Norman E (2016) Remote sensing and reflectance profiling in entomology. Annu Rev Entomol 61:139–58
Wu D, Sun D-W (2013) Advanced application of hyperspectral imaging technology for food quality and safety analysis and assessment: a review—part 1: fundamentals. Innovative Food Sci Emerg Technol 19:1–14
Roux O, Gers C, Legal L (2008) Ontogenetic study of three Calliphoridae of forensic importance through cuticular hydrocarbon analysis. Med Vet Entomol 22:309–17
Zhu GH, Ye GY, Hu C, Xu XH, Li K (2006) Development changes of cuticular hydrocarbons in Chrysomya rufifacies larvae: potential for determining larval age. Med Vet Entomol 20:438–44
Butler SM, Moon RD, Hinkle NC, Millar JG, McElfresh JS, Mullens BA (2009) Characterization of age and cuticular hydrocarbon variation in mating pairs of house fly, Musca domestica, collected in the field. Med Vet Entomol 23:426–442
Chapman RF (2012) The insects. Structure and function. 5th ed. Cambridge University Press.
Frere B, Suchaud F, Bernier G et al (2014) GC-MS analysis of cuticular lipids in recent and older scavenger insect puparia. An approach to estimate the postmortem interval (PMI). Anal Bioanal Chem 406:1081–8
Nansen C, Sidumo AJ, Capareda S (2010) Variogram analysis of hyperspectral data to characterize the impact of biotic and abiotic stress of maize plants and to estimate biofuel potential. Appl Spectrosc 64:627–36
Grassberger M, Freidrich E, Reiter C (2003) The blowfly Chrysomya albiceps (Wiedemann) (Diptera: Calliphoridae) as a new forensic indicator in Central Europe. Int J Legal Med 117:75–81
Davies K, Harvey ML (2013) Internal morphological analysis for age estimation of blow fly pupae (Diptera: Calliphoridae) in postmortem interval estimation. J Forensic Sci 58:79–84
Fraenkel G, Bhaskaran G (1973) Pupariation and pupation in cyclorrhaphous flies (Diptera): terminology and interpretation. Ann Entomol Soc Am 66:418–22
Smith KGV (1986) A manual of forensic entomology. Trustees of the British Museum, Natural History and Cornell University Press, London
Amendt J, Campobasso C, Gaudry E, Reiter C, LeBlanc H, Hall M (2007) Best practice in forensic entomology - standards and guidelines. Int J Legal Med 121:90–104
Zhang X, Nansen C, Aryamanesh N, Yan G, Boussaid F (2015) Importance of spatial and spectral data reduction in detection of internal defects in food products. Appl Spectrosc 69:473–80
Nansen C, Geremias LD, Xue Y, Huang F, Parra JR (2013) Agricultural case studies of classification accuracy, spectral resolution, and model over-fitting. Appl Spectrosc 67:1332–8
Krushkal JB, Wish M (1978) Multidimensional scaling. Sage, Beverley Hills
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–43
Fisher RA (1936) The use of multiple measurements in taxonomic problems. Ann Eugenics 7:179–88
Richards C, Villet M (2008) Factors affecting the accuracy and precision of thermal summation models of insect development used to estimate post-mortem intervals. Int J Legal Med 122:401–8
Malewski T, Draber-Monko A, Pomorski J, Los M, Bogdanowicz W (2010) Identification of forensically important blowfly species (Diptera: Calliphoridae) by high resolution melting PCR analysis. Int J Legal Med 124:277–85
Acknowledgments
We are grateful to Mike Johnson and Yvette Hitchen at The University of Western Australia, School of Animal Biology, for access to a dissecting microscope with image capture capabilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Voss, S.C., Magni, P., Dadour, I. et al. Reflectance-based determination of age and species of blowfly puparia. Int J Legal Med 131, 263–274 (2017). https://doi.org/10.1007/s00414-016-1458-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-016-1458-5