Abstract
The present study investigated the molecular pathology of atrial and brain natriuretic peptides (ANP and BNP) in the myocardium to evaluate terminal cardiac function in routine forensic casework with particular regard to fatal drug intoxication (n = 18; sedative–hypnotics, n = 10; methamphetamine, n = 8), hypothermia (cold exposure, n = 13), and hyperthermia (heatstroke, n = 10), compared with that in acute ischemic heart disease (AIHD, n = 35) and congestive heart disease (CHD, n = 11) as controls (total n = 87; within 48 h postmortem). Quantitative analyses of myocardial ANP and BNP messenger RNA demonstrated that their expressions in bilateral atrial and ventricular walls were high in methamphetamine intoxication and hypothermia, comparable to those in AIHD and CHD, but were low in sedative–hypnotic intoxication and hyperthermia. In pericardial fluid, both ANP and BNP levels were increased in hypothermia, while CHD cases had an elevated BNP level, and ANP level showed a tendency to increase in hyperthermia; however, immunohistochemistry showed no evident differences in myocardial ANP and BNP among the causes of death. These findings suggest terminal high cardiac strain in methamphetamine intoxication, decreased cardiac strain in sedative–hypnotic intoxication and hyperthermia (heatstroke), and persistent congestion in hypothermia (cold exposure).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In forensic casework, difficulties remain in determining the causes of death involving functional deterioration without specific pathologies, which include fatal intoxication and thermal disorders, such as cold exposure and heat stroke. In such cases, investigation of characteristic functional changes of life-supporting organs, including the brain, heart, and lungs, may be helpful to reinforce pathological and toxicological findings, excluding the contribution of any other traumas and diseases to the death process. For this purpose, previous studies suggested possible application of postmortem molecular biological evaluation of the brain, heart, and lung [1–5]. As for markers of cardiac function, atrial and brain natriuretic peptides (ANP and BNP) in the myocardium rapidly respond to increased cardiac strain [6–9]; the application of molecular biological procedures to these markers may be useful to investigate terminal cardiac function, especially in “functional death,” which presents with poor morphological findings [1].
The present study compared terminal cardiac function in fatal intoxication, hypothermia (cold exposure), and hyperthermia (heatstroke) to that of acute and chronic heart diseases, using molecular pathology of atrial and brain natriuretic peptides (ANP and BNP) in the myocardium as markers of cardiac strain.
Materials and methods
Materials
Forensic autopsy cases of fatal drug intoxication (n = 18; sedative–hypnotics, n = 10; and methamphetamine, n = 8), hypothermia (cold exposure, n = 13), and hyperthermia (heatstroke, n = 10), as well as acute ischemic heart disease (AIHD, n = 35) and congestive heart disease (CHD, n = 11) as controls, were examined (total n = 87; within 48 h postmortem). Case profiles are summarized in Table 1. For these groups, cases where the causes of death were established on the basis of complete autopsy and well-established circumstantial evidence were included, and those with significant complications were excluded. The inclusion criteria for hypothermia were typical pathologies including frost erythema and hemorrhagic gastric erosions (Wischnewski spots) as well as biochemical signs of elevated serum urea nitrogen and/or acetonemia [10–12], and those for hyperthermia were pathological and biochemical findings of multiple organ tissue damage [multiple organ dysfunction syndrome (MODS)] involving rhabdomyolysis [13–15], excluding those of drug abusers, chronic alcoholics, and death during bathing.
Pericardial fluid was collected aseptically using a syringe after opening the pericardial cavity at autopsy, centrifuged and stored at –20 °C until use. Routine heart tissue specimens were preserved in formalin for histopathology. Myocardial tissue specimens for messenger RNA (mRNA) measurements (about 50 mg) were taken from consistent sites of the bilateral atrial walls, anterior and posterior walls of the left ventricle, and right ventricular wall during autopsy, then submerged in 1 ml of RNA stabilization solution (RNAlaterTM, Ambion, Austin) and stored at 4 °C for <1 week until RNA extraction. The sample collections and analyses described below were performed within the framework of our routine casework, following the autopsy guidelines (2009) and ethics guidelines (1997 and 2003) of the Japanese Society of Legal Medicine, approved by our institutional ethics committee.
Methods
Measurements of ANP and BNP levels in pericardial fluids
The pericardial ANP and BNP concentrations were measured by chemiluminescent enzyme immunoassay using MIO2 Shionogi ANP and MIO2 Shionogi BNP assay kits (Shionogi Co. Ltd., Osaka), respectively. The ranges of measurement were <2,000 pg/ml for both ANP and BNP. Samples were diluted (×10 and ×100, respectively) to measure higher concentrations (>2,000 pg/ml), and measurements were performed in duplicate to exclude possible interference due to contaminants. The clinical serum reference ranges were 10–40 pg/ml for ANP and 2–20 pg/ml for BNP, and postmortem pericardial cut-off values were estimated to be 30 pg/ml for ANP and 150 pg/ml for BNP [16].
Immunostaining of ANP and BNP in the myocardium
Serial sections of 5-μm thickness were prepared from formalin-fixed, paraffin-embedded heart tissue specimens. Polyclonal rabbit anti-human ANP IgG (0780-0179; AbD Serotec, Oxford, UK; diluted 600-fold) and rabbit anti-human BNP IgG (16162; IBL, Takasaki, Japan; diluted 25-fold) as well as mouse monoclonal antihuman ANP IgG (0200-0648; AbD Serotec; diluted 50-fold) and antihuman BNP IgG (MCA2642; AbD Serotic; diluted 20-fold) were used. Following overnight incubation with the primary antibodies described above at room temperature, immunoreactions were visualized by the polymer method (ChemMate Envision; Dako Japan, Tokyo) and color was developed with 3,3′-diamino benzidine tetrahydrochloride (DAB liquid system, Dako Japan), according to the manufacturer’s instructions (counterstaining with hematoxylin). Endogenous peroxide was inactivated by incubation with 3 % hydrogen peroxide for 10 min. For a control study to confirm the specificity of immunostaining, phosphate-buffered saline, rabbit IgG (Vector Laboratories, Burlingame, CA, USA) or mouse IgG (Vector Laboratories) was substituted for the primary antibody.
Quantification of mRNA in the myocardium
Total RNA was isolated with ISOGEN (Nippon Gene, Toyama) according to the manufacturer’s instructions and stored at −80 °C until use. The extraction yield was quantified spectrophotometrically, and the quality (integrity) of total RNA was assessed by electrophoresis in agarose gels stained with ethidium bromide; 18S and 28S rRNA bands were visualized under UV illumination.
Reverse transcription PCR (RT-PCR) was performed using the TaqMan Gold RT-PCR Core Reagents kit on an ABI PRISM 7000 sequence Detection System (Applied Biosystems, Foster City, CA, USA). The contents of the amplification mix (20 μl/tube), including total mRNA (1.0 μl of 0.08–0.28 μg/100 μl solution), and the thermal cycling conditions were set according to the accessory protocols. Amplification of ANP and BNP mRNAs, together with the endogenous references described below, was performed. Primers and probes for these mRNAs were synthesized according to previous reports [2, 3, 17–23] and the GenBank nucleotide database, with probes spanning the junction of bordering exons. According to the manufacturer’s instructions, the relative quantification of mRNA transcripts was carried out using the comparative threshold method. Each mRNA level was expressed as the ratio of the target normalized against endogenous references, for which potential quantitative references for normalizing real-time PCR data were generated for each sample using five common housekeeping genes: glyceraldehyde-3-phosphate dehydrogenase (GAPDH), β2-microglobulin (β2M), β-actin, TATA box-binding protein (TBP), and cyclophilin A (CYCA) [19, 21]. The calibrator was obtained from a case of peracute death with accidental decapitation (ca. 24 h postmortem).
Statistical analyses
Regression equation analysis was used to study the relationships between pairs of parameters. Steel–Dwass test was used for nonparametric multiple comparison among groups. In addition, comparisons between individual groups were performed using the nonparametric Mann–Whitney U test. A p value of <0.05 was considered statistically significant.
Results
ANP and BNP levels in pericardial fluids
Pericardial ANP level (cut-off value, 30 pg/ml) was elevated in most cases of hypothermia and hyperthermia, while BNP level (cut-off value, 150 pg/ml) was markedly increased in hypothermia and CHD (Table 2).
Immunostaining of ANP and BNP in the myocardium
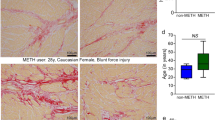
Immunostaining demonstrated ANP and BNP in the cardiomyocytes (Fig. 1). The staining intensity was lower in bilateral ventricular walls than in atrial walls, showing a varied intensity and distribution by case; however, differences were not evident among the causes of death.
Immunostaining of ANP (1) and BNP (2) in the cardiomyocytes in cases of methamphetamine intoxication (P-2), hypothermia (H), hyperthermia (C), and congestive heart disease (CHD), using monoclonal anti-human ANP and BNP. The atrial walls were more intensely positive than bilateral ventricular walls, showing varied intensity and distribution by case; however, differences were not evident among the causes of death. The findings were similar when polyclonal reagents were used. a Anterior wall of left ventricle; b right ventricular wall; c right atrial wall. P-2 (62-year-old man; survival time, about 15 h; about 26 h postmortem); H (64-year-old man; survival time, about 3 h; about 28 h postmortem); C (64-year-old woman; survival time, about 6 h; about 30 h postmortem); CHD (52-year-old woman, survival time unknown; about 36 h postmortem)
ANP and BNP mRNA expressions in the myocardium
Site difference in target and reference mRNA expression
When raw data of mRNA amplification were compared for all cases, C T values for ANP mRNA were evidently lower in atrial walls (left, 16.2–32.0 with a median of 21.6; right, 16.0–33.7 with a median of 19.7) than in bilateral ventricles (19.2–37.9 with medians of 29.0–30.5; p < 0.05); ANP mRNA expression was generally higher in the atria than in the ventricles. BNP mRNA expression was similar in the atria and ventricles (17.5–38.3 with medians of 23.3–30.2); however, C T values were slightly lower in the left/right atrial wall (median, 25.9/23.3) than in the ventricular walls (median, 29.0–30.2), and lower in the right atrial wall than in the left (p < 0.05), indicating higher expression in the atria, especially in the right. In bilateral atria, mRNA expression was higher for ANP than for BNP (p < 0.0001). Among five housekeeping genes, C T values of GAPDH (19.4–30.1 with medians of 22.7–23.9), β2M (19.2–30.3 with medians of 22.9–23.1), and CYCA (19.5–30.4 with medians of 22.3–23.7) were similar at each site, and those of β-actin were slightly higher (19.9–34.1 with medians of 23.0–25.2; insignificant), showing no site differences; however, C T values of TBP (25.9–36.7 with medians of 28.9–29.5) were higher than those of others at each site (p < 0.05), indicating lower mRNA expression.
Stability of relative mRNA quantification with regards to gender, age, survival period, postmortem interval, and endogenous reference genes
Simultaneous RT-PCR of mRNA of five housekeeping genes (GAPDH, β2M, β-actin, TBP, and CYCA) showed high correlations of C T values of GAPDH to others at each site (r = 0.90–0.96, p < 0.0001), showing almost equivalent values. β2M and CYCA also showed high and almost equivalent correlations to others (r = 0.93–0.95, p < 0.0001). Correlations of β-actin or TBP to others were partly lower (r = 0.77–0.96, p < 0.0001) than those of others, and C T values were not equivalent; β-actin and TBP showed higher C T values, indicating lower mRNA expressions.
For all cases, an age-dependent increase was partly detected for β2M, β-actin, TBP, and CYCA mRNAs, especially in right atrial and ventricular walls (r = 0.32–0.50, p = 0.10–p < 0.01; partly significant), but was insignificant for GAPDH. No gender-related difference was detected for each housekeeping gene expression. A slight survival time-dependent decrease was detected for CYCA mRNA in bilateral atrial walls (r = 0.38, p < 0.05 for the left; r = 0.50, p < 0.01 for the right). A tendency toward a postmortem decrease was significant for TBP and CYCA mRNA in left anterior and posterior ventricular walls, respectively, but was otherwise insignificant within 48 h postmortem.
For ANP mRNA, there was no age-dependent, gender-related difference, or survival time or postmortem time dependency. BNP mRNA showed a slight age-dependent increase in the right ventricle (r = 0.42, p < 0.05), and a survival time-dependent increase in right atrial and ventricular walls (r = 0.39 and r = 0.40, respectively, p < 0.05). No gender-related difference or postmortem time dependency was detected. When neighboring portions of the myocardium at each site were compared (n = 29), correlations of ANP/BNP mRNA measurements were high (r = 0.91/0.87, p <0.0001), showing no significant differences, irrespective of the site; local differences did not affect the findings. The stability of ANP and BNP mRNA assays was partly confirmed by re-examination of the same RNA samples (r = 0.93 and r = 0.99, respectively, p < 0.0001, n = 50); the assay-to-assay deviation was insignificant and did not affect the findings
Relative quantification of ANP and BNP mRNA levels in the myocardium with regard to the cause of death
When ANP and BNP mRNA expressions were normalized against GAPDH, β2M, or CYCA mRNA, in consideration of the stabilities and equivalencies of the housekeeping genes mentioned above, similar findings were seen with regard to the cause of death, as follows. In fatal methamphetamine abuse and hypothermia cases, myocardial ANP and BNP mRNA levels in bilateral atrial and ventricular walls were as high as those in AIHD and CHD (medians for ANP/BNP: left ventricle, 1.75–25.28/2.40–21.56; right ventricle, 0.33–1.23/0.46–23.05; bilateral atria, 786.88–2,336.28/30.19–337.79) (Fig. 2). Overall, these markers were low in sedative–hypnotic intoxication (medians for ANP/BNP: left anterior and posterior ventricular wall, 0.23 and 0.20/0.13 and 0.10; right ventricle, 0.14/0.30; left and right atria, 452.86 and 560.33/12.04 and 21.54) and hyperthermia (medians for ANP/BNP: left anterior and posterior ventricular wall, 0.35 and 0.19/0.29 and 0.23; right ventricle, 0.09/0.04; left and right atria, 63.70 and 412.50/1.10 and 4.18), compared with those in other groups; significant differences were detected for left ventricular ANP and BNP mRNA in sedative–hypnotic intoxication, and bilateral atrial and posterior left ventricular BNP in hyperthermia.
ANP and BNP mRNA quantification on the logarithmic scale with regard to the cause of death. ANP atrial natriuretic peptide, BNP brain natriuretic peptide, GAPDH glyceraldehyde-3-phosphate dehydrogenase, P-1 sedative–hypnotic intoxication, P-2 methamphetamine intoxication, H hyperthermia, C hypothermia, AIHD acute ischemic heart disease (including acute myocardial infarction and acute ischemic heart disease without apparent myocardial necrosis), CHD chronic congestive heart disease. 1 ANP mRNA quantification, normalized against GAPDH. Dagger Significantly lower: a anterior wall of left ventricle, sedative–hypnotic intoxication (P-1) vs. other groups except for hyperthermia (H) (p < 0.05); b posterior wall of left ventricle, sedative–hypnotic intoxication (P-1) vs. methamphetamine intoxication (P-2) and hypothermia (C) (p < 0.05); by Steel–Dwass test. The results of individual comparisons by nonparametric Mann–Whitney U test were as follows. Significantly lower: a anterior wall of left ventricle, P-1 vs. other groups except for H (p < 0.005); H vs. other groups except for P-1 (p < 0.005); b posterior wall of left ventricle, P-1 vs. other groups except for H (p < 0.01-p < 0.005); H vs. other groups except for P-1 and P-2 (p < 0.05-p < 0.005); c) right ventricular wall, P-1 vs. AIHD and CHD (p < 0.05); e right atrial wall, P-1 vs. AIHD (p < 0.05) and C (p < 0.01); H vs. AIHD (p < 0.05). These findings were similar when normalized against β2-microglobulin (β2M) or cyclophilin A (CYCA). 2 BNP mRNA quantification, normalized against GAPDH. Dagger Significantly lower: a anterior wall of left ventricle, sedative–hypnotic intoxication (P-1) vs. other groups except for hyperthermia (H) (p < 0.05); b posterior wall of left ventricle, sedative–hypnotic intoxication (P-1) vs. other groups except for hyperthermia (H) (p < 0.05), and hyperthermia (H) vs. methamphetamine intoxication (P-2) and acute ischemic heart disease (AIHD) (p < 0.05); d left atrial wall, hyperthermia (H) vs. chronic congestive heart disease (CHD) (p < 0.05); e right atrial wall, hyperthermia (H) vs. hypothermia (C) (p < 0.05) by Steel–Dwass test. The results of individual comparisons by nonparametric Mann–Whitney U test were as follows. Significantly lower: a anterior wall of left ventricle, P-1 vs. other groups except for H (p < 0.005-p < 0.001); H vs. P-2, AIHD and CHD (p < 0.05); b posterior wall of left ventricle, P-1 vs. other groups except for H (p < 0.005-p < 0.001); H vs. other groups except for P-1 (p < 0.05-p < 0.005); c right ventricular wall, P-1 vs. P-2 (p < 0.05); H vs. P-2 and CHD (p < 0.05); d left atrial wall, P-1 vs. CHD (p < 0.05); H vs. C, AIHD and CHD (p < 0.05-p < 0.005); e right atrial wall, P-1 vs. P-2 (p < 0.05); H vs. C, AIHD and CHD (p < 0.05-p < 0.005). These findings were similar when normalized against β2-microglobulin (β2M) or cyclophilin A (CYCA)
Expression of ANP mRNA was evidently higher in bilateral atria than in the ventricles, and a similar tendency was detected for BNP. Site-to-site correlations depended on the cause of death and were significant: in sedative–hypnotic intoxication, for ANP mRNA between right and left anterior ventricular walls as well as for BNP mRNA between bilateral atria, and between right and left posterior ventricular walls (r = 0.85–0.99, p < 0.01–0.0001); in fatal methamphetamine abuse, for ANP mRNA between bilateral atria as well as for BNP mRNA between bilateral atria, between anterior and posterior walls of the left ventricle, and between right and left anterior/posterior ventricular walls (r = 0.91–0.99, p < 0.01–0.0001); in hyperthermia, for ANP mRNA between bilateral atria, between anterior and posterior walls of the left ventricle, and between the left atrium and left anterior/posterior ventricular walls as well as for BNP mRNA between right and left posterior ventricular walls (r = 0.66–0.95, p < 0.05–0.0001); in hypothermia, for ANP/BNP mRNA between bilateral atria (r = 0.85, p < 0.001/r = 0.87, p < 0.0001); in AIHD, for ANP mRNA between bilateral atria, between the left atrium and left posterior ventricular wall, and between right and left anterior ventricular walls (r = 0.35–0.90, p < 0.05–0.0001) as well as for BNP mRNA between bilateral atria, between anterior and posterior walls of the left ventricle, between the right atrium and ventricle, and between the left atrium and left posterior ventricular wall (r = 0.35–0.90, p < 0.05–0.0001); and in CHD, for ANP mRNA between bilateral atria, between anterior and posterior walls of the left ventricle, and between right and left anterior ventricular walls (r = 0.64–0.96, p < 0.05–0.0001).
The heart weight was relatively large in AIHD and small in sedative–hypnotic intoxication (significantly different between these groups), compared with those in other groups (insignificant), and combined lung weight was increased in most cases other than hypothermia (Table 1). In fatal methamphetamine abuse, anterior left ventricular ANP mRNA level correlated with the heart weight (r = 0.73, p < 0.05), and CHD showed correlations of right ventricular ANP, and left and right atrial BNP mRNA levels to the heart weight (r = 0.769, p < 0.01, r = 0.697, p < 0.02, and r = 0.670, p < 0.05, respectively). Correlations with the combined lung weight were detected for anterior left ventricular ANP mRNA in AIHD and CHD. Otherwise, there was no correlation of ANP or BNP mRNA to the heart or lung weight.
Discussion
Various ancillary procedures have been published for determining deaths due to hypothermia (cold exposure) and hyperthermia (heat stroke), including immunohistochemical and biochemical markers related to stress responses, metabolic deterioration, and systemic tissue damage [11, 13, 14, 24–31]. These markers can demonstrate metabolic deterioration and persistent heart failure without substantial damage to life-supporting organ tissues, predispositions and complications in death from cold exposure, and dehydration and/or advanced multiple organ tissue damage (MODS) in death from heat stroke, as well as different stress responses in these causes of death [11, 13, 25–29]. These procedures are also useful for investigating systemic dysfunction in fatal intoxication [14, 30–34]. In addition, previous studies have suggested that relative mRNA quantification can be used for postmortem investigation of molecular biological alterations in the death process [1–5, 17, 18, 20, 22, 23].
In the present study, the stability of relative mRNA quantification using RT-PCR for autopsied myocardium specimens was established for target and reference genes; endogenous reference markers (GAPDH, β2M, β-actin, TATA, and CYCA) showed high correlations in simultaneous assays. Among these reference markers, however, expressions of GAPDH, β2M, and CYCA mRNAs showed high correlations and equivalencies without evident age-dependent or gender-related difference, and their expression levels were similar to those of target mRNAs (ANP and BNP). Using these housekeeping genes, relative mRNA expression levels of individual target genes (ANP and BNP), normalized against each endogenous reference, showed no significant deviation between neighboring sites or in re-examination, representing mRNA expressions at the site of sampling; target mRNA expression levels could be successfully evaluated within 2 days postmortem.
BNP is secreted by both cardiac atria and ventricles in response to persistent cardiac strain, although the main source is the ventricle, whereas ANP is secreted from the atrium in a physiological state, and the induction of ANP mRNA is seen in a pathological state involving heart failure [35–38]. In the present study, immunostaining of the myocardium detected no differences among the examined causes of death; however, biochemical and molecular pathological analyses demonstrated significant differences among these causes of death, as described below. Overall expression of BNP mRNA and higher ANP mRNA expression in bilateral atria than in the ventricles was consistent with the major site of physiological secretion and induction in response to increased cardiac strain, described above [35–38]. Generally higher ANP mRNA expressions in bilateral atria may represent increased central venous (right atrial) and pulmonary capillary (left atrial) pressure as a sign of terminal heart failure, thus showing minor differences among the causes of death.
In intoxication, high myocardial ANP and BNP mRNA expressions in the whole heart in fatal methamphetamine abuse, which were similar to those of AIHD, suggested high cardiac strain as a sign of cardiac dysfunction accompanied by intense pulmonary congestion [39]. The correlation of anterior left ventricular ANP mRNA with the heart weight suggested the contribution of pre-existing left ventricular strain related to cardiac hypertrophy, whereas complication of advanced hyperthermia may reduce these mRNA expressions, as described below [40, 41]. In sedative–hypnotic intoxication, however, overall lower ANP and BNP mRNA expressions, especially in the left ventricle, in sedative–hypnotic intoxication indicated decreased left ventricular strain, which can be the consequence of reduced left ventricular filling due to advanced pulmonary edema, accompanied by diffuse myocardial damage [42]. Meanwhile, no significant increase in pericardial ANP or BNP level was detected in these intoxication cases. These findings were consistent with pathological findings of increased lung weight in methamphetamine and sedative–hypnotic intoxication, mainly accompanied by acute congestion and edema, respectively, showing differences in these drug toxicities. Determination of fatal intoxication depends on toxicological data; however, blood drug levels varied by case and were often below lethal levels, especially in cases of a longer survival combined with drug abuse. With respect to this, previous studies using biochemical and immunohistochemical markers have indicated systemic deterioration of nervous systems accompanied by myocardial and skeletal muscle damage, suggesting toxic or adverse effects of drugs, including methamphetamine and sedative–hypnotics [26, 30, 43]. The present study demonstrated a difference in terminal cardiac function between methamphetamine and sedative–hypnotic intoxication, which may be used to interpret major drug effects in combined drug abuse; the molecular pathology of myocardial natriuretic peptides can provide additional findings for evaluating terminal cardiac function related to fatal intoxication.
Hypothermia (cold exposure) presented with characteristic findings, involving higher expressions of myocardial ANP and BNP mRNA in the whole heart, accompanied by elevated pericardial ANP and BNP levels, indicating cardiac dysfunction accompanied by persistent heart failure without substantial damage to the myocardium [16, 44], ready for recovery by adequate medical management [44–46]. In hyperthermia (heatstroke) cases, however, especially lower expressions of posterior left ventricular and bilateral atrial BNP mRNA, accompanied by an increase in pericardial ANP may represent the terminal cardiac status as a consequence of reduced circulatory blood volume due to generalized vasodilatation and dehydration, accompanied by increased venous return and high-output cardiac failure, as well as diffuse myocardial damage involved in multiple organ tissue damage (multiple organ dysfunction syndrome), followed by circulatory collapse [47–49]. These observations indicate a difference in terminal cardiac function between hypothermia and hyperthermia as well as between hypothermia and sedative–hypnotic intoxication and also between hyperthermia and methamphetamine intoxication, which can contribute to analysis of terminal cardiac dysfunction on a case-by-case basis in individual fatalities due to extreme environmental temperatures under possible influence of drugs.
In relative mRNA quantification, the amount of tissue sample or the site of sampling does not immediately affect the assay. In the present study, site-to-site correlations were detected depending on the cause of death, suggesting parallel responses to bilateral atrial and ventricular strain in fatal methamphetamine abuse and AIHD as well as to bilateral atrial strain in sedative–hypnotic intoxication and hypothermia (cold exposure), and the ANP-dominant responses to cardiac strain in the death process of hyperthermia (heatstroke) and CHD. Interindividual differences in ANP and BNP mRNA expressions in each cause of death, showing partial overlap with other groups, may represent the severity of terminal cardiac dysfunction. Because of microanalysis, however, the findings may depend on the status of tissue at the site of sampling; thus, at least histological evaluation of the sampling site is needed. Double sampling at each site, as well as site-to-site comparisons of ANP and BNP mRNAs, may be helpful to establish the findings in individual case studies.
In conclusion, the present study demonstrated terminal high cardiac strain in methamphetamine intoxication, decreased cardiac strain in sedative–hypnotic intoxication and hyperthermia, and persistent congestion in hypothermia, suggesting the possible application of postmortem molecular biological analysis of myocardial ANP and BNP to demonstrate terminal cardiac dysfunction as part of systemic functional changes of life-supporting organs related to fatal intoxication, hypothermia (cold exposure), and hyperthermia (heatstroke), which may be helpful to reinforce pathological and toxicological findings.
References
Maeda H, Zhu BL, Ishikawa T et al (2010) Forensic molecular pathology of violent deaths. Forensic Sci Int 203:83–92
Zhao D, Zhu BL, Ishikawa T et al (2006) Real-time RT-PCR quantitative assays and postmortem degradation profiles of erythropoietin, vascular endothelial growth factor and hypoxia-inducible factor 1 alpha mRNA transcripts in forensic autopsy materials. Leg Med (Tokyo) 8:132–136
Ishida K, Zhu BL, Maeda H (2000) Novel approach to quantitative reverse transcription PCR assay of mRNA component in autopsy material using the TaqMan fluorogenic detection system: dynamics of pulmonary surfactant apoprotein A. Forensic Sci Int 113:127–131
Bauer M (2007) RNA in forensic science. Forensic Sci Int Genet 1:69–74
Zhao D, Ishikawa T, Quan L et al (2009) Evaluation of pulmonary GLUT1 and VEGF mRNA levels in relation to lung weight in medicolegal autopsy cases. Leg Med (Tokyo) 11:S290–S293
Mannix ET, Farber MO, Aronoff GR et al (1991) Regulation of atrial natriuretic peptide release in normal humans. J Appl Physiol 71:1340–1345
de Bold AJ, Bruneau BG, Kuroski de Bold ML (1996) Mechanical and neuroendocrine regulation of the endocrine heart. Cardiovasc Res 31:7–18
LaPointe MC (2005) Molecular regulation of the brain natriuretic peptide gene. Peptides 26:944–956
McGrath MF, de Bold AJ (2005) Determinants of natriuretic peptide gene expression. Peptides 26:933–943
Tsokos M, Rothschild MA, Madea B et al (2006) Histological and immunohistochemical study of Wischnewsky spots in fatal hypothermia. Am J Forensic Med Pathol 27:70–74
Zhu BL, Ishikawa T, Michiue T et al (2007) Differences in postmortem urea nitrogen, creatinine and uric acid levels between blood and pericardial fluid in acute death. Leg Med (Tokyo) 9:115–122
Mizukami H, Shimizu K, Shiono H et al (1999) Forensic diagnosis of death from cold. Leg Med (Tokyo) 1:204–209
Zhu BL, Ishida K, Quan L et al (2001) Post-mortem urinary myoglobin levels with reference to the causes of death. Forensic Sci Int 115:183–188
Maeda H, Zhu BL, Bessho Y et al (2008) Postmortem serum nitrogen compounds and C-reactive protein levels with special regard to investigation of fatal hyperthermia. Forensic Sci Med Pathol 4:175–180
Bouchama A, Knochel JP (2002) Heat stroke. N Engl J Med 346:1978–1988
Zhu BL, Ishikawa T, Michiue T et al (2007) Postmortem pericardial natriuretic peptides as markers of cardiac function in medico-legal autopsies. Int J Legal Med 121:28–35
Ishida K, Zhu BL, Maeda H (2005) TaqMan fluorogenic detection system to analyze gene transcription in autopsy material. Methods Mol Biol 291:415–421
Ishida K, Zhu BL, Maeda H (2002) A quantitative RT-PCR assay of surfactant-associated protein A1 and A2 mRNA transcripts as a diagnostic tool for acute asphyxial death. Leg Med (Tokyo) 4:7–12
Vandesompele J, De Preter K, Pattyn F et al (2002) Accurate normalization of real-time quantitative RT-PCR data by genometric averaging of multiple internal control genes. Genome Biol 3:1–11
Zhu BL, Tanaka S, Ishikawa T et al (2008) Forensic pathological investigation of myocardial hypoxia-inducible factor-1 alpha, erythropoietin and vascular endothelial growth factor in cardiac death. Leg Med (Tokyo) 10:11–19
Koppelkamm A, Vennemann B, Fracasso T et al (2010) Validation of adequate endogenous reference genes for the normalization of qPCR gene expression data in human post mortem tissue. Int J Legal Med 124:371–380
Chen JH, Michiue T, Ishikawa T et al (2012) Difference in molecular pathology of natriuretic peptides in the myocardium between acute asphyxial and cardiac deaths. Leg Med (Tokyo)14:177–182
Miyazato T, Ishikawa T, Michiue T et al (2012) Molecular pathology of pulmonary surfactants and cytokines in drowning compared with other asphyxiation and fatal hypothermia. Int J Legal Med 126:581–587
Preuss J, Dettmeyer R, Poster S et al (2008) The expression of heat shock protein 70 in kidneys in cases of death due to hypothermia. Forensic Sci Int 176:248–252
Ishikawa T, Yoshida C, Michiue T et al (2010) Immunohistochemistry of catecholamines in the hypothalamic-pituitary-adrenal system with special regard to fatal hypothermia and hyperthermia. Leg Med (Tokyo) 12:121–127
Ishikawa T, Michiue T, Maeda H (2011) Evaluation of postmortem serum and cerebrospinal fluid growth hormone levels in relation to the cause of death in forensic autopsy. Hum Cell 24:74–77
Ishikawa T, Quan L, Li DR et al (2008) Postmortem biochemistry and immunohistochemistry of adrenocorticotropic hormone with special regard to fatal hypothermia. Forensic Sci Int 179:147–151
Ishikawa T, Miyaishi S, Tachibana T et al (2004) Fatal hypothermia related vacuolation of hormone-producing cells in the anterior pituitary. Leg Med (Tokyo) 6:157–163
Yoshida C, Ishikawa T, Michiue T et al (2011) Postmortem biochemistry and immunohistochemistry of chromogranin A as a stress marker with special regard to fatal hypothermia and hyperthermia. Int J Legal Med 125:11–20
Quan L, Ishikawa T, Hara J et al (2011) Postmortem serotonin levels in cerebrospinal and pericardial fluids with regard to the cause of death in medicolegal autopsy. Leg Med (Tokyo) 13:75–78
Wang Q, Michiue T, Ishikawa T et al (2011) Combined analyses of creatine kinase MB, cardiac troponin I and myoglobin in pericardial and cerebrospinal fluids to investigate myocardial and skeletal muscle injury in medicolegal autopsy cases. Leg Med (Tokyo) 13:226–232
Quan L, Ishikawa T, Michiue T et al (2005) Ubiquitin-immunoreactive structures in the midbrain of methamphetamine abusers. Leg Med (Tokyo) 7:144–150
Ishikawa T, Zhu BL, Miyaishi S et al (2007) Increase in clusterin-containing follicles in the adenohypophysis of drug abusers. Int J Legal Med 121:395–402
Zhu BL, Ishida K, Oritani S et al (2001) Immunohistochemical investigation of pulmonary surfactant-associated protein A in fatal poisoning. Forensic Sci Int 117:205–212
Potter LR (2011) Natriuretic peptide metabolism, clearance and degradation. FEBS J 278:1808–1817
Xu-Cai YO, Wu Q (2010) Molecular forms of natriuretic peptides in heart failure and their implications. Heart 96:419–424
Daniels LB, Maisel AS (2007) Natriuretic peptides. J Am Coll Cardiol 50:2357–2368
Hama N, Itoh H, Shirakami G et al (1995) Rapid ventricular induction of brain natriuretic peptide gene expression in experimental acute myocardial infarction. Circulation 92:1558–1564
Pappas L, Filippatos G (2011) Pulmonary congestion in acute heart failure: from hemodynamics to lung injury and barrier dysfunction. Rev Esp Cardiol 64:735–738
Patel MM, Belson MG, Longwater AB et al (2005) Methylenedioxymethamphetamine (ecstasy)-related hyperthermia. J Emerg Med 29:451–454
Freedman RR, Johanson CE, Tancer ME (2005) Thermoregulatory effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 183:248–256
Ferslew KE, Hagardorn AN, Harlan GC et al (1998) A fatal drug interaction between clozapine and fluoxetine. J Forensic Sci 43:1082–1085
Iacovelli L, Fulceri F, De Blasi A et al (2006) The neurotoxicity of amphetamines: bridging drugs of abuse and neurodegenerative disorders. Exp Neurol 201:24–31
Turk EE (2010) Hypothermia. Forensic Sci Med Pathol 6:106–115
Aslam AF, Aslam AK, Vasavada BC et al (2006) Hypothermia: evaluation, electrocardiographic manifestations, and management. Am J Med 119:297–301
Wolfe CS (1993) Severe hypothermia associated with prolonged cardiorespiratory arrest and full recovery. J Am Board Fam Pract 6:594–596
Broessner G, Beer R, Franz G et al (2005) Case report: severe heat stroke with multiple organ dysfunction - a novel intravascular treatment approach. Crit Care 9:R498–R501
Holman ND, Schneider AJ (1989) Multi-organ damage in exertional heat stroke. Neth J Med 35:38–43
Ali SZ, Taguchi A, Rosenberg H et al (2003) Malignant hyperthermia. Best Pract Res Clin Anaesthesiol 17:519–533
Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science and the Ministry of Education, Culture, Sports, Science and Technology, Japan (grant no. 22590642).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, JH., Michiue, T., Ishikawa, T. et al. Molecular pathology of natriuretic peptides in the myocardium with special regard to fatal intoxication, hypothermia, and hyperthermia. Int J Legal Med 126, 747–756 (2012). https://doi.org/10.1007/s00414-012-0732-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-012-0732-4