Abstract
Thanatophilus micans is capable of finding corpses at least as quickly as most fly species and, as the most widespread species of the Silphidae in Africa, offers a useful model for estimating post-mortem interval. Larvae were reared at ten constant temperatures from 15°C to 35°C and their length measured at 4, 8, or 12-h intervals depending on their instar. Length generally increased with increased rearing temperature, but decreased at extremely high temperatures. Note was made of the age at which individuals progressed past developmental milestones. Development took longer at lower temperatures. These results are presented as a combined isomegalen and isomorphen diagram. Developmental constants were generated for each milestone using major axis regression. Developmental threshold values did not differ significantly between milestones. Development took longer than in blow flies, but was faster than in Dermestidae. The three models presented here, therefore, cover an important time frame in estimating minimum PMI once fly larvae have matured to the point of leaving a corpse, and, therefore, provide a tool that was not previously available to forensic entomologists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of carrion-breeding insects is widely accepted as a useful indicator of minimum post-mortem interval (mPMI) [1, 2, 4, 18]. Most estimates of mPMI are made using the development of maggots because adult flies are capable of finding corpses within hours of death [18]. For this reason, most of the research into the development of carrion-related insects has focused on maggots (fly larvae), and beetles have been neglected.
Adults of Thanatophilus micans (Silphidae) and Dermestes maculatus (Dermestidae) have been observed on animal carcasses within 24 h of death (personal observation), and larvae of T. micans have been observed on these carcasses within a few days of death, but larvae of D. maculatus do not normally appear until the carcass has dessicated substantially. This implies that some silphids, at least, have some of the desirable forensic characteristics of blowflies. Beetles typically develop more slowly than flies, and, therefore, offer an opportunity to estimate mPMI from developmental data after maggots have left the carcass. Estimates of mPMI based on beetle development are reputedly less precise (days or weeks) than those based on maggot development (hours or days) [3], but once maggots are no longer present on a carcass, they are still suitable. In addition, using live beetle samples instead of old fly puparia collected at crime scenes is likely to reduce the contamination concerns raised by Archer et al. [21].
Given that T. micans is the most common and widespread silphid species in Africa [17] and that it can locate carcasses soon after death, a development model for this species is of forensic value. This study presents such a model, developed from the growth rate of T. micans at ten constant temperatures, to illustrate the value of coleopteran developmental data to forensic entomology in general.
Materials and methods
A culture of T. micans was established by collecting adults from various dead animals in the Grahamstown district of South Africa. Pairs of adults were placed at a range of temperatures (15°C, 17°C, 18°C, 19°C, 20°C, 25°C, 28.4°C, and 35°C) with a small amount of food (Shallow-water hake, Merluccius capensis) and oviposition substrate (damp sand). Shallow-water hake was chosen because it is the most readily available frozen fish in South Africa, and fish or fish meal is commonly used to raise carcass beetles [5, 14]. Frozen fish was chosen as it is readily available, and freezing of food does not influence insect development [22]. Freshly hatched first instar larvae were separated into plastic Petri dishes with pupation substrate (damp sand) and food ad libitum and reared at ten constant temperatures (oviposition temperatures, plus 22.5°C and 32.5°C), ten individuals per temperature, until adults emerged from the pupation substrate. The Petri dishes were closed using elastic bands and turned 90° onto their sides. This arrangement was narrow enough to allow monitoring of the pupal chamber, allowing pupation and eclosion times to be noted, but also providing enough space for the larvae to move and feed.
Developmental milestones were identified and the period between milestones noted by checking all individuals at regular intervals based on development stage (eggs 8-hourly; 1st instar larvae 4-hourly; 2nd instar larvae 8-hourly; 3rd instar larvae, pupae and adults 12-hourly). Beetle larvae were measured using a measurement triangle [19] at the same intervals, giving a total of 3,398 measurements. The developmental milestones identified were: Oviposition; Dig 1 (when 1st instar larvae dug out of the oviposition substrate); Ecdysis 1; Ecdysis 2; Dig 2 (when 3rd instar larvae dug into the pupation substrate); Pupation; Eclosion and Dig 3 (when the adults dug out of the pupation substrate). Mortality was monitored at the same intervals to monitor optimum temperature for survival.
Lengths were compared between temperatures using ANCOVA with age as a covariate to control for the effect of differing time to grow. Only temperatures where development was completed were used in the analysis. An isomegalen diagram was prepared from the length and age data using spline interpolation in Statistica v.8. Developmental constants for each milestone were estimated using major axis regression as described by Ikemoto and Takai [13]. Data from the 28.4°C experiment were not used in calculations due to incubator malfunctions during the experiment, but are still presented because the malfunction occurred only once, for a short period (1 day), during the experiment.
Results
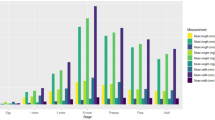
Growth curves at all temperatures were sigmoidal (Fig. 1). Mortality ranged from 20% to 100%, with the highest mortality at extreme temperatures and lower mortality at intermediate temperatures (Fig. 2). Larval length differed significantly between temperatures (F = 355.34, p < 0.001), showing five distinct groups (Fig. 3). Larvae reared at low temperatures (15°C and 17°C) were significantly shorter than those reared at higher temperatures and larvae reared at 15°C were also significantly shorter than larvae reared at 17°C. The larvae reared at higher temperatures formed three overlapping groups (Table 1), showing an increase in body length with increased temperature (Fig. 3), even after taking age into account. This is seen in the isomorphen diagram, where developmental contours intersect several body length contours on the isomegalen diagram (Fig. 4). Ecdysis 1 and Ecdysis 2 have the fewest intersections (three contours) and Dig 2 the most (four contours).
Growth curves of Thanatophilus micans larvae at constant temperatures: a 15°C, 17°C, 18°C, and 19°C and b 20°C, 22.5°C, 25°C, 28.4°C, 32.5°C and 35°C. All curves are sigmoidal, showing slow initial growth, followed by an accelerated growth phase and tailing off as development is completed. Note the range of ages at any given length, particularly at the lowest temperatures. Data for development at 32.5°C and 35°C should be interpreted with care as development was not completed
Means (±95% CI) of body length of mature larvae of T. micans at different constant temperatures. Letters above the points (A–E) indicate statistically different groups (Table 1). Length at low temperatures (15°C, 17°C) was significantly shorter than all other temperatures, while higher temperatures formed overlapping groups, showing a more gradual increase in length
Overlaid isomorphen and isomegalen diagrams showing intersections of developmental and body length contours. The isomorphen diagram was constructed using the median times to each developmental event at each temperature; error bars denote range of time at given event and temperature. The isomegalen contours were constructed using spine interpolation
Egg development took 5.33 days (128 h) at 17°C to 1.66 days (40 h) at 35°C. Development from hatching to adult took between 63.16 days (1,516 h) at 15°C and 19.33 days (464 h) at 28.4°C to be completed. Development was not completed above 28.4°C, and at 28.4°C larval mortality was high (Table 1). At 17°C and 15°C mortality was also high (Table 1).
Thermal summation models were constructed for each previously identified landmark, using as many co-linear temperatures as possible (Table 2, Fig. 5). Because oviposition was not achieved at all temperatures, eggs were transferred between temperature conditions and development time for all other landmarks, therefore, represents the time between digging to the surface (Dig 1) and the specific landmark, and not time from oviposition to the landmark. To obtain an accurate linear regression model, only data points that fall in the near-linear relationship should be used, and points that deviate from this linear relationship should be excluded in the generation of developmental models [11, 13]. Of the 59 data points presented, 50 were suitable in the seven models, three points were rejected because they did not form a linear relationship and six points were rejected because they were from the less reliable 28.4°C data set.
Major axis regression lines and 95% confidence intervals for the seven development milestones identified for Thanatophilus micans, Dig 1, Ecdysis 1, Ecdysis 2, Dig 2, Pupation, Eclosion and Dig 3. In the first diagram (Dig 1), Days is the time from oviposition to the developmental milestone. In all other diagrams, Days is the time from Dig 1 to the developmental milestone. In all diagrams, DT is the rearing temperature multiplied by the time to reach the milestone. Fifty of the 59 points generated were used in the analyses: closed symbols were used in the calculations and open symbols were excluded
Discussion
Development of Thanatophilus micans from oviposition to pupation took 15.7 days at 25°C. This is longer than several forensically important Diptera, including Sarcophaga tibialis, which takes 4.5 days at 25°C [20], Lucilia sericata, which takes 6.6 days at 25°C [9] and Protophormia terraenovae, which takes 9.6 days at 25°C [10]. Development also took longer than Chrysomya albiceps, which takes 22.5 days at 17.5°C [15], while T. micans takes 29.5 days at 17°C. Other forensically important Coleoptera take longer to develop than T. micans, including Dermestes haemorrhoidalis, which takes 40.6 days at 25°C [5] and Dermestes peruvianus, which takes 52.5 days at 25°C [5]. Dermestes maculatus takes 59.2 days at 25°C to complete development from egg to adult [16] while T. micans takes 22.2 days at 25°C. The development time of Silphidae therefore fills an important gap in our ability to estimate mPMIs. In field observations around Grahamstown, T. micans larvae were found on carcasses within 4 days of death. This corresponds to the egg development times in this study; given that egg development took 3 days at 25°C, this implies that like blowflies, T. micans can lay eggs on freshly dead carcasses and corpses.
Intermediate temperatures produced lower mortality, with the exception of 22.5°C, where mortality was 50% (Table 1). This suggests a wide range of temperatures where T. micans can survive well. The optimal temperature for survival was 20°C, where mortality was lowest. Larval length was significantly shorter at 15°C and 17°C than at all other temperatures, but the remaining temperatures formed three overlapping groups, showing a steady increase in larval length through the temperature range at which T. micans survives well. Larger female beetles tend to produce more offspring than smaller beetles [6, 12], which means that while the optimal temperature for individual survivorship is 20°C; population growth might well be higher at increased temperatures. Further work is needed to determine the optimal temperature for population growth (as opposed to individual growth) in T. micans.
The intersected contours on the overlaid isomorphen and isomegalen diagrams (Fig. 4) provide good evidence that length is an ambiguous indicator of physiological age in this species. This is especially true at low temperatures, where the ranges of body sizes at each developmental event were very large (Fig. 4). This has been noted in Diptera by previous authors [7, 8, 15], and the same is probably true for Coleoptera in general.
Developmental threshold (D 0) values ranged by 1.6°C between developmental landmarks, but the D 0 values for the life stages that occur above ground ranged by only 0.4°C, while the stages below ground ranged by 1.0°C (Table 2). These two groups also showed no overlap, with the epigeal stages having D 0 values 0.2°C higher than the hypogeal groups. The observed differences are not significant, as the 95% confidence intervals overlap in all cases. It is likely that the observed differences are due to the subsurface environment buffering the organisms from temperature fluctuations that occur above ground. The difference between carcass and soil temperatures needs to be taken into account when using the models presented here for estimating mPMIs.
The D 0 values are not unexpected for an African insect, but cannot be compared to other silphid species, as no relevant data are published. Richardson and Goff [16] present some developmental data for D. maculatus, from which a D 0 value for D. maculatus can be calculated. The D 0 value calculated from these data is 12.48°C, almost 2.5°C higher that the value for T. micans. This value is, however, not statistically robust, as only three data points fall on the linear section of the graph. Coombs [5] presents data for both D. haemorrhoidalis and D. peruvianus, from which D 0 values can also be calculated, but these are also not statistically robust, with only four and three points on the linear section, respectively. The D 0 value for D. haemorrhoidalis is 12.99°C, which is almost 3°C higher than T. micans; and 12.32°C for D. peruvianus, which is almost 2.4°C higher. Given that the three D0 values calculated for the Dermestes species are all not statistically robust, it is not wise to compare them too rigorously because they cover a relatively small range of temperatures. Definitive data should be generated before definitive comparisons can be made.
While comparisons between many parameters of this model are possible, the most valuable aspect of this study is that it provides a model for a time period where forensic entomologists were previously unequipped to make development-based mPMI estimates.
References
Amendt J, Campobasso CP, Gaudry E, Reiter C, LeBlanc H, Hall M (2007) Best practice in forensic entomology—standards and guidelines. Int J Leg Med 121:90–104
Byrd JH, Castner JL (2001) Insects of forensic importance. In: Byrd JH, Castner JL (eds) Forensic entomology: the utility of arthropods in legal investigations. CRC, Boca Raton, pp 43–80
Catts EP (1992) Problems in estimating the postmortem interval in death investigations. J Agr Entomol 9:245–255
Catts EP, Goff ML (1992) Forensic entomology in criminal investigations. Annu Rev Entomol 37:253–272
Coombs CW (1979) The effect of temperature and humidity upon the development and fecundity of Dermestes haemorrhoidalis (Kuster) and Dermestes peruvianus (Laporte de Castelnau) (Coleoptera: Dermestidae). J Stored Prod Res 15:43–52
Czypionka K, Hill MP (2007) The relationship between female pupal mass and fecundity of Gratiana spadicea (Klug, 1829) (Coleoptera: Chrysomelidae). Afr Entomol 15:380–382
Dadour IE, Cook DF, Fissioli JN, Bailey WJ (2001) Forensic entomology: application, education and research in Western Australia. Forensic Sci Int 120:48–52
Gaudry E, Myskowiak JB, Chauvet B, Pasquerault T, Lefebvre F, Malgorn Y (2001) Activity of the forensic entomology department of the French Gendarmerie. Forensic Sci Int 120:68–71
Grassberger M, Reiter C (2001) Effect of temperature on Lucilia sericata (Diptera: Calliphoridae) development with special reference to the isomegalen- and isomorphen-diagram. Forensic Sci Int 120:32–36
Grassberger M, Reiter C (2002) Effect of temperature on development of the forensically important holarctic blow fly Protophormia terraenovae (Robineau-Desvoidy) (Diptera: Calliphoridae). Forensic Sci Int 128:177–182
Higley LG, Haskell NH (2001) Insect development and forensic entomology. In: Byrd JH, Castner JL (eds) Forensic entomology: the utility of arthropods in legal investigations. CRC, Boca Raton, pp 287–302
Honêk A (1993) Intraspecific variation in body size and fecundity in insects: a general relationship. Oikos 66:483–492
Ikemoto T, Takai K (2001) A new linearized formula for the law of total effective temperature and the evaluation of line-fitting methods with both variables subject to error. Environ Entomol 29:671–682
Lambkin TA, Khatoon N (1990) Culture methods for Necrobia rufipes (Degeer) and Dermetes maculatus Degeer (Coleoptera: Cleridae and Dermestidae). J Stored Prod Res 26:59–60
Richards CS, Paterson ID, Villet MH (2008) Estimating the age of immature Chrysomya albiceps (Diptera: Calliphoridae), correcting for temperature and geographical latitude. Int J Legal Med 122:271–279
Richardson MS, Goff ML (2001) Effects of temperature and intraspecific interaction on the development of Dermestes maculatus (Coleoptera: Dermestidae). J Med Entomol 38:347–351
Schawaller W (1987) Faunistische und systematische Daten zur Silphiden-Fauna Sudafrikas (Coleoptera, Silphidae). Entomofauna 8:277–288
Smith KGV (1986) A manual of forensic entomology. British Museum (Natural History), London, p 205
Villet MH (2007) An inexpensive geometrical micrometer for measuring small, live insects quickly without harming them. Entomol Exp Appl 122:279–280
Villet MH, MacKenzie B, Muller WJ (2006) Larval development of the carrion-breeding flesh fly, Sarcophaga (Liosarcophaga) tibialis Maquart (Diptera: Sarcophagidae), at constant temperatures. Afr Entomol 14:357–366
Archer MS, Elgar MA, Briggs CA, Ranson DL (2006) Fly pupae and puparia as potential contaminants of forensic entomology samples from sites of body discovery. Int J Legal Med 120:364–368
Day DM, Wallman JF (2006) A comparison of frozen/thawed and fresh food substrates in development of Calliphora augur (Diptera: Calliphoridae) larvae. Int J Legal Med 120:391–394
Acknowledgments
We thank Sue Abraham for her assistance in creating the isomegalen/isomorphen diagram; Terence Bellingan, Cameron Richards and Kendall Crous for assistance in the laboratory and Rhodes University and the National Research Foundation for funding. Any opinion, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Midgley, J.M., Villet, M.H. Development of Thanatophilus micans (Fabricius 1794) (Coleoptera: Silphidae) at constant temperatures. Int J Legal Med 123, 285–292 (2009). https://doi.org/10.1007/s00414-008-0280-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-008-0280-0