Abstract
Carisoprodol is commonly prescribed as a centrally acting muscle relaxant, but it is also subject to abuse. The literature describing fatal intoxications with the drug is limited to a relatively small number of cases, and there are inconsistencies with regard to which concentration levels that are toxic. We therefore investigated all forensic autopsies at the Norwegian Institute of Public Health during the period 1992–2003 where carisoprodol was detected. The median concentrations of carisoprodol in intoxication with carisoprodol only or with only minor other analytical findings was 36 mg/l (range 8–65 mg/l; n=5). In the rest of the intoxications, the relevance of carisoprodol relative to the other drugs detected was variable (n=93). When the number of intoxications with carisoprodol each year were divided by the number of defined daily doses (DDD) sold, a fatal toxicity index between 5.6 and 6.9 deaths/1 million DDD was obtained. The total number of cases where carisoprodol was detected increased during the period studied, which correlated to sales figures for the drug. We conclude that carisoprodol can be fatal in concentrations below those indicated in some of the previously published literature. There were, however, only a small number of cases where the cause of death can be attributed to use of carisoprodol alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carisoprodol is a commonly prescribed centrally acting skeletal muscle relaxant, introduced in 1959 as an alternative to the barbiturate-like drug meprobamate. Later, it became clear that carisoprodol is almost totally metabolised to meprobamate in humans [1]. The recommended adult dose of carisoprodol is 350 mg, given up to four times a day for acute low back pain, but in clinical practice, single doses of 700 mg are frequently used. The effect of carisoprodol for short-term treatment of low back pain [2] has been documented in three high quality studies [3–5], but there is insufficient evidence for the use of carisoprodol in other conditions [6].
Case reports on carisoprodol abuse are numerous [7–10], and there are also several reports on carisoprodol intoxication [11–14], but the extent and possible seriousness of this problem is not known in detail. Meprobamate probably has a barbiturate-like mode of action [15], whereas carisoprodol’s mode of action is not known. The symptoms of carisoprodol intoxication indicate that it might act differently from meprobamate, and we have suggested earlier the possible involvement of the serotonin system in carisoprodol toxicity [11]. Carisoprodol is a potentially lethal drug, but there are inconsistencies in the literature with regard to which concentration levels are toxic. Some articles reported that concentrations of carisoprodol need to be as high as 100 mg/l to be lethal [16], whereas published autopsy materials and case reports indicate that fatal concentrations can be much lower [17–21]. The number of cases in these publications is quite small, and a systematic report from a larger material of carisoprodol deaths would give valuable information.
The aim of this study was to investigate fatal intoxications with carisoprodol, especially in looking at lethal concentrations. We also wanted to calculate a fatal toxicity index (FTI) for the drug. Finally, we would like to see how the number of forensic autopsies, where carisoprodol was detected, developed in the period 1992–2003, and how this correlated with the sales figures for carisoprodol.
Materials and methods
Material (1992–2003)
The Norwegian Institute of Public Health, Division of Forensic Toxicology and Drug Abuse routinely analyses drugs in more than 90% of the forensic autopsies performed in Norway, as requested by the police in unnatural deaths. The material in the present study was based on all cases with carisoprodol and/or meprobamate detected in the period 1992–2003. Meprobamate is rarely used as a drug, as such, in Norway, and when meprobamate was detected alone in the autopsy cases, the finding was considered to represent intake of carisoprodol.
Data on wholesale of drugs (1992–2003) [22–24] were collected from all drug wholesalers in Norway. These data represent total sales to pharmacies, institutions etc. The sales were calculated and presented in number of defined daily doses (DDD) per 1,000 inhabitants per day.
Characteristics of carisoprodol intoxications (2000–2003)
For the period 2000–2003, the autopsy cases where carisoprodol and/or meprobamate were detected were studied in detail. For these years, the official cause of death in each case was collected from the cause of death register at Statistics Norway after permission from the Norwegian data inspectorate and the directorate for health and social affairs. Based on the classification from the death register, the cases were divided into those with intoxication as primary cause of death with detection of carisoprodol (group A) and deaths from other causes where carisoprodol was detected (group B).
In group A, the relevance of carisoprodol as the cause of death was further estimated as follows:
-
Group A1
Intoxication with findings of carisoprodol/meprobamate only or only with one other additional drug present in therapeutic concentrations, or only additional ethanol in a concentration below 1 g/l.
-
Group A2
Intoxication with other drugs present but not in lethal concentrations, according to the criteria described below.
-
Group A3
Intoxication with one or more other drugs present in a lethal concentration. The limits of lethal concentrations of other drugs were set after consulting three different sources: the compilation of fatal concentrations of drugs by Druid and Holmgren [17], The International Association of Forensic Toxicologist reference blood level list [25] and the information from the Poisindex in the Micromedex [26]. We used the lowest lethal concentration reported in these three sources multiplied by 2, not to get a too low level, as the lowest lethal concentration for each particular drug. For ethanol, the level was set to 3 g/l. If two or more drugs were present in concentrations above 50% of the defined lethal levels, the combined effects of these drugs were considered lethal.
Group B (deaths from other causes) was used as a reference group for group A.
The samples in group A2 and A3 were central post-mortem blood in one case, pericardial fluid in one case and serum and plasma respectively sampled at hospital before death in two cases. In group B, the samples were central blood in two cases and muscle in one case. In group A1 and the rest of groups A2, A3 and B, the specimen was peripheral post-mortem blood in all cases. The blood/serum partition coefficients are 0.88 and 0.95 for carisoprodol and meprobamate, respectively (unpublished results).
A second reference group consisted of 356 suspected drugged drivers, from the period 1985–1998, in whom carisoprodol or meprobamate was detected in their blood (group C) [41].
Calculation of fatal toxicity index
The FTI was calculated for the years 2000–2003 after the model of Reith et al. [27]. The number of deaths associated with carisoprodol intoxications each year was divided by the total number of DDD carisoprodol sold in the same year. Like Reith et al., we also used the number of all deaths from intoxication where carisoprodol was detected and not only the cases where carisoprodol was considered the primary agent. The DDD for carisoprodol is 1.4 g. Wholesale of drugs were expressed as DDD/1,000 inhabitants/day, and to calculate the total amount of DDD sold, the number was multiplied both by 365 and midyear population of Norway in each year.
Total number of cases (1992–2003)
For the whole period, the cases with carisoprodol or meprobamate in blood were counted but were not described in more detail. This was done to see if the total number of cases had increased over a longer time period and how this was related to the sales figures for carisoprodol. These cases included both intoxications as well as deaths from other causes.
Analytical method
From 1992 until early 2003, all blood samples from autopsy cases were screened for medicinal drugs, including carisoprodol and meprobamate, using capillary gas-chromatography (GC) combined with nitrogen-phosphor and flame ionization detectors. The blood samples (200 μl) were extracted with butyl acetate after adding internal standard solution (methqualone) and saturated potassium dihydrogen phosphate (pH 5). Confirmation and quantification analyses for carisoprodol and meprobamate were performed by GC using alternative capillary column compared to screening and the extraction procedure described above. For other medicinal drugs, confirmation and quantification were performed through high-performance liquid chromatography combined with UV or electrochemical detectors or with GC using an alternative capillary column compared to screening. From early 2003, all samples were screened by liquid chromatography–mass spectrometry (LC/MS-EI mode), covering 62 medicinal drugs, including carisoprodol, meprobamate and a number of antidepressants, antipsychotics, analgesics, antiepileptics, benzodiazepines and related compounds. The blood cells were precipitated with acetonitrile, followed by centrifugation and injection of the supernatant on the LC-column. Methqualone was used as internal standard. Confirmation and quantification were performed with a LC/MS system using an alternative LC-column and sample preparation procedure compared to screening. The limit of quantification (LOQ) for carisoprodol was 0.34 mg/l with the GC method and 0.78 mg/l with the LC/MS method. An administrative cut-off value for carisoprodol was set at 2.6 mg/l until July 1996 and then changed to 1.3 mg/l, which has been used during the rest of the study period. Only values above these levels were reported as positive results. The LOQ for meprobamate was 1.35 mg/l with the GC method and 1.1 mg/l with the LC/MS method. An administrative cut-off for meprobamate was unchanged at 2.2 mg/l during the whole study period. Only values above this level were reported as positive results. All changes of analytical methods were based on validation programs including comparison of the old and new methods before the new analytical program could be accepted. The between-day precisions at cut-off levels were approximately 16% for both carisoprodol and meprobamate during the period using GC quantification. When changed to LC/MS quantification, the between-day precisions were approximately 7 and 8% for meprobamate and carisoprodol, respectively.
The standard analytical program for autopsy cases also includes alcohols and acetone, analysed by head-space GC and an enzymatic method (alcohol dehydrogenase) for ethanol and illegal drugs screened by an immunological method (enzyme multiplied immunoassay technique), followed by confirmation and quantification using GC/MS. These analytical programs have been unchanged during the period covering samples included in this study.
Results
Fatal carisoprodol intoxications (2000–2003)
A total of 5,001 forensic autopsies were analysed during the period 2000–2003. Carisoprodol and meprobamate (92%), or meprobamate alone (8%), were detected in 156 cases, of which official causes of death records were available for 150 cases. Of these, 98 had died from intoxication; 27 of these were classified as suicides. The remaining 52 persons died of varying causes, the most frequent being violent deaths from fire, hanging, drowning, shooting or traffic accidents (23 cases) or cardiac disease (12 cases).
Of the 98 intoxications, carisoprodol and meprobamate were the only drugs detected in only one case; in another four cases, there were only minor other toxicological findings. These five cases constituted group A1. There were 34 cases of mixed intoxications where no other drugs or combinations were thought to be present in fatal concentrations (group A2). The remaining 59 cases were mixed intoxications where the other drugs were considered to be present in fatal concentrations (group A3). Concentrations of carisoprodol and meprobamate, as well as age, sex and percent of cases with additional drugs detected in the carisoprodol intoxications, were compared with the two reference groups, and the results are shown in Table 1. All but one case were mixed intoxications, and the most frequent additional drugs detected were codeine and morphine (64 cases), diazepam (43 cases), ethanol (33 cases) and selective serotonergic re-uptake inhibitors (SSRI; 22 cases). There were no SSRIs detected in group A1, 7 in group A2 and 15 in group A3. There was a non-significant tendency towards lower concentrations of carisoprodol in the intoxications where SSRI was present in addition (data not shown). Paracetamol was the only drug detected, in addition to carisoprodol and meprobamate, in only two cases. In the rest of the intoxications, drugs with an abuse potential were present in addition to carisoprodol/meprobamate. Illegal drugs like cannabis and amphetamine were only present in five and three cases, respectively.
Fatal toxicity index
FTI [27] was calculated for the years 2000–2003. The number of fatal intoxications with carisoprodol and the total prescription each year are shown in Table 2. When the total number of fatal intoxications involving carisoprodol each year was divided by the total amount of DDD, it gave a FTI between 5.6 and 6.9 deaths/1 million DDD sold (Table 2).
Number of autopsy cases (1992–2003) and sales figures
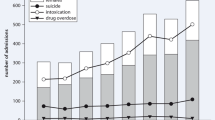
An average number of 1,710 forensic autopsies were analysed each year during the period 1992–2003. The number of forensic autopsy cases where carisoprodol was detected increased from 1992 to 2003, both in absolute numbers and when adjusted for total number of cases analysed. These cases include both intoxications and deaths from other causes. There was also an increase in the sales of carisoprodol during the period. The number of cases was related to the amount of carisoprodol sold in Norway (Pearson’s test, r=0.805, p<0.01), as shown in Fig. 1.
Discussion
In this study, we investigated fatal carisoprodol intoxications regarding concentrations, FTI and development related to sales figures during the last decade.
The classification of deaths might be a weakness of the present study. By gathering the official cause of death from Statistics Norway, we believe the classification into “intoxications” or “deaths from other causes” was as reliable as possible, but the importance of other drugs relative to carisoprodol was subject to interpretation. Each case was, however, classified after strict criteria based on well-known references to minimize this source of error. The cause of death register in Norway has a near 2 year delay, and deaths from 2004 and 2005 were therefore not included in the study. A second possible weakness was that the post-mortem concentrations of carisoprodol and meprobamate may deviate from pre-mortem values because of post-mortem redistribution. This is, however, most significant for drugs with a high volume of distribution (V D) [28–30], whereas carisoprodol and meprobamate have a low V D [19]. A third methodological problem was the administrative cut-off for carisoprodol, which was lowered in 1996, and could explain some of the increase in number of cases after this time. However, there was a continuous rise in number of carisoprodol detections from 1995 and throughout the study.
Reference studies and textbooks consider different levels of carisoprodol to be lethal. Some sources claim that carisoprodol is lethal only at concentrations above 100 mg/l [16]. This may, however, be due to a misinterpretation from an earlier work where carisoprodol and meprobamate levels were added together [31]. Published autopsy materials [17, 21] and case-reports [18–20] indicate fatal concentrations to be much lower at around 25 mg/l. The results from group A1 in our study, with a median concentration of 36 mg/l and a range of 8–65 mg/l (Table 1), were in accordance with these data. Considering that the maximum serum or blood concentration after administration of 700 mg carisoprodol is in the range of 3–6 mg/l [32, 33], our study gave an impression of the therapeutic index of carisoprodol to be around 10, which is relatively low compared to, for instance, most benzodiazepines [34]. We classified the cases according to the toxic potential of the other drugs found and assumed the importance of carisoprodol to be higher when no other drugs were present in lethal concentrations. The median concentration of carisoprodol in group A2, where carisoprodol must be considered the most relevant drug, was 9.4 mg/l, which is significantly higher than in group A3 (5.7 mg/l; p=0.028). In the control groups B and C, the concentrations of carisoprodol were lower as expected (Table 1). These concentrations are comparable to what has been seen after clinically used doses.
The mechanism of deaths from carisoprodol overdose is not known in detail. Despite genetic polymorphism [33] and possible saturation of enzymes after ingestion of large doses [11], the ratio between carisoprodol and meprobamate in group A, especially group A1, in the present study indicated relatively recent ingestion of carisoprodol [1, 32, 33] compared to group A3 and the reference groups B and C. This may indicate two situations. Firstly, it would seem that the deaths cannot be attributed to the effects of meprobamate alone, as the concentrations of meprobamate differ less than carisoprodol between the intoxications and the reference groups. Secondly, a death from the barbiturate-like meprobamate would be expected to be more protracted, although it seems that carisoprodol has a mechanism that leads to a more sudden death. Previous studies have reported symptoms after carisoprodol intoxication resembling those of the serotonin syndrome unlike the CNS-depressing symptoms produced by meprobamate [11–14]. In theory, the combination of carisoprodol and SSRIs could be especially dangerous, and the frequent finding of SSRIs (n=22) in the present study, as well as the tendency of lower concentrations of carisoprodol in these cases, might strengthen this assumption.
This study confirmed carisoprodol as an agent that is often used in combination with other drugs. Previous studies of fatal carisoprodol intoxications have also reported frequent detection of additional drugs [17, 21]. A large degree of polydrug abuse is also reported from fatal intoxications with other drugs, where an average of 2.4–3.5 drugs are found [35, 36], as well as studies of drugged drivers showing an average number of 2.6 additional drugs [37].
Our material had a clear overrepresentation of women, with 56% in group A. Among all autopsy cases sent to the Division of Forensic Toxicology and Drug Abuse during 2004, only 27% were women. This low fraction of women could be due to the reason for forensic autopsies, which are often violent deaths and suicides, where men are represented two to four times more frequently than women [35, 38–40]. Group C consisted of 40% women, but, as previously published, female drivers only constituted 10–14% of the drivers suspected of drugged driving [41]. The present study therefore confirmed the position of carisoprodol as a “women’s drug”. This is in accordance with the Drug Abuse Warning Network data, which estimates all intoxications in emergency rooms in the USA. Of all patients admitted to emergency rooms because of carisoprodol intoxications, 72% were women [42].
We calculated a FTI that was higher than for many other drugs, but this was based on all intoxications with carisoprodol and not only the ones where it was the primary agent [43]. This was, however, strictly based on the model of Reith et al. [27] to be able to make comparisons with the previous published data. The FTI between 5.6 and 6.9 deaths/1 million DDD for carisoprodol was higher than their findings for diazepam (5.2), oxazepam (4.9), nitrazepam (2.8) and zopiclon (1.9) but lower than for alprazolam (16.0) and klonazepam (16.1) [27]. This trend is also seen in the Drug Abuse Warning Network data, where carisoprodol was at position 17 of the most frequently mentioned drug in emergency rooms, after alprazolam and clonazepam. Other drugs mentioned more often than carisoprodol were the schedule 1 substances cocaine, cannabis and heroin, as well as paracetamol and ibuprofen [42].
The development of forensic autopsies involving carisoprodol from the period 1992–2003 also increased together with the sales figures for carisoprodol. We only had information about cause of death for the last 4 years, but the majority (65%) of the deaths were intoxications. As the importance of carisoprodol was variable in these cases, carisoprodol could have been a spurious finding in persons who died from intoxication with other drugs. The FTI and the development of forensic autopsies with carisoprodol over time, therefore, do not necessarily indicate a marked toxicity of carisoprodol, but carisoprodol was at least often ingested together with toxic drugs.
References
Dalen P, Alvan G, Wakelkamp M, Olsen H (1996) Formation of meprobamate from carisoprodol is catalysed by CYP2C19. Pharmacogenetics 6:387–394
van Tulder MW, Touray T, Furlan AD, Solway S, Bouter LM (2003) Muscle relaxants for nonspecific low back pain: a systematic review within the framework of the cochrane collaboration. Spine 28:1978–1992
Hindle TH III (1972) Comparison of carisoprodol, butabarbital, and placebo in treatment of the low back syndrome. Calif Med 117:7–11
Boyles W, Glassmann J, Soyka J (1983) Management of acute muskuloskeletal conditions: thoracolumbar strain and pain. A double-blind evaluation comparing the efficacy and safety of carisoprodol with diazepam. Today’s Ther Trends 1:1–16
Rollings H, Glassmann J, Soyka J (1982) Management of acute muskuloskeletal conditions—thoracolumbar strain and sprain: a double-blind evaluation comparing the efficacy and safety of carisoprodol with cyclobenzadrine hydrochloride. Curr Ther Res 34:917–928
Boothby L, Doering P, Hatton R (2003) Carisoprodol: a marginally effective skeletal muscle relaxant with serious abuse potential. Hosp Pharm 38:337–345
Wyller TB, Korsmo G, Gadeholt G (1991) Dependence on carisoprodol (Somadril)? A prospective withdrawal study among prisoners [Avhengig av karisoprodol (Somadril)? En prospektiv seponeringsundersøkelse blant fengselsinnsatte]. Tidsskr Nor Laegeforen 111:193–195
Reeves RR, Carter OS, Pinkofsky HB, Struve FA, Bennett DM (1999) Carisoprodol (soma): abuse potential and physician unawareness. J Addict Dis 18:51–56
Bailey DN, Briggs JR (2002) Carisoprodol: an unrecognized drug of abuse. Am J Clin Pathol 117:396–400
Sikdar S, Basu D, Malhotra AK, Varma VK, Mattoo SK (1993) Carisoprodol abuse: a report from India. Acta Psychiatr Scand 88:302–303
Bramness JG, Morland J, Sorlid HK, Rudberg N, Jacobsen D (2005) Carisoprodol intoxications and serotonergic features. Clin Toxicol 43:39–45
Siddiqi M, Jennings CA (2004) A near-fatal overdose of carisoprodol (SOMA): case report. J Toxicol Clin Toxicol 42:239–240
Roth BA, Vinson DR, Kim S (1998) Carisoprodol-induced myoclonic encephalopathy. J Toxicol Clin Toxicol 36:609–612
Roberge RJ, Lin E, Krenzelok EP (2000) Flumazenil reversal of carisoprodol (Soma) intoxication. J Emerg Med 18:61–64
Roache JD, Griffiths RR (1987) Lorazepam and meprobamate dose effects in humans: behavioral effects and abuse liability. J Pharmacol Exp Ther 243:978–988
Winek CL, Wahba WW, Winek CL Jr, Balzer TW (2001) Drug and chemical blood-level data 2001. Forensic Sci Int 122:107–123
Druid H, Holmgren P (1997) A compilation of fatal and control concentrations of drugs in postmortem femoral blood. J Forensic Sci 42:79–87
Chung H, Park M, Hahn E, Choi H, Choi H, Lim M (2004) Recent trends of drug abuse and drug-associated deaths in Korea. Ann N Y Acad Sci 1025:458–464
Backer RC, Zumwalt R, McFeeley P, Veasey S, Wohlenberg N (1990) Carisoprodol concentrations from different anatomical sites: three overdose cases. J Anal Toxicol 14:332–334
Adams HR, Kerzee T, Morehead CD (1975) Carisoprodol-related death in a child. J Forensic Sci 20:200–202
Davis GG, Alexander CB (1998) A review of carisoprodol deaths in Jefferson County, Alabama. South Med J 91:726–730
Ronning M (2001) Drug consumption in Norway 1996–2000 [Legemiddelforbruket i Norge 1996–2000], Norwegian Medicinal Depot, Oslo
Ronning M, Sanner T, Boe GH, Litleskare I, Strøm H, Granum T (2005) Drug consumption in Norway 2000–2004 [Legemiddelforbruket i Norge 2000–2004], Norwegian Institute of Public Health, Oslo
Oydvin K, Ronning M, Sakshaug S, Blix H, Ullerud T (1997) Drug consumption in Norway 1992–1996 [Legemiddelforbruket i Norge 1992–1996], Norsk Medisinaldepot AS, Oslo
TIAFT (2006) http://www.tiaft.org/tmembers/ttvidx.html. Cited 7 Feb 2006
Micomedex (2006) http://www.thomsonhc.com/hcs/librarian/PFPUI/Tq4CzCw1heeLZm. Micromedex. Cited 7 Feb 2006
Reith DM, Fountain J, McDowell R, Tilyard M (2003) Comparison of the fatal toxicity index of zopiclone with benzodiazepines. J Toxicol Clin Toxicol 41:975–980
Hilberg T, Rogde S, Morland J (1999) Postmortem drug redistribution—human cases related to results in experimental animals. J Forensic Sci 44:3–9
Hilberg T, Morland J, Bjorneboe A (1994) Postmortem release of amitriptyline from the lungs; a mechanism of postmortem drug redistribution. Forensic Sci Int 64:47–55
Pelissier-Alicot AL, Gaulier JM, Dupuis C et al (2006) Post-mortem redistribution of three beta-blockers in the rabbit. Int J Legal Med 120:226–232
Schulz M, Schmoldt A (1997) Therapeutic and toxic blood concentrations of more than 500 drugs. Pharmazie 52:895–911
Olsen H, Koppang E, Alvan G, Morland J (1994) Carisoprodol elimination in humans. Ther Drug Monit 16:337–340
Bramness JG, Skurtveit S, Gulliksen M, Breilid H, Steen VM, Morland J (2005) The CYP2C19 genotype and the use of oral contraceptives influence the pharmacokinetics of carisoprodol in healthy human subjects. Eur J Clin Pharmacol 61:499–506
Woods JH, Katz JL, Winger G (1992) Benzodiazepines: use, abuse, and consequences. Pharmacol Rev 44:151–347
Steentoft A, Teige B, Ceder G et al (2001) Fatal poisoning in drug addicts in the Nordic countries. Forensic Sci Int 123:63–69
Jonsson A, Holmgren P, Ahlner J (2004) Fatal intoxications in a Swedish forensic autopsy material during 1992–2002. Forensic Sci Int 143:53–59
Morland J (2000) Driving under the influence of medication and various substances other than alcohol [Kjøring under påvirkning av medikamenter og andre rusmidler enn alkohol]. Tidsskr Nor Laegeforen 120:2148–2150
Meel BL (2004) Incidence and patterns of violent and/or traumatic deaths between 1993 and 1999 in the Transkei region of South Africa. J Trauma 57:125–129
Qin P, Agerbo E, Westergard-Nielsen N, Eriksson T, Mortensen PB (2000) Gender differences in risk factors for suicide in Denmark. Br J Psychiatry 177:546–550
Demetriades D, Gkiokas G, Velmahos GC, Brown C, Murray J, Noguchi T (2004) Alcohol and illicit drugs in traumatic deaths: prevalence and association with type and severity of injuries. J Am Coll Surg 199:687–692
Bramness JG, Skurtveit S, Grung M, Morland J (2000) Centrally acting muscle relaxants and traffic hazards [Sentralt virkende muskelrelakserende midler i trafikken]. Tidsskr Nor Laegeforen 120:1966–1969
Substance Abuse and Mental Health Service Administration (2003) Emergency department trends from the drug abuse warning network, final estimates 1995–2002. DAWN Series D-24, Rockville, MD
Koski A, Vuori E, Ojanpera I (2005) Newer antidepressants: evaluation of fatal toxicity index and interaction with alcohol based on Finnish postmortem data. Int J Legal Med 119:344–348
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Høiseth, G., Bramness, J.G., Christophersen, A.S. et al. Carisoprodol intoxications: a retrospective study of forensic autopsy material from 1992–2003. Int J Legal Med 121, 403–409 (2007). https://doi.org/10.1007/s00414-006-0139-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-006-0139-1