Abstract
To establish reliable methods to aid the timing of brain damage after traumatic brain injury (TBI), brain tissue from 56 autopsy cases with TBI and known survival times, ranging from a few minutes to 126 days, were tested for apoptotic changes to the neuronal and glial cells. Apoptosis was established using the TdT-mediated dUTP nick end labelling (TUNEL) method of in-situ labelling and immunohistochemical reaction of caspase 3. In addition, cellular reaction and astroglial cell differentiation were investigated using histological and immunohistochemical markers. From a survival time of 120 min up to 12 days, TUNEL-positive apoptotic neuronal cells were frequently detected in the contusion zone. The earliest positive caspase 3 reaction in cortical neurons was evident after a posttraumatic interval of 80 min. Detection of apoptotic glial cells using the TUNEL technique showed that as in the case of neuronal cells, the earliest positive TUNEL reaction was obtained after 110 min. In cases of survival times of 120 min up to 4 days, apoptotic glial cells could frequently be detected. However, the first caspase 3-positive glial cells appeared 5 h after injury. Cerebral apoptosis was significantly associated with TBI cases as compared to control cases (P<0.001). The reference histological findings of neutrophilic granulocytes, CD3-positive T-lymphocytes, CD68-positive activated microglial cells/macrophages and TUNEL-positive neuronal cells increases the degree of certainty in determining the probable age of traumatic brain injury to 87.5%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past, the loss of neuronal and glial cells as a result of traumatic head injury has generally been regarded as a consequence of necrosis combined with the appearance of inflammatory cells. The death of brain cells can occur both primarily, in and around the cerebral contusion, and secondarily, as a result of hypoxic changes, due to increased pressure in the brain caused by edema or because of microcirculatory disturbances, for example. However, in recent years, research has increasingly demonstrated the significance of programmed cell death or apoptosis. Apoptosis is a genetically determined, active (i.e., energy-consuming) death programme without any accompanying inflammatory reaction [1, 2]. These recent studies have shown that apoptosis contributes to neuronal and glial cell loss following both acute neurological disorders and in traumatic brain injuries (TBI) [3]. Further data indicate that effector mechanisms of apoptotic cell death include the activation of cysteine proteinases, called caspases 1–9 [4–7]. If TBI occurs, this enzyme cascade is activated. A distinction is made between the initiator caspases, which are activated at the start of the signalling cascade (e.g., caspases 8 and 9), and the effector caspases that they cause to be activated (e.g., caspases 3, 6 and 7) [8–10]. The active effector caspases cleave a large number of cytoplasmatic and nuclear proteins and activate DNA-cleaving endonucleases [11, 12] with caspase 3 being a common end point of apoptotic signalling in the CNS [4].
We performed a prospective case study to investigate the extent to which apoptosis of neuronal and glial cells can be used to determine the age of traumatic brain injuries (TBI). We documented tissue damage by the evaluation of apoptosis using enzymatic labelling of DNA breaks (TUNEL-TdT-mediated dUTP nick end labelling) and immunohistochemistry for caspase 3. We found that combined consideration of apoptosis and several other criteria allowed for a reasonably accurate assessment of the time course of TBI. This information is important for the neuropathological assessment of TBI extent and a reliable forensic determination of the age of injuries, which may have a significant influence on the legal proceedings in a given case.
Materials and methods
In 56 autopsy cases with TBI and known survival times, a sample of fresh brain tissue was taken from the macroscopically visible contusion zone in the cerebral cortex (10 × 10 × 5 mm). The ages of the individuals ranged from 14 to 86 years (average age: 47.3 years). There were 48 males (85.7%) and eight females (14.3%). The survival time (time after injury) ranged from several minutes to 126 days. Of the individuals with a survival time of less than 24 h (n = 29), 17 died of an isolated TBI, while 12 persons had multiple injuries (polytrauma). Of those patients with a survival time of more than 24 h (n = 27), 18 died of cerebral dysregulation resulting from brain damage. In nine cases, death was caused by secondary complications such as pneumonia (n = 8) and pulmonary thromboembolism (n = 1). These complications mainly affected patients with a survival time of more than 10 days (n = 6). The postmortal interval has a maximum of 8 days with adequate cooling (approximately 4°C). For the control group, brain tissues were taken from ten autopsy cases without trauma in which death had resulted from sudden cardiac arrest (e.g., acute myocardial infarction or rhythmogenic heart failure). The ages and gender distribution of the control group was comparable to that of the TBI group (12–90 years—average age, 47.8 years, seven males and three females).
Histological and immunohistochemical methods and the in situ labelling technique
After a maximum of 24-h fixation (neutral buffered 4% formalin), the brain tissue samples were embedded in paraffin and serial sections with a thickness of 4 μm were produced. Subsequently, the tissue sections were first stained using haematoxylin and eosin (H&E) to assess morphology and to detect hypoxic changes of neurons, Prussian blue and subjected to enzymohistochemical treatment using chloracetatesterase (CE) to detect neutrophilic granulocytes.
Immunohistochemical staining was then used for the further characterisation and differentiation of the neuronal, glial and inflammatory cells. Serial sections from paraffin-embedded tissues were cut and processed for immunohistochemistry, which was performed using the indirect avidin–biotin–peroxidase complex method (ABC, Vector Laboratories). Primary antibodies were applied at a concentration of 5–25 μg/ml for 1 to 12 h. These were monoclonal anti-GFAP (astrocytes, clone 6F2, Dako), monoclonal anti-CD68 (macrophages and microglia, clone KP1, Dako), monoclonal MIB-1 (proliferating cells, clone Ki 67, Dako), and polyclonal rabbit-anti-CD3 (T-lymphocytes, Dako). The antibodies required pretreatment by microwave heating at 600 W for 25 min, in 10 mM sodium citrate, pH 6.0. Biotinylated preadsorbed secondary antibodies anti-mouse from sheep (Amersham) and anti-rabbit from swine (Dako) were used at a concentration of 10 μg/ml. Diaminobenzidine (0.05%, DAB, Sigma) in 0.02% hydrogen peroxide/phosphate-buffered saline (PBS) served as chromogen. Counterstaining was performed with hematoxylin (Harris) 7 g/l. For blocking of nonspecific binding sites, nonfat dry milk (1–10%), bovine serum albumin (1–10%) and normal goat serum (1–25%) in PBS were used. To block the endogenous peroxidase, the sections were immersed for 10–20 min in PBS containing 1% hydrogen peroxide.
To establish apoptosis of neuronal and glial cells, a polyclonal antibody to caspase 3 (CPP 32, Dako) was used and detected by means of the indirect alkaline phosphatase method with fast red as chromogen (Chemmate/Ventana, Illkirch, France). DNA fragmentation and apoptosis bodies were detected by means of the TUNEL method of in situ labelling (TdT-mediated dUTP nick end labelling; Apop-Detect kit; Chemicon Southampton, UK) with an anti-digoxigenin–peroxidase system (Chemicon) on sections of paraffin-embedded tissues. After deparaffinisation and rehydration, the tissue sections were pretreated with pronase (Dakocytomation) for 15 min at room temperature followed by blocking the endogenic peroxidase with 3% hydrogen peroxide for 10 min. The enzymatic marking of the free 3’OH DNA end using TdT was carried out at 37°C for 60 min. After application of the stop reagent, tissue sections were incubated with the antibody for 30 min at room temperature, followed by application of the ABC–peroxidase system (Vector) and DAB was used for detection.
Tissue samples from lymph nodes, small intestine, brain and granulation tissue containing histiocytes were used as positive controls, and for negative controls, the primary antibodies were omitted. Calibration of the TUNEL method was conducted on partially necrotic lymphoma tissue with known numbers of apoptotic cells to avoid false positive staining in areas of necrosis.
Histomorphological evaluation
Evaluation was conducted microscopically using the Olympus BX 51 optical microscope (magnification, ×20–×40). The contusion zones, mostly located in layers 2 to 3 of the cerebral cortex, were semiquantitatively assessed according to general histopathological criteria and the extent of the cellular reaction in the brain tissue (neutrophilic granulocytes, siderophages, fat-granule cells) and hematoidin depositions. Semiquantitative evaluation of immunostaining for CD3, GFAP and CD68 was performed without knowledge of the diagnosis on 4-μm thick sections at a magnification of ×20 in the light microscope. We established a semiquantitative scoring system using a 4-grade scale, specified for CD3 and CD68 as follows: 0=negative; 1=single perivascular intraparenchymatous cells (not in Virchow–Robin spaces) or single cells surrounding hematomas, 2=small clusters of focal cellular infiltrates, 3=dense cellular infiltrates the size of at least one high power field (high power field [1 HPF] = 0.238 mm2). The scoring system for GFAP had to account for the often extensive normal staining depending on the anatomical region studied. Here, score values reflected not only increased staining but also anomalies of the anatomical distribution of GFAP which typically was evaluated within the zones surrounding the lesion: (0=normal anatomical distribution and intensity; 1=slightly above normal intensity, more pronounced perivascular staining, slight increase of astrocytic cytoplasm; 2=clearly increased staining intensity in astrocytes with increased cytoplasm and plumper processes and moderate alteration of the anatomical distribution; 3=strongly increased staining intensity massive increase of astrocytic cytoplasm, gemistocytic astrocytes, with plump processes and diffuse staining of increased numbers of astrocytic processes, severe disturbance of the anatomical distribution). At least two of the criteria described above had to be present for the accordance of a specific score value.
MIB-1, caspase 3 and TUNEL were evaluated quantitatively. The number of neurons and glial cells in the contusion zone were established by quantifying an average of ten high-power fields (magnification, ×40). TUNEL-positive and caspase 3-positive neuronal and glial cells and Mib-1-positive glial cells, were then quantified in the same way, so that the apoptosis or proliferation index could be calculated as a percentage of the positive cells (TUNEL positive: >2%, caspase 3 positive: >3%, Mib-1 positive: >1%). Only a strong nuclear reaction or the staining of apoptotic bodies was rated as a TUNEL-positive cell, and for the caspase 3 reaction, the decisive factor was distinct staining of the cytoplasm. The overall staining pattern was also controlled for plausibility and for obvious false positive reactions (e.g., all cells in a given region were positive).
Statistics
For practical and statistical reasons, the five indices (TUNEL-positive neuronal and glial cells, caspase 3-positive neuronal and glial cells, and Mib-1-positive glial cells) were divided into two (negative/positive) and the survival times into three categories (<2 h, 2 h–4 days, >4 days). The Fisher’s exact test was used to analyse association between the categorical indices and the survival times and to check the homogeneity of the indices between the test groups. The influence of the postmortem interval (PMI) on the apoptotic indices were tested with the correlation and regression analysis. The dependence of the individual indices on survival time was described by means of linear regression analysis. Relationships between the indices and the quantities established were described by Spearman’s rank correlation coefficient and checked for noncorrelation. The predictive value of the inflammation indicators for the three categories of survival time was investigated with the aid of linear discriminant analysis. As a result, a classification scheme was derived using the stepwise variable entry and removal [13].
Results
Uninjured brain tissue (control group)
No caspase 3-positive neuronal and glial cells were detectable in any of the tissue samples of uninjured brain tissue (Fig. 5a). TUNEL-positive neurons were absent in eight out of ten samples and TUNEL-positive glial cells could not be detected in nine samples. In two specimens from very elderly patients (89 and 90 years old), a few TUNEL-positive neuronal cells were detected (index, 0.5 and 0.625%, respectively), and in one case (89 years), a few TUNEL-positive microglial cells (index, 0.465%) were found. Evidently, in these cases, the apoptotic changes detected were due to neurodegenerative processes in one case (morphologically confirmed Pick’s disease) and to diffuse hypoxia with ubiquitous distribution of eosinophilic pyknotic degeneration of neurons in the other case. Therefore, both cases had to be excluded from the control group. The proliferation index (Mib-1) was 0 in the neurons as was to be expected. In four out of ten cases, the microglial cells displayed minimal proliferation activity (index, 0.2–0.5%), and glial neoplasms could be excluded by morphology. The semiquantitative evaluation of the cellular inflammatory reaction and astroglial cell activation resulted in a score value of 0 (negative) in all cases (Figs. 1a, 2a).
Pathomorphological changes in cerebral contusions after varying survival times, 4-μm thick serial sections of paraffin-embedded tissues from macroscopically recognisable contusion n areas. Staining with H&E and immunohistochemistry or enzymatic tagging-immunohistochemistry (TUNEL) using either the indirect peroxidase technique (brown reaction product) or the indirect alkaline phosphatase technique (red reaction product, see Materials and methods section for a detailed description)
Varying reactive changes including astrocytosis at different levels of survival time. 1a Cortical area of a control brain without contusion from a 12-year-old male with sudden cardiac arrest (H&E, Magnification orig. ×10), see also Fig. 2a. b Cortex of a 17-year-old male with a survival time of 13 h after contusion (H&E, Magnification orig. ×10) without major reactive changes, see also Fig. 2b. c Cortex of a 54-year-old male with a survival time of 138 h after contusion (H&E, Magnification orig. ×10) with beginning reactive changes, see also Fig. 2c. d Cortex of a 31-year-old male with a survival time of 136 h after contusion (H&E, Magnification orig. ×10) with pronounced reactive changes, see also Fig. 2d
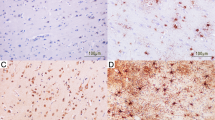
Reactive immunohistochemical changes for GFAP of astrocytosis. a Cortical area of the control brain without contusion from a 12-year-old male with sudden cardiac arrest in Fig. 1a, regular staining results (score=0), denoting the habitually increased GFAP reaction in the first molecular layer of the cortex (Immunocytochemistry, indirect Peroxidase, DAB, Magnification orig. ×10). b Cortex of the 17-year-old male with a survival time of 13 h after contusion in Fig. 1b with slightly increased perivascular GFAP (score=1) immunoreaction (Immunohistochemistry, indirect Peroxidase, DAB, Magnification orig. ×10). c Cortex of the 54-year-old male with a survival time of 138 h after contusion in Fig. 1c with increased cortical astrogliosis (GFAP, score=2) in areas surrounding the contusion (Immunohistochemistry, indirect Peroxidase, DAB, Magnification orig. ×10). d Cortex of the 31-year-old male with a survival time of 136 h after contusion with very strong astrogliosis (GFAP, score=3) featuring extended cytoplasm and increased numbers of diffusely staining astrocytic processes (Immunohistochemistry, indirect Peroxidase, DAB, Magnification orig. ×10)
Serial sections from the cortex of a 39-year-old male with a survival time of 440 h after contusion with extensive cortical defects, inflammation and gliosis, also showing extensive neuronal and glial apoptosis and large numbers of macrophages. a Extensive reactive changes (H&E, Magnification orig. ×10). b Strong astrogliosis (GFAP, Score=3) in areas surrounding the damaged tissue regions. (Immunohistochemistry, indirect Peroxidase, DAB, Magnification orig. ×10). c Numerous CD68 positive macrophages and microglia (Immunohistochemistry, indirect Peroxidase, DAB, Magnification orig. ×10). 3d CD3-positive T-cells with diffuse distribution within the tissue (Immunohistochemistry, indirect Peroxidase, DAB, Magnification orig. ×10)
Serial sections from the cortex of the 39-year-old male with a survival time of 144 hours in Fig. 3, showing extensive glial and neuronal apoptosis. a MIB-1, few proliferating lymphocytes and microglia with diffuse distribution (Immunohistochemistry, indirect Peroxidase, DAB, Magnification orig. ×10). b Caspase-3 with numerous positive cells within the lesion and the surrounding tissues. (Immunohistochemistry, indirect alkaline phosphatase, New-Fuchsin, Magnification orig. ×10). c Detail of the area depicted in 4b with caspase 3-positive cytoplasm mainly in macrophages and glial cells (Immunohistochemistry, indirect alkaline phosphatase, New-Fuchsin, Magnification orig. ×20). d TUNEL reaction with immunohistochemical detection showing positive nuclei mostly in glial cells (Indirect Peroxidase, DAB, Magnification orig. ×10)
Serial sections from the cortex of a 22-year-old female with a survival time of 130 min after contusion denoting the time point where the first reproducible results for staining with caspase 3 were obtained. a Negative control for caspase 3 (Immunohistochemistry, indirect alkaline phosphatase, New-Fuchsin/Hematoxylin, Magnification orig. ×20). b Strong positive staining for caspase 3 of the cytoplasm of most neurons in the lesion area and few glial cells (Immunohistochemistry, indirect alkaline Phosphatase, New-Fuchsin, Magnification orig. ×20). c TUNEL reaction with strong positive nuclear staining in neurons. The numbers of positively staining cells are considerably lower when compared with caspase 3 (Immunohistochemical detection with indirect Peroxidase, DAB, Magnification orig. ×20). d TUNEL reaction, detail of the area depicted in 5c demonstrating exclusively nuclear staining and morphology consistent with apoptosis (Immunocytochemical detection with indirect Peroxidase, DAB, Magnification orig. ×40)
Injured brain tissue (TBI-case group)
Cellular reaction
We found several pathomorphological changes in the contusion area after varying survival times (Figs. 1b–d, 2, 3, 4, 5 and 6). Infiltration of neutrophilic granulocytes (CE-positive cells) was observed after only 30 min in one case and frequently after 120 min. CD68-positive cells in the form of activated microglia or immigrant macrophages appeared after 120 min. Earlier slight staining for CD68 shows only activated microglia. From a survival time of 14 h upwards, CD68-positive cells were always detectable in the area of the lesion, with increased values after day 4 (Figs. 3c and 6a). A lymphocytic inflammation reaction (CD3-positive T-lymphocytes, outside of Virchow–Robin-spaces) was detectable for the first time after 4 days. Lymphocyte infiltration mostly consisted of thin perivascular intraparenchymatous infiltrates within the injured area which occurred frequently from day 5 and regularly from day 9 onwards (Figs. 3d and 6a). Fat-granule cells and siderophages were detectable from day 5 at the earliest and, haematoidin was not detectable before day 9 (Fig. 6a). GFAP is expressed to some extent in normal brain tissues. Especially the molecular layer (first layer) of the cortex and the white matter, considerable constitutive staining of small stellate astrocytes with numerous long processes is shown (Figs. 2a and 6a). GFAP was frequently seen after 3 h and regularly after 13 h of survival time in areas surrounding the lesion. The score value of GFAP-positive actrocytes increased gradually over time, most evidently after day 4 (Figs. 2b–d, 3b and 6a).
Development of pathological changes after TBI. The cases have been assigned to three groups of survival time: 0–2 h, 2 h–4 days and >4 days. Semiquantitatively evalued markers (a) and quantitatively evalued markers (b) are depicted separately. A detailed description of the evaluation process is provided in the Materials and methods section. Error bars show the standard error of means (SEM)
Proliferation
As expected, there was no proliferation of neurons in the TBI group. Increased proliferation activity of glial cells containing mostly microglia (Mib-1 index over 1%) was not observed until day 4 at the earliest. In 11 out of 24 cases (45.8%) with survival times of 4 days or more, a raised Mib-1 index (minimum, 1.3%–maximum, 15%; average, 3.02%) was established (Figs. 4a and 6b). Neoplastic proliferation was ruled out by morphology.
Apoptosis
The earliest observation of apoptotic neurons using the TUNEL technique occurred after a posttraumatic interval of 110 min with an index value of 17.6%. From a survival time of 120 min up to 12 days, apoptotic neurons were frequently detectable in the contusion zone (in 31 out of 37 cases, index values from 5.5 to 33.5%, Figs. 5c,d and 6b). The earliest positive caspase 3 reaction in cortical neurons was already detectable after a posttraumatic interval of 80 min. After that, caspase 3-positive neurons were detectable in only 39% of the cases (nine out of 23 cases, index, 6.0–38.4%) with survival times of up to 4 days, and afterwards, their numbers were greatly reduced (index, 1.3–26.0%, three out of 22 cases, Fig. 5b and 6b). Similarly to neurons, the first apoptotic glial cells were detected after 110 min with the TUNEL technique. From a survival time of 120 min up to 93 days, apoptotic glial cells were frequently detectable (in 35 out of 40 cases) in the contusion zone (Figs. 4d and 6b). Index values for TUNEL-positive glial cells of between 2.4 and 25.0% were established. Caspase 3-positive glial cells did not occur until after 5 h and were then frequently detected up to the 18th day after trauma (in 15 out of 34 cases, index values ranging from 2.0 to 28.2%, Figs. 4b,c and 6b). Apoptotic glial cells consisted mainly of microglia with low numbers of oligodendroglia and astrocytes.
Morphological examination of six cases with very high index values of 40% or more showed clustered apoptosis conjunction with neuronal changes indicative of diffuse neuronal hypoxia such as eosinophilic cytoplasm and pyknotic nuclei. In three cases, handling artefacts (triangular compressed neurons without eosinophilia) of the cortical region were also evident by morphology. It could be argued whether the TUNEL reactions should be regarded as genuine in these areas. When these cases with survival times of 4 or more days in which the patients died of secondary complications (pneumonia or pulmonary thromboembolism) were excluded (as they were for the purpose of biostatistics), TUNEL staining of neurons showed a decrease of TUNEL-positive neurons after day 4, in parallel with the values observed for caspase 3 (Fig. 6b). The apoptosis index values for the studied periods of time are given in Table 1. Time course of all markers after TBI is illustrated in a summarizing graph for the first 24 h (Figs. 6a,b and 7a) and up to day 26 (Fig. 7b).
Time course of all investigated markers after TBI, related to the wound age, for the first 24 h (a) and up to day 26 (b). A detailed description of earliest positive (+), frequently positive (- - -), and regularly positive (—) observation of the different markers is provided in the Results section. Abbreviations for the investigated markers: CE-positive neutrophilic granulocytes (CE + PMN), TUNEL-positive neurons (TUNEL + NC), TUNEL-positive glia cells (TUNEL+GC), caspase 3-positive neurons (Casp 3+ NC), caspase 3-positive glia cells (Casp 3+GC), CD68-positive activated microglia and macrophages (CD68+aC), GFAP-positive activated glia cells (GFAP+aGC), Mib 1-positive proliferating glia cells (Ki 67+ GC), CD3-positive T-lymphocytes (CD3+LC), siderophages-iron-positive macrophages (Fe + C), fat-granule cells (FGC) and hematoidin depositions (hematoidin)
Biostatistics
TUNEL and the immunohistochemical index of caspase 3 in neuronal and glial cells and postmortem interval have been analysed and compared, revealing no correlation between these two parameters (Table 2). However, extended postmortem intervals sometimes lead to increased background staining which remains non-problematic as evaluation and cell counts have been performed manually.
We now consider a set of classical inflammation indicators (e.g., the occurrence of neutrophilic granulocytes, macrophages and lymphocytes) along with apoptotic markers to predict the cases to one of three classes of survival time categories (<2 h, 2–4 h, >4 h). Linear discriminant analysis was used to select the most important predictor variables by a step-wise procedure. The procedure extracted the infiltration of the brain lesion by CE-positive neutrophilic granulocytes, CD3-positive T-lymphocytes, CD68-positive activated microglial cells/macrophages and TUNEL-positive neuronal cells. The information of these four markers was used to assign cases to survival time categories. All of 15 cases with a lifetime less than 2 h were classified correctly (100%). Fifteen of 18 cases (83%) with a lifetime of 2 h to 4 days and 19 of 23 cases (83%) with a lifetime of more than 4 days could be assigned correctly. The overall percentage of cases classified correctly was 87.5%.
Up to 24 h after TBI, the neuronal cells in the injured area of autopsy cases only showed a linear correlation (R 2 = 0.393) to the apoptotic state. As the age of the injury increases, the number of apoptotic neuronal cells (TUNEL positive) increases statistically significantly (Fig. 8), but more than half of the variability is not explained.
Regression analysis for the apoptosis index of positive neuronal cells (NC) in relation to a survival time of up to 24 h. Apoptosis was detected using the TUNEL method as described in the Materials and methods section
Discussion
The aim of this prospective study was to establish reference data concerning the age of a TBI when the age of the injury is unknown. We have shown that a combination of morphology, cellular markers and detection of apoptosis in neurons and glial cells allows for a reasonably accurate evaluation of the age of brain lesions after TBI. Biostatistical analysis of the indices showed that taking account of classical inflammation indicators (e.g., the occurrence of neutrophilic granulocytes, macrophages, lymphocytes and astroglia) along with apoptotic markers provides the greatest degree of certainty, i.e., 87.5%, when correctly cross-validated with the survival time categories (<2 h; 2 h–4 days; >4 days). TUNEL-positive neurons were detected in our assay after a posttraumatic interval of 110 min at the earliest. In cases of survival times ranging from 120 min up to 12 days, apoptotic neurons were frequently detected in the contusion zone. The earliest positive caspase 3 reaction in cortical neuronal cells was already evident after a posttraumatic interval of 80 min. TUNEL-positive glial cells showed a similar time scale as neurons; however, caspase 3-positive glial cells did not appear until at least 5 h after injury.
Additionally, inflammatory and reactive changes in the brain were described within the range of survival time. The most significant changes over time were observed after 2 h in the markers of brain infiltration such as CE-positive neutrophilic granulocytes, CD3-positive T-lymphocytes, CD68-positive activated microglial cells/macrophages and in GFAP-positive astroglia. These changes are also partially accessible by morphology, although mostly with a lower degree of accuracy. TUNEL staining and immunohistochemistry for caspase 3-positive neurons proved to be the most important early markers. The vitality of injured brain tissue has been characterized by means of classic histomorphological and immunohistochemical methods [14–17]. Our results are mostly in accordance with earlier studies although with a slightly different time course. Namely, increased GFAP expression appears somewhat earlier (after 6 h) than in most studies [18–20], which may be due to different evaluation criteria emphasizing perivascular alteration of anatomical distribution or to local differences in the treatment of TBI. We tested the establishment of apoptosis by two different methods as an additional criterion to improve the accuracy of age estimates [21–23].
Using the TUNEL method of DNA fragment in situ labelling, apoptotic neuronal and glial cells can be specifically identified by means of the characteristic staining of the nucleus, whereas, positive immunohistochemical reactions of the cytoplasm to a caspase 3 antibody indicate the progress of the apoptotic cascade [1, 22]. Our results concur with those obtained by Hausmann et al. [22] insofar as these authors also found an increasing number of apoptotic neuronal cells (in situ nick translation (ISNT) positive) up to a posttraumatic interval of 14 days but detected the first apoptotic reaction products after only 45 min. In our assay, too, caspase 3-positive neurons appeared earlier than apoptotic cells detectable by means of the TUNEL technique, but at a later time than published by Hausmann et al. [22]. In practice, this finding would indicate that a reliable diagnosis of TBI may be possible only at the earliest 80–90 min after the trauma. The earlier appearance of caspase 3-positive neurons may be due to the fact that the activation of caspase 3 is an upstream event in the apoptotic reaction cascade, while DNA fragmentation and apoptotic bodies delineate the endpoints of apoptosis [2, 24]. The divergence between caspase 3 and TUNEL product in glial cells and neurons and the different behaviour of these markers after 4 days of survival time indicate the involvement of several distinct mechanisms of apoptosis. Especially, glial cells are more resistant to injury than neurons [25, 26] and may undergo apoptosis by slightly different mechanisms [27] or on a different timescale. It can be argued that the apoptosis of neuronal and glial cells after TBI is not initiated exclusively by effector caspases [4, 27, 28]. This hypothesis is indirectly substantiated by the fact that with increasing time after injury—i.e., in case group >4 days—no differences were found in the apoptotic cell status between the ipsilateral contusion zone and the contralateral brain tissue without a primary lesion, as was also established in the investigations conducted by Hausmann et al. [22]. Caspase 3 was no longer detectable in the neuronal cells of patients with survival times of >4 days nor in the glial cells in cases of survival times of >24 days. We also cannot exclude that some caspase 3-positive cells do not complete the death process.
Regression analysis of our results showed a significant decrease in the number of caspase 3-positive neuronal and glial cells after a period of 4 days after injury. A striking result consisted in the finding that after day 4, especially high TUNEL values were often associated with secondary hypoxia. In some of these cases, it is questionable whether TUNEL staining indeed represents apoptosis. If these cases are discounted, TUNEL values recede after day 4 in parallel to caspase 3, while the numbers of apoptotic neurons and glial cells rise when these cases are included. These findings indicate, at least, that different mechanisms are involved in late hypoxia associated with apoptosis, which are not dependent on caspase 3 activation [28]. One could argue that secondary hypoxia has to be rated among the more important complications of TBI and should, therefore, be included. In this case, the results are concordant with the increased number of apoptotic neuronal cells up to 22 weeks (154 days) described by Hausmann et al. [22] which may, like our results, be related to secondary hypoxic changes in the tissues. In such cases, we registered additional non-nucleus-related, i.e., positive, immunohistochemical reactions and high index values of up to 54% for apoptotic neuronal cells. This applied only to cases with secondary complications involving other organ systems (e.g., pneumonias). Owing to these influencing factors, we considered it advisable to observe these cases critically and in comparison with H&E staining when evaluating the results [29].
Neurodegenerative brain disorders, in particular, Alzheimer’s disease [30] and Parkinson’s disease [31], constitute an additional influencing factor. No evidence that might have indicated the existence of these conditions was found in the case histories of the patients from whom our samples were taken. No confirmation was found in our case group (TBI) that apoptotic changes increase with age, as has been suggested and discussed in the literature: the chi2 test of the contingency tables did not result in the establishment of any significant correlation between the age of the patient and the apoptotic index of the neuronal and glial cells. This corresponds to the findings of Fowler et al. [32] who likewise found no influence of age on DNA fragmentation after TBI. Only in the investigated control group was any indication found of a slightly raised neuronal apoptosis rate in two patients of more than 80 years of age. In view of the significance of this finding for diagnosing the age of an injury, a pathologically relevant index of TUNEL-positive neuronal and/or glial cells should only be used upwards of a value of 2%, particularly in the context of legal medicine.
In our experience, TUNEL staining has proven to be a reliable method for the detection of apoptosis in autopsy tissues when certain caveats are observed. Similar results have been obtained by several other groups [33–35]. The limits of the TUNEL technique are reflected in the inclusion criteria used for this study and in careful calibration of the staining technique using tissues containing necrosis. In addition, it was necessary to control the staining pattern in each case for plausibility. False positive staining tends to be recognizable by a clustering of TUNEL-positive cells of various cell types unrelated to the morphology of cellular damage.
The assay material consisted exclusively of cerebral cortex specimens obtained from autopsies conducted at the Institute of Legal Medicine. From these samples, paraffin sections were produced on which immunohistochemical methods and the TUNEL technique could easily be applied after testing the antibodies and conducting calibration on lymphoma tissue. Thus, the use of the morphological methods did not require any unusually complicated or costly laboratory procedures, as can be the case when using molecular genetic polymerase chain reaction (PCR) techniques. The methods described were used on bodies up to 8 days after death. Critical evaluation by the investigators in exceptional cases is, however, essential despite the fact that no significant differences were found in comparison with brain samples taken from bodies immediately after death [34, 36]. The relative autolysis resistance of the findings, provided cold-storage conditions are adequate, is significant for postmortem histology.
For forensic purposes, the combination of biological parameters used here to study traumatic injuries of unknown age provided acceptable results that can be used in reporting practice. However, the necessary degree of forensic certainty—a 95% probability of correctness as defined by Hummel et al. [37] in paternity being very probable, or at 99.9% practically proven—is not achieved by the markers relating to the apoptosis indices alone. In each specific case, it is, therefore, useful to take into account other established immunohistochemical and morphological findings, such as the detection of the expression of cytokines and adhesion proteins and the assessment of hypoxia, to increase the degree of certainty behind any statement [38].
References
Ng I, Yeo TT, Tang WY, Soong R, Ng PY, Smith DR (2000) Apoptosis occurs after cerebral contusions in humans. Neurosurgery 46:949–956
Padosch SH, Vogel P, Böttiger BW (2001) Neuronale Apoptose nach zerebraler Ischämie. Anaesthesist 50:905–920
Raghupathi R, Graham DI, Mcintosh TK (2000) Apoptosis after traumatic brain injury. J Neurotrauma 17:927–938
Springer JE, Nottingham SA, Mcewen ML, Azbill RD, Jin Y (2001) Caspase-3 apoptotic signalling following injury to the central nervous system. Clin Chem Lab Med 39:299–307
Suzuki A, Shiraki K (2001) Tumor cell “dead or alive”: caspase and surviving regulate cell death, cell cycle and cell survival. Histol Histopathol 16:583–593
Ridout HJ, Stefenis L (2001) Caspase inhibitation. A potential therapeutic strategy in neurological diseases. Histol Histopathol 16:895–908
Yakovlev AG, Faden AI (2001) Caspase dependent apoptotic pathways in CNS injury. Mol Neurobiol 24:131–144
Kam PCA, Ferch NI (2000) Apoptosis: mechanisms and clinical implications. Anaesthesia 55:1081–1093
Lewen A, Matz P, Chan PH (2000) Free radical pathways in CNS injury. J Neurotrauma 17:871–890
Oehmichen M (2001) Brain hypoxia and ischemia. Schmidt-Römhild, Lübeck
Eldadah BA, Faden AI (2000) Caspase pathways, neuronal apoptosis, and CNS injury. J Neurotrauma 17:811–829
Harter L, Keel M, Hentze H, Leist M, Ertel W (2001) Caspase-3 activity is present in cerebrospinal fluid from patients with traumatic brain injury. J Neuroimmunol 121:76–78
Fisher LD, van Belle G (1993) Biostatistics—a methodology for the health sciences. Wiley, New York
Oehmichen M (1990) Die Wundheilung. Springer, Berlin Heidelberg New York
Hausmann R, Kaiser A, Lang C, Bohnert M, Betz P (1999) A quantitative immunohistochemical study on the time-dependent course of acute inflammatory cellular response to human brain injury. Int J Legal Med 112:227–232
Hausmann R, Betz P (2000) The time course of the vascular response to human brain injury—an immunohistochemical study. Int J Legal Med 113:288–292
Hausmann R, Betz P (2001) Course of glial immunoreactivity for vimentin, tenascin and α1 antichymotrypsin after traumatic injury of human brain. Int J Legal Med 114:338–342
Crooks DA, Scholtz CL, Vowles G, Greenwald S, Evans S (1991) The glial reaction in closed head injuries. Neuropathol Appl Neurobiol 17:407–414
Hausmann R, Riess R, Fieguth A, Betz P (2000) Immunohistochemical investigations on the course of astroglial GFAP expression following human brain injury. Int J Legal Med 113:70–75
Onaya M (2002) Neuropathological investigation of cerebral white matter lesions caused by closed head injury. Neuropathology 22:243–251
Williams S, Raghupathi R, Mackinnon MA, Mcintosh TK, Saatman KE, Graham DI (2001) In situ DNA fragmentation occurs in white matter up to 12 months after head injury in man. Acta Neuropathol 102:581–590
Hausmann R, Biermann T, Wiest I, Tübel J, Betz P (2004) Neuronal apoptosis following human brain injury. Int J Legal Med 118:32–36
Dressler J, Hanisch U, Busuttil A (2005) Comments on Hausmann et al: neuronal apoptosis following human brain injury. Int J Legal Med 119:177–178
Ang BT, Yap E, Lim J, Tan WL, Ng PY, Ng I, Yeo TT (2003) Poly(adenosine diphosphate-ribose) polymerase expression in human traumatic brain injury. J Neurosurg 99:125–130
Raghupathi R (2004) Cell death mechanisms following traumatic brain injury. Brain Pathol 14:215–222
Giffard R, Ouyang Y (2004) Effect of overexpression of protective genes on mitochondrial function of stressed astrocytes. J Bioenerg Biomembr 36:313–315
Hostettler ME, Knapp PE, Carlson SL (2002) Platelet-activating factor induces cell death in cultured astrocytes and oligodendrocytes: involvement of caspase-3. Glia 38:228–239
Bonini P, Cicconi S, Cardinale A, Vitale C, Serafino AL, Ciotti MT, Marlier LN (2004) Oxidative stress induces p53-mediated apoptosis in glia: p53 transcription-independent way to die. J Neurosci Res 75:83–95
Hausmann R, Vogel C, Seidel S, Betz P (2006) Value of morphological parameters for grading of brain swelling. Int J Legal Med 120:219–225
Rohn TT, Head E, Su JH, Anderson AJ, Bahr BA, Cotmann CW, Cribbs DH (2001) Correlation between caspase activation and neurofibrillary tangle formation in Alzheimer’s disease. Am J Pathol 158:189–198
Hartmann A, Hunot S, Michel PP et al (2000) Caspase-3: a vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson’s disease. Proc Natl Acad Sci USA 97:2875–2880
Fowler J, Mackinnon MA, Raghupathi R, Saatman KE, Mcintosh TK, Graham DI (2002) Age does not influence DNA fragmentation in the hippocampus after fatal traumatic brain injury in young and aged humans compared with controls. Clin Neuropathol 21:156–162
Witeside G, Cougnon N, Hunt SP, Munglani R (1998) An improved method for detection of apoptosis in tissue sections and cell culture, using the TUNEL technique combined with Hoechst stain. Brain Res Brain Res Protoc 2:160–164
Davison FD, Groves M, Scaravilli F (1995) The effects of formalin fixation on the detection of apoptosis in human brain by in-situ end-labelling of DNA. Histochem J 12:983–988
Schallock K, Schulz-Schaefer WJ, Giese A, Kretzschmar HA (1997) Postmortem delay and temperature conditions affect the in situ end-labeling (ISEL) assay in brain tissue of mice. Clin Neuropathol 16:133–136
Geiger KD, Bloom FE, Sarvetnick NE (1997) Methods for the detection of apoptosis in the CNS. In: Poirier J (ed) Neuromethods apoptosis techniques and protocols. Humana 29, Totowa, NJ, pp 217–235
Hummel K, Ihm P, Schmidt V (1972) Biostatistische Abstammungsbegutachtung. Tabellenwerk. Fischer, Stuttgart
Dressler J, Koch R, Bachmann L, Müller E (1998) Modell zur Schätzung des Wundalters mit Hilfe des Expressionsgrades von Adhäsionsmolekülen. Rechtsmedizin 8[Suppl I]:A 25
Acknowledgement
We wish to thank Doreen Kuechler for the excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dreßler, J., Hanisch, U., Kuhlisch, E. et al. Neuronal and glial apoptosis in human traumatic brain injury. Int J Legal Med 121, 365–375 (2007). https://doi.org/10.1007/s00414-006-0126-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-006-0126-6