Abstract
In previous studies, it was shown that there is a gunshot-related transport of skin particles and microorganisms from the entrance region into the depth of the bullet path. The present study deals with the question of whether gunshots may also cause a retrograde transport of skin particles and microorganisms from the bullet exit region back into the bullet path. For this purpose, we used a composite model consisting of rectangular gelatin blocks and pig skin. The skin pieces were firmly attached to the gelatin blocks on the side where the bullet was to exit. Prior to the test shots, the outer surface of the pig skin was contaminated with a thin layer of a defined bacterial suspension. After drying the skin, test shots were fired from a distance of 10 m using cartridges calibre .38 spec. with different bullet types. Subsequent analyses showed that in all shots with full penetration of the composite model, the bullet path contained displaced skin particles and microorganisms from the skin surface at the exit site. These could be regularly detected in the distal 6–8 cm of the track, occasionally up to a distance of 18 cm from the exit hole. The distribution of skin particles and microorganisms is presented and the possible mechanism of this retrograde transport is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The gunshot exit wound is typically a laceration of the skin without actual tissue loss. In most cases the margins can be brought into apposition. The exit wound can be slit-like or star-like, sometimes also roundish or completely irregular in shape. On the other hand, the gunshot entrance wound is typically characterised by a skin defect that could be shown to be caused by anterograde and retrograde displacement of macroscopically visible and microscopically small skin particles [5]. Furthermore, it has been demonstrated that microorganisms from the skin of the entrance site are carried along the whole length of the bullet track and may therefore constitute a source of infection in gunshot victims [6].
Under normal conditions the skin of the entrance and exit regions is colonised by resident microbial flora. Therefore, the present study was undertaken to investigate whether, in experimental gunshots, microorganisms can be displaced in a retrograde direction from the skin of the exit region back into the bullet path, thus creating a further source of infection in gunshot wounds. As in our previous experiments [6], we used composite models of gelatin and pig skin for test shots with different bullet types. A preliminary test was performed using gelatin blocks and pig skin contaminated with green fluorescent protein (GFPuv)-labelled bacteria to investigate whether a retrograde transport of microorganisms back into the bullet path can be demonstrated. In the main test series the presence of macroscopically visible skin particles and the topographical distribution of microorganisms within the wound track were investigated using Staphylococcus epidermidis to contaminate the pig skin of the exit region.
Materials and methods
Experimental set-up
For the composite models, gelatin blocks measuring 26×12×12 cm were prepared as previously described [5, 10]. For each test shot, a piece of skin (25×10 cm in size) from the belly region of slaughtered pigs was pre-treated as described and firmly attached to the gunshot exit site on the back of a gelatin block. For this purpose, the pig skin was fixed in a wooden rack. Then the gelatin block was placed in front of it and firmly pressed against the fixed pig skin (Fig. 1b). The test shots were fired at the gelatin side of the 26-cm-long composite model.
Schematic illustration of the experimental studies: a disinfected blue-coloured skin from a pig is wetted with a defined bacterial suspension; b shots are fired into the composite model of gelatin and skin from the gelatin side; c after firing the shot, the gelatin block is laminated in 1-cm-thick layers; d the sections with the bullet track are examined for skin particles visible to the naked eye, then cut out, liquefied and spread on culture media
Preliminary test with green fluorescent protein (GFPuv)-labelled Escherichia coli
One piece of pig skin was disinfected prior to firing the shot, then air-dried and covered with 1 ml of a bacterial suspension of recombinant Escherichia coli DH5α K12 [108 colony forming units (CFU)/ml], which constitutively expressed GFPuv after transformation with the respective plasmid. After air-drying the skin specimen again, it was attached to the bullet exit side of a rectangular gelatin block as described above.
The test shot was fired from a revolver (six-shot double-action revolver; Smith & Wesson, Springfield, MA, USA; model 14-1; calibre .38 spec.) from a distance of 5 m using a round-nose lead bullet cartridge (Hirtenberger, Hirtenberg, Austria). The inside of the barrel, the cartridge chamber and the cartridge surface were disinfected before firing the shot.
After the shot, the gelatin block was laminated in 1-cm-thick layers under sterile conditions, and the area of the bullet track was cut out of each gelatin slice. The excised gelatin pieces had a volume of approximately 500 μl. They were liquefied at 37°C, then spread on Luria-Bertani (LB) agar with 100 mg/l ampicillin and incubated at 36±1°C. After 24 and 48 h, the culture media were examined for bacterial growth, and smears of bacterial colonies were investigated by fluorescence microscopy.
Main test series with Staphylococcus epidermidis
Nine pieces of pig skin, each measuring 25×10 cm, were stained in a haematoxylin bath, dried and disinfected as described in a previous study [6]. Directly before the test shots, 1 ml of a bacterial suspension (S. epidermidis, DSM 1798, 108 CFU/ml) was applied to the skin surface (Fig. 1a). The pieces of skin were air-dried and attached to the back of the gelatin blocks.
For firing the shots, a calibre .38 spec. mount and different cartridges were used, where the surface had been disinfected with alcohol (Table 1). After lamination of the gelatin blocks, each layer was examined for the presence of macroscopically visible blue-coloured pig skin particles (Fig. 1c). Then, the central area containing the bullet track was cut out of each gelatin layer and processed using Columbia blood agar as culture medium (Fig. 1d). After an incubation time of 24 h, the bacterial colonies were counted and representative colonies from each block were subcultured onto Columbia blood agar and subsequently classified as previously described [6]. After 48 h the Columbia blood agar plates were inspected again for bacterial growth.
As a negative control, one shot was fired into a composite model with uncontaminated pig skin on the exit side. The bullet track was subsequently processed in an identical manner. As further negative controls, gelatin specimens were taken from areas far away from the bullet tracks in the nine test shots with contaminated skin and examined for the presence of CFUs. The nine test shots of the main series were documented using a high-speed motion camera (2,000 frames per second, MotionXtra HG-100K, Redlake, San Diego, CA, USA).
Results
Preliminary test for bacterial growth
The round-nose projectile passed through the whole length of the composite model of gelatin and pig skin. After laminating the 26-cm-long block and cutting out the gelatin immediately surrounding the bullet track of each slice, 26 smear cultures were incubated for 24–48 h and examined for the presence of GFP-labelled E. coli colonies. Along the distal 10 cm of the bullet track, growth of GFP-labelled bacterial colonies was demonstrated by fluorescence microscopy. No contaminating microorganisms were found.
Main test series
Distribution of macroscopically visible skin particles along the bullet track
The five test shots using round-nose lead bullets and the test shot using the truncated cone semi-jacketed bullet all penetrated the full length of the composite model and perforated the pig skin at the exit site. The hollow-point bullet and the flat-nose bullet passed through the gelatin block but failed to penetrate the skin at the exit site. The characteristics of the projectiles used are shown in Table 1.
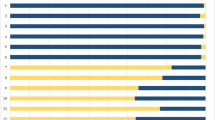
In the distal 6–8 cm of the bullet tracks, blue-coloured skin particles visible to the naked eye were found in all six test shots producing exit holes in the pig skin. Occasionally, skin particles were present at even greater distances of up to 15 cm from the exit site (Fig. 2, dark bars). The track produced by the truncated cone bullet showed a distribution of skin particles similar to those of the round-nose projectiles. In general, skin particles were present not only in the permanent bullet track but were also trapped inside the radial gelatin fissures corresponding to the temporary cavity (Fig. 3). The tracks caused by the non-penetrating bullets (hollow point and flat nose) did not contain any skin particles visible to the naked eye.
Distribution of bacteria along the bullet track
All six fully penetrating bullet tracks showed growth of S. epidermidis colonies after an incubation time of 24 h. The number of bacterial colonies was highest in the track area close to the exit region. Bacterial growth was regularly observed in the distal 6–8 cm of the bullet track. Out of the six perforating test shots, three led to bacterial growth at distances of up to 18 cm away from the exit region (Fig. 2). The absolute number of CFUs varied from block to block even if the same type of projectile was used (Fig. 2).
The negative controls—one bullet track of a test shot with uncontaminated pig skin at the exit site and several gelatin specimens taken far away from the bullet tracks—did not exhibit growth of S. epidermidis. The bullet tracks caused by the non-penetrating hollow point and the flat-nose projectile did not contain S. epidermidis, either. Representative colonies from each block were identified as S. epidermidis with an identical biochemical profile as the strain S. epidermidis, DSM 1789, used for contamination of the skin. Minor contamination was detected in all bullet tracks, which could be clearly differentiated morphologically from the skin bacteria applied for test purposes. At the second inspection, after an incubation period of 48 h, there was only a minor increase in contamination.
High-speed camera documentation
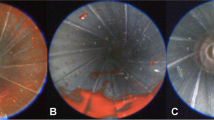
The test shots of the main test series were documented using a high-speed motion camera. Figure 4 shows selected pictures taken from a test shot using a round-nose lead bullet (r n 1). During the penetration of the bullet, the temporary cavity develops and greatly exceeds the diameter of the bullet (Fig. 4a–c). When the still undeformed round-nose bullet perforates the pig skin on the exit side, the temporary cavity extends from the entrance to the exit (Fig. 4c). Then the cavity partly collapses in an anterograde direction (Fig. 4d, e), and again expands towards the entrance (Fig. 4f) followed by another collapse. During these pulsations, small (skin) particles spatter from the exit (Fig. 4g).
Discussion
Gunshot wounds can never be regarded as sterile. Local infections of gunshot wounds in surviving victims are a common problem, and numerous clinical studies have been undertaken to optimise prophylaxis and treatment [1–4, 8, 9, 11, 15, 17, 19]. In a previous study it was shown that local contamination of gunshot tracks can be caused by an anterograde displacement of skin bacteria from the entrance region [6]. Macroscopically visible and microscopically small skin particles may serve as a transport vehicle of microorganisms [6].
The question of whether and under what circumstances bacteria can be transported from the skin of the exit region back into the bullet path has not yet been examined using tissue simulants. Luff [14] investigated the wound dynamics at the gunshot exit site by experimental gunshots to different organ tissues that had been covered with sawdust in the exit region. He was able to demonstrate a retrograde displacement of sawdust into the bullet path in almost all test shots and argued that pressure differences between the temporary wound cavity and the outer atmosphere were responsible for this effect. Tian et al. [18] covered dog legs with bacterially contaminated cloth (Bacterium prodigiosum) on the side where the bullet exited. After test shots, they were able to detect bacteria displaced from the exit wound to the middle of the wound track and, like Luff [14], proposed a suction mechanism to be the cause of this retrograde transport.
With our experiments we clearly show that bacteria applied to the skin on the exit site of a composite model were displaced in a retrograde direction back into the bullet path. The preliminary test was performed to unambiguously identify bacteria within the wound track as being those previously applied to the pig skin on the bullet exit site. UV-fluorescent GFP-labelled bacteria could be detected in the posterior 10 cm of the bullet track. There was no growth of contaminating microorganisms on the ampicillin-containing LB agar. Negative controls were not considered necessary, as fluorescence-labelled bacteria do not occur in the natural environment so that an unintentional contamination with these bacteria is not possible.
With the main test series, we investigated the topographical distribution of the bacteria inside the bullet track. As in our previous study [6], we used S. epidermidis because these bacteria are commensal inhabitants of healthy human skin. In the six through-and-through gunshots with the round-nose lead bullets and the truncated cone bullet, we were able to detect S. epidermidis in the last 6–8 cm of the bullet tracks, and up to a distance of 15–18 cm from the exit site in three out of six cases. In general, the number of detectable bacteria was highest close to the exit site and decreased towards the centre of the composite model. The variations in the distribution and the absolute numbers of CFUs detected could be explained by the inhomogeneous drying of the contaminating bacterial suspension on the uneven pig skin. Species identification of representative colonies as S. epidermidis and lack of growth of this species in the negative controls showed that the bacteria detected inside the bullet path were those previously applied to the skin surface.
The distribution of skin particles visible to the naked eye roughly corresponded to the distribution of displaced microorganisms in such a way that gelatin layers with macroscopically visible skin particles usually contained numerous CFUs. This suggests that the skin particles may serve as transport vehicle for the contaminating bacteria [6]. The presence of macroscopically visible skin particles in gelatin layers without bacterial growth (Fig. 2, r n 1) could be explained by the displacement of corium fragments not covered by bacterially contaminated epidermis.
The high-speed motion camera documentation of the test shots suggests that a suction mechanism due to pulsations of the temporary cavity is responsible for the observed retrograde transport of skin particles and microorganisms. Furthermore, we could photographically demonstrate movable (skin) particles in the gunshot exit area being thrown out of the exit hole during the pulsations of the cavity. The number of pulsations, also called “breathing” of the temporary cavity, depends on the medium and the energy released to the medium. In water, between three and eight pulsations have been observed [12], and the time interval between two pressure maxima is about 20 ms in water [13]. The pressure pulsations during the passage of missiles through the abdominal cavity have also been investigated [7]. The presence of skin particles at the end of radial slits in gelatin supports the assumption that the dynamics of the temporary cavity are causal for the displacement of skin particles. The number and length of the gelatin fissures allow an estimation of the maximum extent of the temporary cavity [5, 16].
The cartridges used in this study were chosen in accordance with our previous studies [5, 6]. For practical reasons, the tests had to be confined to a small number of cartridges. Further studies with a larger number of test shots and additional types of cartridges, especially those carrying full metal jacketed bullets, will be performed in the near future. If the suction due to pressure differences between the temporary cavity and the surrounding atmosphere is the main mechanism of bacterial displacement, the extension of the temporary cavity and its position relative to the exit site is probably of great importance for the degree of this suction effect. Therefore, we expect this suction effect to be dependent on the properties of the respective bullet, the local energy transfer to the tissue or simulant and the length of a particular missile track.
In summary, the findings presented in this study clearly show that the infection of a gunshot wound may be caused by bacteria resident on the skin of the exit site.
References
Albreht M, Scepanovic D, Ceramilac A, Milivojevic V, Berger S, Tasic G, Tatic V, Todoric M, Popovic D, Nanusevic N (1979) Experimental soft tissue wounds caused by standard military rifles. Acta Chir Scand 489:185–198 (Suppl)
Czymek R, Lenz S, Dusel W (1999) Prevention of infection in war wounds. Chirurg 70:1156–1162
Dahlgren B, Almskog B, Berlin R, Nordstrom G, Rybeck B, Schantz B, Seeman T (1982) Local effects of antibacterial therapy (benzyl-penicillin) on missile wound infection rate and tissue devitalization when debridement is delayed for twelve hours. Acta Chir Scand 508:271–279 (Suppl)
Gonul E, Baysefer A, Kahraman S, Ciklatekerlioglu O, Gezen F, Yayla O, Seber N (1997) Causes of infections and management results in penetrating craniocerebral injuries. Neurosurg Rev 20:177–181
Große Perdekamp M, Vennemann B, Mattern D, Serr A, Braunwarth R, Pollak S (2005) Tissue defect at the gunshot entrance wound: what happens to the skin? Int J Legal Med 119:217–222
Große Perdekamp M, Kneubuehl BP, Serr A, Vennemann B, Pollak S (2006) Gunshot-related transport of microorganisms from the skin of the entrance region into the bullet path. Int J Legal Med (in press) DOI: 10.1007/s00414-005-0073-7
Harvey EN, Whiteeley AH, Grundfest H, McMillen JH (1946) Piezoelectric crystal measurements of pressure changes in the abdomen of deeply anaesthetized animals during the passage of HV missiles. Mil Surg 98:509–528
Hecimovic I, Dmitrovic B, Kurbel S, Blagus G, Vranes J, Rukovanjski M (2000) Intracranial infection after missile brain wound: 15 war cases. Zentralbl Neurochir 61:95–102
Jacob E, Setterstrom JA (1989) Infection in war wounds: experience in recent military conflicts and future considerations. Mil Med 154:311–315
Jussila J (2004) Preparing ballistic gelatine—review and proposal for a standard method. Forensic Sci Int 141:91–98
Korzinek K (1993) War injuries of the extremities. Unfallchirurg 96:242–247
Liu Y, Guo R, Wu B, Li S, Wang D (1988) Pressure variation in temporary cavities trailing three different projectiles penetrating water and gelatin. J Trauma 28:9–13 (Suppl)
Liu Y, Li S, Wu B, Wang D, Jiang S, Cheng X et al. (1988) Characteristics of cavities trailing different projectiles penetrating water. J Trauma 28:13–16 (Suppl)
Luff K (1968) Untersuchungen zur Frage des Druckdifferenzausgleichs im Schußkanal. Beitr Gerichtl Med 24:108–113
Matheson JM (1968) Infection in missile wounds. Ann R Coll Surg Engl 42:347–366
Ragsdale BD, Josselson A (1988) Predicting temporary cavity size from radial fissure measurement in ordnance gelatin. J Trauma 28:5–9 (Suppl)
Thurston AJ (2000) Of blood, inflammation and gunshot wounds: the history of the control of sepsis. Aust N Z J Surg 70:855–861
Tian HM, Huang MJ, Liu YQ, Wang ZG (1982) Primary bacterial contamination of wound tract. Acta Chir Scand 508:265–269 (Suppl)
Tikka S (1982) The contamination of missile wounds with special reference to early antimicrobial therapy. Acta Chir Scand 508:281–287 (Suppl)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vennemann, B., Große Perdekamp, M., Kneubuehl, B.P. et al. Gunshot-related displacement of skin particles and bacteria from the exit region back into the bullet path. Int J Legal Med 121, 105–111 (2007). https://doi.org/10.1007/s00414-006-0107-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-006-0107-9