Abstract
The histamine content in vital wounds is known to increase, with a zenith after 3 h, and then decrease until 24 h after wounding. We addressed whether this biochemical alteration has a morphological counterpart. Since the main source of skin histamine are mast cells, the distribution and number of these cells was assessed upon labeling with fluorescent avidin and with antibodies to the mast cell specific enzymes, chymase and tryptase. Analyses were performed on skin from 15 healthy controls (from surgical biopsies), from 15 post-mortem lesions and 75 vital lesions, obtained at autopsy from subjects who had survived from a few seconds to 24 h. The number of mast cells per unit area of section surface increased progressively with survival time, up to a maximum in subjects who survived 1–3 h (p<0.01), and decreased thereafter becoming less than in the controls if lesions had occurred earlier than 6 h before death (p<0.01). Samples from post-mortem lesions had significantly fewer mast cells than those of any other groups of samples (p<0.01). We suggest that in association to other histological and circumstantial evidence the analysis of mast cells by affinity cytochemistry can help to discriminate vital from post-mortem lesions and to estimate survival time after lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The evolution of human skin lesions with time, hence the relationships between the aspect of lesions and the time from trauma and between the time from trauma and death, is a major problem in forensic medicine which is addressed on the basis of the normal wound healing process [1, 2].
Histamine levels in skin vary appreciably after wounding [3, 4]; these levels increase significantly between 5 min and 3 h after trauma and decrease afterwards until 24 h [5].
We have recently demonstrated that the density of mast cells (MC) within 2 cm of pre-mortem lesions is significantly higher than in healthy skin and in post-mortem lesions [6]. We have addressed here the time course of MC alterations in skin wounds by immunohistochemistry, trying to elucidate the possible advantages of this method to estimate wound age.
Material and methods
Protocol
A total of 105 lesions were examined: 15 tissue samples were taken during surgical treatment for wounds from patients who gave written consent after having been informed of the purpose of the study. The procedures were carried out in adherence to Italian law and to the ethical guidelines of the Italian National Medical Council. All the other tissue samples were obtained at autopsies performed at the Department of Anatomy, Histology, and Forensic Medicine, section of Forensic Medicine, of the University of Florence (Italy). Cadavers were routinely kept at +4°C from the moment they arrived at the department morgue until autopsy. The time between death and autopsy was 24 h. The samples were divided into groups of 15 samples each, as follows:
- Group 1.:
-
Biopsies of clinically healthy skin that had been excised at surgery. These samples were used as controls.
- Groups 2–6.:
-
Samples of vital skin lesions (surgical wounds, lacerations and abrasions), with different survival periods between injury and death as estimated from records of the injury and medical treatment. In particular:
- Group 2.:
-
Time between lesion and death from a few seconds to 1 h. Subjects were 14 men and 1 woman aged between 22 and 79 years (mean age 46.8 years).
- Group 3.:
-
Time between lesion and death from 1 to 3 h. Subjects were 12 men and 3 women aged between 15 and 84 years (mean age 44.7 years)
- Group 4.:
-
Time between lesion and death from 3 to 6 h. Subjects were 10 men and 5 women aged between 19 and 97 years (mean age 46.6 years).
- Group 5.:
-
Time between lesion and death from 6 to 12 h. Subjects were 7 men and 8 women aged between 15 and 83 years (mean age 53.3 years).
- Group 6.:
-
Time between lesion and death from 12 to 24 h. Subjects were 12 men and 3 women aged between 10 and 87 years (mean age 49.2 years).
- Group 7.:
-
Samples of post-mortem lesions. Subjects were 11 men and 4 women aged between 15 and 87 years (mean age 43.8 years).
Histochemistry and morphometry
The specimens were prepared following in detail a previously published method [6]. Briefly, they were fixed in Bouin’s or Carnoy’s fluids [7] and embedded in paraplast. Sections were stained with hematoxylin and eosin, with an indirect immunofluorescence method for tryptase and chymase [8, 9] and with fluoresceinated avidin to selectively tag the MCs [10].
Statistical analysis
Neutrophil infiltration as shown in hematoxylin and eosin stained sections was graded on a 0–3 arbitrary scale for each biopsy site.
The numbers of MCs per unit section surface area were counted by one of the authors (SB) in fluorescent avidin labeled sections, at magnification ×200. The cells were counted and the surface area was measured in one section per biopsy. Differences were subjected to analysis of variance, using StatView 512+ program (Abacus Concepts, Berkeley, CA) [11], values of p<0.01 were considered to be significant.
Results
Neutrophils increased in vital lesions progressively and significantly with the time elapsed between lesion and death (p<0.01). Post-mortem lesions contained significantly less neutrophils than control skin (Table 1).
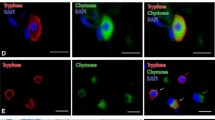
Mast cells were labeled by fluorescent avidin and were immunostained for tryptase and chymase. They were scattered in the upper dermis especially along the blood vessels and around adnexa (Fig. 1a). Mast cell density appeared to vary significantly with time in vital lesions (p<0.01). This density increased progressively to a maximum in lesions which occurred 1–3 h before death (Fig. 1b, Table 2) and decreased thereafter, until in lesions which occurred earlier than 6 h it became lower than in controls. MC density in post-mortem lesions was significantly less than in controls and in any other group of specimens (Fig. 1c).
Fig. 1a–c Mast cells tagged by fluorescent avidin. a Control skin: mast cells are scattered in the lamina propria and around adnexa. b Lesion occurred 3 h before death: mast cells appear increased in number, especially near the epidermis. c Lesion occurred after death: only very few mast cells are still present in the dermis. Fluorescence microscopy, ×200
Discussion
As a result of this study, detailed information was obtained on the number and distribution of MCs in vital and post-mortem skin lesions. We could show that transient infiltration of MCs occurs in the dermis for 3 h upon vital lesions and is followed by protracted decrease until 24 h. We could also confirm that infiltration of neutrophils occurs in vital lesions of subjects with survival time longer than 3 h, as expected from the literature [2, 12, 13, 14], and that post-mortem damage to skin leads to a decrease in MC numbers as compared with healthy skin and vital lesions, as also expected from the literature [6]. Since previous biochemical studies showed that in lesions skin histamine first increases, with a zenith after 3 h, then decreases [5], it seems reasonable to conclude that variations in the numbers of MCs within the dermis, as shown here, are largely responsible for the reported variations in histamine content [5].
The findings of this study show that an increase in dermal MCs occurs within a few hours from trauma and is transient. The possible origin of MCs for this increase may be the migration of nearby cells, differentiation of precursors already present in the tissue or the influx of precursors and their differentiation to MCs; this last mechanism is considered to be at work at later time points, i.e. 4–10 days after wounds, when MCs increase again [15]. The time needed for the influx and differentiation of circulating precursors to MCs is not known exactly but perhaps could be within hours, as for other cell types [16]. The stimulation of the above mentioned processes can be mediated by molecules secreted by keratinocytes, pre-existing MCs, nerve terminals and other cells in the dermis [17, 18, 19].
The late decrease in MC numbers may follow apoptosis of these cells once they have performed their function or just protracted degranulation which makes these cells no longer labeled because of lack of specific granules. The available data do not allow to discriminate between these hypotheses, which moreover are not alternatives to each other. Decrease in MC numbers in post-mortem lesions indicates that trauma itself can damage these cells, which obviously would not be compensated by any of the above proposed mechanisms, because of death.
The time course of the variations in the number of MCs in the skin upon trauma, as shown here, casts light on the dynamics of these processes and, from the forensic pathology stand point, offers an indication of the time elapsed between lesion and death. This indication needs to be taken with caution, because it is made on a statistical basis, however it can implement the information gained from the analysis of neutrophil infiltration and from circumstantial evidence. Other recently proposed methods, such as the immunohistochemical detection of chemokines and ubiquitin, seem to be of little help to discriminate lesions less than 12 h old [20, 21]. On the contrary the identification of apoptotic keratinocytes [22] and of some cytokines within epidermal and dermal cells [23] can help within this time range although each approach is exposed to limitations inherent to the methods and to interindividual variations in baseline values. Therefore, the possibility of addressing the issue of lesion age through multiple and independent methods should be greatly welcome. The approach proposed here offers some advantages over the levels of histamine in tissues. It can be performed on routinely fixed and stored tissue samples instead of requiring dedicated procedures, which should be carefully planned and performed to offer reliable results and therefore are difficult to propose as routine. It does not require destruction of the tissue and can be combined with other morphological analyses on the same tissue block. The reagents, in particular labeled avidin, are relatively cheap and the procedure can be performed in any forensic pathology laboratory.
References
Betz P (1994) Histological and enzyme histochemical parameters for the age estimation of human skin wounds. Int J Legal Med 107:60–68
Hernandez-Cueto C, Girela E, Sweet DJ (2000) Advances in the diagnosis of wound vitality: a review. Am J Forensic Med Pathol 21:21–31
Fazekas GY, Virágos-Kis E (1965) Der Gehalt der Erhängungsfurche an freiem Histamin als vitale Reaktion. Dtsch Z Ges Gerichtl Med 56:250–268
Raekallio J (1973) Estimation of the age of injuries by histochemical and biochemical methods. Z Rechtsmed 73:83–102
Berg S, Ditt J, Friedrich D, Bonte W (1968) Möglichkeiten der biochemischen Wundalterbestimmung. Dtsch Z Ges Gerichtl Med 63:183–198
Bonelli A, Bacci S, Vannelli GB, Norelli GA (2003) Immunohistochemical localization of mast cells as a tool for the discrimination of vital and post-mortem lesions. Int J Legal Med 117:14–18
Pearse AG (1980) Histochemistry. Theoretical and applied. Volume one: preparative and optical technology. Churchill Livingstone, Edinburgh
Irani AM, Bradford TR, Kepley CL, Shechter NM, Schwartz LB (1989) Detection of MCT and MCTC types of human mast cells by immunohistochemistry using new monoclonal anti-tryptase and anti-chymase antibodies. J Histochem Cytochem 37:1509–1515
Fineschi V, Gambassi R, Gherardi M, Turillazzi E (1998) The diagnosis of amniotic fluid embolism: an immunohistochemical study for the quantification of pulmonary mast cell tryptase. Int J Legal Med 111:238–243
Tharp MD, Seelig LL, Tigelaar RE, Bergstresser PR (1985) Conjugated avidin binds to mast cell granules. J Histochem Cytochem 33:27–32
Murray GD (1991) Statistical aspects of research methodology. Br J Surg 78:777–781
Tyler M, Watts A, Perry EM, Roberts A, McGrouther A (2001). Dermal cellular inflammation in burns, an insight into the function of dermal microvascular anatomy. Burns 27:433–438
Janssen W (1985) Forensic histopathology. Springer, Berlin Heidelberg New York Tokyo, pp 88–97
Haussmann R, Kaiser A, Lang C, Bohnert M, Betz P (1999). A quantitative immunohistochemical study on the time-dependent course of acute inflammatory cellular response to human brain injury. Int J Legal Med 112:227–232
Trautmann A, Toksoy A, Engelhardt E, Bröcker EB, Gillitzer R (2000) Mast cell involvement in normal human skin wound healing: expression of monocyte chemoattractant protein-1 is correlated with recruitment of mast cells which synthesize interleukin-4 in vivo. J Pathol 190:100–106
Bacci S, Romagnoli P, Streilein JW (1998). Reduction in number and morphologic alterations of Langerhans cells after UVB radiation in vivo are accompanied by an influx of monocytoid cells into the epidermis. J Invest Dermatol 111:1134–1139
Marone G (1988) Control mechanisms of mediator release in human basophils and mast cells. Immunol Invest 17:707–745
Bienenstock J, MacQueen G, Sestini P, Marshall JS, Stead RH, Perdue MH (1991) Mast cell/nerve interactions in vitro and in vivo. Am Rev Resp Dis 143:S55–58
Kock A, Schwarz T, Kirnbauer R, Urbansky A, Perry P, Ansel JC, Luger TA (1990) Human keratinocytes are a source for tumor necrosis factor alpha: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J Exp Med 172:1609–1614
Kondo T, Tanaka J, Ishida Y, Mori R, Takayasu T, Ohshima T (2002) Ubiquitin expression in skin wounds and its application to forensic wound age determination. Int J Legal Med 116:267–272
Kondo T, Ohshima T, Mori R, Guan DW, Ohshima K, Eisenmenger W (2002) Immunohistochemical detection of chemokines in human skin wounds and its application to wound age determination. Int J Legal Med 116:87–91
Suarez-Penaranda JM, Rodriguez-Calvo MS, Ortiz-Rey JA et al. (2002) Demonstration of apoptosis in human skin injuries as an indicator of vital reaction. Int J Legal Med 116:109–112
Grellner W (2002) Time-dependent immunohistochemical detection of proinflammatory cytokines (IL-1beta, IL-6, TNF-alpha) in human skin wounds. Forensic Sci Int 130:90–96
Acknowledgements
This work was supported by a grant from the Italian Ministry of Universities and Research in Science and Technology. The authors are indebted to Prof. P. Romagnoli for discussion of the manuscript, to Prof. P. Bechi for providing part of the material under study and to Prof. G.B. Vannelli for help with fluorescence microscopy. The technical assistance of A. Forestieri, T. Venturi and P. Venturi is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonelli, A., Bacci, S. & Norelli, G.A. Affinity cytochemistry analysis of mast cells in skin lesions: a possible tool to assess the timing of lesions after death. Int J Legal Med 117, 331–334 (2003). https://doi.org/10.1007/s00414-003-0396-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-003-0396-1