Abstract
Telomeres are important contributors to genome stability, as they prevent linear chromosome end degradation and contribute to the avoidance of telomeric fusions. An important component of the telomeres is the heterochromatin protein 1a (HP1a). Mutations in Su(var)205, the gene encoding HP1a in Drosophila, result in telomeric fusions, retrotransposon regulation loss and larger telomeres, leading to chromosome instability. Previously, it was found that several proteins physically interact with HP1a, including dXNP and dAdd1 (orthologues to the mammalian ATRX gene). In this study, we found that mutations in the genes encoding the dXNP and dAdd1 proteins affect chromosome stability, causing chromosomal aberrations, including telomeric defects, similar to those observed in Su(var)205 mutants. In somatic cells, we observed that dXNP and dAdd1 participate in the silencing of the telomeric HTT array of retrotransposons, preventing anomalous retrotransposon transcription and integration. Furthermore, the lack of dAdd1 results in the loss of HP1a from the telomeric regions without affecting other chromosomal HP1a binding sites; mutations in dxnp also affected HP1a localization but not at all telomeres, suggesting a specialized role for dAdd1 and dXNP proteins in locating HP1a at the tips of the chromosomes. These results place dAdd1 as an essential regulator of HP1a localization and function in the telomere heterochromatic domain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maintenance of chromosomal stability is an essential feature required for correct cell proliferation and overall cell survival. Specialized heterochromatic regions, such as telomeric and pericentromeric regions, are required for correct chromosome segregation (Mathieu et al. 2004; Deng and Chang 2007). The DNA sequences of these regions are rich in repetitive sequences, such as satellite DNA, and may also contain different kinds of LTR and non-LTR retrotransposons. These sequences are very promiscuous and in principle could align with similar sequences in other chromosomes, generating chromosomal aberrations. In somatic cells, the transcription of these sequences must be silenced; in particular, retrotransposon sequences must be silenced to avoid retrotransposition mechanisms that could lead to the integration of these sequences into other genomic regions (Elbarbary et al. 2016; Goodier 2016). Several protein complexes participate in the establishment and maintenance of heterochromatin, and these complexes include proteins such as HP1a (CBX5 in mammals), which have specialized domains that recognize different histone post-translational modifications. In particular, HP1a is able to recognize the H3K9me3 mark on nucleosomes. The recognition of this mark and the oligomerization of HP1a are steps required for the establishment of a heterochromatic state (Canzio et al. 2011). In the last 10 years, different laboratories have reported HP1a protein interactors obtained through several experimental approaches (Brower-Toland et al. 2007; Alekseyenko et al. 2014; Eissenberg and Elgin 2014). The ATRX protein has emerged as one of these interacting factors. In mammalian cells, ATRX recruits CBX5 to telomeric regions, and both proteins cooperate to maintain pericentric heterochromatin (Wong et al. 2010). In Drosophila, the homolog of the ATRX gene is divided into the ADD domain of the protein, encoded by the dadd1 gene, which recognizes the trimethylated form of lysine 9 of the histone 3, and the ATPase-SNF2 domain, encoded by the dxnp gene (also called datrx) (Bassett et al. 2008; López-Falcón et al. 2014). The dadd1 gene encodes three isoforms generated by differential splicing (López-Falcón et al. 2014). The three isoforms contain the ADD domain, but two of these isoforms contain three extra MADF domains that are not present in the human ATRX gene (López-Falcón et al. 2014). We have previously reported that the products of these genes interact physically and genetically (López-Falcón et al. 2014). Additionally, the dxnp gene encodes two protein isoforms, the dXNP L isoform which has a heterochromatic localization and the dXNP s isoform which is observed at heterochromatic and euchromatic regions in polytene chromosomes (Bassett et al. 2008; Schneiderman et al. 2009). The fact that the main domains of ATRX are divided in Drosophila provides us with a useful tool to study the possible specific roles of these domains. In the present study, we analyzed in further detail the contribution of each of these domains to the maintenance of heterochromatin. We found that organisms carrying mutant alleles of both dadd1 and dxnp have up to threefold more chromosomal aberrations, including telomeric fusions, than wild-type individuals. We decided to analyze the telomeric fusion phenotype in more detail. First, we demonstrated that the dAdd1 and dXNP proteins play an important role in the maintenance of the silenced state of the telomeric HTT array in somatic cells, preventing aberrant transcription and integration of the retrotransposons, and second, we found they are necessary to maintain HP1a localization at the telomeric regions. Finally, through rescue experiments, we found that the dAdd1 isoforms play a differential role in the maintenance of telomeric heterochromatin. These experiments helped us to determine that the dAdd1a isoform is responsible for targeting HP1a to the telomeric regions.
Materials and methods
Fly stocks
Flies were maintained at 25 °C with standard food (for 1 L: 100 g live baker’s yeast, 100 g molasses, 9 g agar, propionic acid 10 ml). All stocks used in this study were crossed with our w 1118; Sp/CyO; MKRS, Sb/ TM6B, Tb, Hu or w 1118; Sp/CyO; MKRS, Sb/ TM2, Ubx 1 stocks for five generations in order to minimize background effects. The wild-type flies used in this study were w 1118 (Bloomington Stock Center). The stocks that carried the dxnp 2 (ID26643, Bloomington Stock Center), dxnp 3 (ID26644, Bloomington Stock Center), and mwh 1 (ID549, Bloomington Stock Center) alleles and the sgs3-Gal4 driver, w 1118; P{w[+mC]=Sgs3-GAL4.PD}TP1 (ID6870, Bloomington Stock Center), were obtained from the Bloomington Drosophila Stock Center NIH P40OD018537. The dxnp alleles (dxnp 2 and dxnp 3) were described by (Bassett et al. 2008). These alleles were generated by an excision of the EP element (EP(3)635) that was present at the beginning of the messenger RNA (mRNA), 470 bp from the start of the open reading frame. The dxnp 2 allele is semi-lethal and removed 238 bp of the promoter region including the beginning of the dXNP mRNA; this allele affects both dXNP protein isoforms. dxnp 3 allele is also semi-lethal, but removed 786 bp including the start codon of the dxnp gene and the first 105 amino acids of the coding region; this allele only affects the dXNPL isoform. The dadd1 2 null allele was kindly donated by Dr. Mitzi Kuroda and has been described in (Alekseyenko et al. 2014).
Generation of transgenic lines for rescue experiments
Complementary DNA (cDNA) from the dAdd1a isoform was obtained from the previously described pAc5.1/V5-HisA vector constructs into the EcoR I-Not I restriction sites of the pUAST plasmid (Brand and Perrimon 1993; López-Falcón et al. 2014). For the dAdd1b isoform, the cDNA was amplified from the LD24316 clone (BDGP Gold collection of Drosophila Genomics Resource Center) and cloned into the pUAST EcoR V-Not I restriction sites. Plasmids were then sequenced to ensure correct expression. Plasmid DNAs were sent to the Bestgene Company to obtain the transgenic UAS-dadd1a or UAS-dadd1b lines. Lines harboring insertions into the third chromosome were saved. Virgin flies from these lines were crossed to our w 1118; Sp/CyO; MKRS, Sb/ TM6B,Tb stock to be able to obtain balancers at the second and third chromosomes. Transgene insertion was followed by white complementation. We obtained the w 1118; Sp/CyO; UAS-dadd1a/TM6B,Tb line and the w 1118; Sp/CyO; UAS-dadd1b/TM6B, Tb line. Then we crossed these lines with the w 1118; dadd1 2 /dadd1 2; TM6B, Tb/MKRS, Sb to obtain w 1118; dadd1 2 /CyO; UAS-dadd1a/TM6B, Tb (or UAS-dadd1b). To achieve conditional expression in salivary glands, males from these lines were then crossed with w 1118; dadd1 2 /dadd1 2; sgs3-Gal4/TM6B, Tb. All rescue experiments were performed at 18 °C. Expression of the dAdd1a or dAdd1b proteins was assayed by immunostaining with the pandAdd1 antibody.
Loss-of-heterozygosity (LOH) assays
The crosses were performed by mating virgin females mwh 1 /mwh 1 to males of 48 h of age of the following genotypes: w 1118 ;dxnp 2 /TM3,Sb,Ser, w 1118 ;dadd1 2 /CyO-GFP, w 1118 ;dadd1 2 /dadd1 2 ;dxnp 2 /TM6B,Tb,Hu to obtain flies homozygous for dadd1 2 and heterozygous for dxnp 2 the previously described line was crossed to the w 1118; dadd1 2 /dadd1 2; mwh 1 /TM6B, Tb, Hu line. Flies heterozygous for a mutation in the mwh gene were used for all experiments on LOH. Standard, genetic crosses were carried out to generate fly transheterocigotes for mwh 1 (Papaconstantinou et al. 2010). To assay LOH, F1 wings were dissected, dehydrated in ethanol 70%, and mounted on slides and coded before scoring, for the presence of cell clones showing mutant wing hairs expressing mwh markers (spots). All intervein wing hair cells were examined and cells with two or more hairs were scored as mwh-phenotype. To eliminate any element of subjectivity when scoring the number of wing cells with multiple hairs, these counts were performed blind by two investigators. For each genotype and condition, adult male and female wings were counted (Supp. Table 2). For the evaluation of LOH, statistical comparisons were made using a contingence table (chi-square test) with the Fisher exact probability test: one tailed. Statistical analyses were performed for the total number of spots recovered.
Mitotic chromosome preparations
Mitotic chromosomes were obtained from third instar larval brains using the protocol described in (Williams et al. 1992) with slight modifications. Briefly, third instar larvae were dissected in saline solution (0.7% NaCl), brains were separated and then incubated for 30 min in saline solution containing 1 μM colchicine (Sigma), brains were then transferred to a drop of hypotonic solution (sodium citrate 0.5%) and incubated for 5 min, then brains were transferred to the fixative solution containing (45% acetic acid and 2% formaldehyde) and incubated for 2 min. Squash was then performed to obtain the metaphase chromosomes. Chromosomes were stained using DAPI (1 μM) and cells and mitotic figures were counted on an epifluorescence microscope (Nikon Eclipse E600, Nikon, Tokyo, Japan) with a 100X 1.3 numeral aperture objective. Images of mitotic figures and aberrations were taken on an Olympus FV1000 confocal microscope (Olympus Optical, Tokyo, Japan) with a 60 × 1.3 numeral aperture objective. Images were processed using ImageJ (Fiji) and Photoshop CS software.
Tunel assay
Larval brains were dissected in cold 1xPBS and fixed with 4% paraformaldehyde for 30 min at room temperature. Brains were washed with PBT (1xPBS with 0.3% TritonX-100). Apoptotic cells in larval brains were detected by TUNEL (terminal deoxynucleotide transferase-mediated dUTP and labeling) assays, which were performed according to the manufacturer’s instructions (In Situ Cell Death Detection Kit Fluorescein, Roche). To quantify the apoptosis, we determined the apoptotic cell number per larval brain analyzing ten brains per genotype and using the Image J (Fiji) software. A t student test was performed using RStudio software to evaluate significative differences between genotypes. Fluorescent images were obtained using an Olympus FV1000 confocal microscope with a 20 × 0.75 numeral aperture objective. Images were processed using ImageJ (Fiji) and Adobe Photoshop CS software.
Antibodies
The pandAdd1 antibody has been previously described (López-Falcón et al. 2014). The HP1a antibody was obtained from the Developmental Studies Hybridoma Bank at the University of Iowa (C1A9). The anti H4K16Ac was obtained from Merck Millipore.
Immunostaining of polytene chromosomes
Salivary glands from third instar larvae were fixed in solution I (PBS, 3.7% paraformaldehyde and 1% Triton X-100) and then in solution II (3.7% paraformaldehyde, 50% acetic acid). Chromosomes were spread on poly-l-Lysine-coated microscope slides. Anti-HP1a antibody was used at 1:100; antiH4K16Ac at 1:100, and pandAdd1 at 1:50. Secondary antibodies Alexa fluor 488 and 568 (Invitrogen) were used at 1:500. Slides of mutant and control genotypes were treated at the same time to maintain equal conditions on concentrations of antibodies and incubation time. Images were obtained using an Olympus FV1000 confocal microscope with a 60 × 1.3 numeral aperture objective. Images were processed using ImageJ (Fiji) and Adobe Photoshop CS software.
Immunostaining of metaphase chromosomes
Immunostaining of metaphase chromosomes was performed as described in (Fanti et al. 1998) with slight modifications. Briefly, brains were dissected in saline solution (NaCl 0.7%) and colchicine treatment was performed as described above. Brains were then transferred to hypotonic solution (0.5% sodium citrate) for 5 min. Fixation of metaphase chromosomes was performed with 45% acetic acid and 2% paraformaldehyde for 7 min. Squashing was performed and metaphase chromosomes were incubated for 10 min in PBST (PBS 1X and 1% Triton X-100). Immunostaining protocol was then followed as in the mentioned reference. Images of mitotic figures were taken on an Olympus FV1000 confocal microscope (Olympus Optical, Tokyo, Japan) with a 60 × 1.3 numeral aperture objective. Images were processed using ImageJ (Fiji) and Photoshop CS software.
In situ hybridization assay
Het-A DNA probes marked with digoxigenin were synthetized using a PCR DIG Probe Synthesis Kit (Roche), and 200 ng of genomic DNA from wild-type males as template. Specific primers were designed: Het-A Forward 5′- TCAATTTTTGCGGCACCCTG -3′ Het-A Reverse 5′- GGTGTGGAGTGGTGGAGATG -3′. We performed In situ hybridization of polytene chromosomes according to the protocol described by (Sullivan et al. 2000). The probe was quantified and 200 ng of DNA probe were used per slide. Detection was performed incubating the slides with 1:200 anti-digoxigenin-rhodamine fab fragments (Roche) antibody. Chromosome DNA staining was performed by using 3.5 μg/μl of DAPI. Slides of mutant and control genotypes were treated at the same time to maintain equal conditions on concentrations and hybridization time. Slides were observed and images were taken using an Olympus FV1000 confocal microscope with a 60 × 1.3 numeral aperture objective. Images were processed using ImageJ (Fiji) and Adobe Photoshop CS software.
Genomic DNA and RNA isolation
Genomic DNA was isolated from young males (from 1 to 5 days post-hatching) without testis as described by (Scott et al. 1983). To avoid contamination with RNA, we treated the isolated DNA with RNAse A (0.1 μg/μl) at 37 °C for 1 h. RNA was isolated using the TRIzol Reagent Total Isolation (Life Technologies) according to the manufacturer’s instructions. Reverse transcription was performed using 1 μg of total RNA, 0.025 μg/μl Oligo(dT) (Invitrogen), 0.025 μg/μl random primers (Invitrogen), and 10 U/μl M-MLV reverse transcriptase (Invitrogen).
Real-time PCR assay
For real-time PCR and real-time RT-PCR, specific primers of Het-A and TART were used: Het-A Forward 5′-ACAGATGCCAAGGCTTCAG-3′ Het-A Reverse 5′- GCCAGCGCATTTCATGC-3′ (Silva-Sousa et al. 2012); TART Forward 5′- CAAACTGCAATGGAAGCCA-3′, TART Reverse 5′- GGGCATCAATATTTAGAATGAACAG -3′ (Walter et al. 2007). We used primers Forward 5′-GACTGGTGGTTCGGCCAAGA-3′ and Reverse 5′- TGTCAGGCCGGCAAGATACG-3′ of the Rap2l gene previously reported as reference gene for real-time RT-PCR (Ling and Salvaterra 2011). Reactions were set up in triplicates, and the LightCycler Fast Start DNA Master SYBR Green 1 was used (Roche). Real-time quantitative PCR was performed by using a LightCycler 1.5 Instrument by Roche. The reaction mixtures were first kept at 95 °C for 10 min, followed by 45 cycles at 95 °C for 10s, alignment temperature for 10s, and 72 °C for 18 s. The alignment temperature was 55 °C for Het-A and 60 °C for TART and Rap2l. Fluorescence output results were captured and analyzed by using LightCycler 1.5 software version 3.5.3 (Roche), and the threshold cycle (Ct) was used for assessing relative levels of HeT-A and TART versus Rap2l transcripts and number of DNA copies. The relative levels on mutant genotypes were compared with the relative levels on wild-type strain to obtain the fold difference using the formula 2-ΔΔCT = [(CT gene of interest − CT internal control) simple A − (CT gene of interest − CT internal control) sampleB] previously reported for transcript relative quantification (Schmittgen and Livak 2008). Quantification of transcript abundance and number of DNA copies were measured by triplicate and three independent biological replicates were analyzed.
Chromatin immunoprecipitation, qPCR, and data analyses
Chromatin immunoprecipitation from salivary glands was performed. Briefly, batches of 100 pairs of salivary glands (per antibody or irrelevant IgG) were dissected in ice-cold PBS with protease inhibitors (complete, Roche plus PMSF 0.2 μM). Cross-linking was performed with 1% formaldehyde in PBS for 15 min at 37 °C. Salivary glands were rinsed twice with 500 μl PBS and snap frozen in 100 μl PBS with protease inhibitors in liquid nitrogen and stored at −70 °C until used. Salivary glands were thawed on ice, PBS was removed, 130 μl of SDS lysis buffer was added (1% SDS, 50 mM Tris-HCl, pH 8.0, 10 mM EDTA), and incubation on ice was performed for 10 min. Sonication was performed by 11 cycles (30 s on/30 s off) on a Diagenode Bioruptor. After sonication, the sample was centrifuged at 10,000 g for 10 min at 4 °C. At this point protein was quantified by Bradford (Bio-Rad). Lysate was diluted 1:5 with ChIP dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-HCl, pH 8.0, 167 mM NaCl) and pre-cleared with rabbit IgG coupled to Dynabeads Protein G for 4 h at 4 °C. After pre-clearing, 10% of the lysate was reserved as input. Lysate corresponding to 360 μg of protein was incubated with 25 μl Dynabeads coupled to 2.5 μg of anti-HP1a antibody (C1A9 clone DSHB) or 2.5 μg irrelevant IgG (purified mouse IgG; Invitrogen) overnight at 4 °C. Beads were then washed once with low-salt wash buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.0, and 150 mM NaCl), once with high-salt wash buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.0, and 500 mM NaCl), once with LiCl wash buffer (0.25 M LiCl, 1% NP40, 1% sodium deoxycholate, 1 mM EDTA, and 10 mM Tris-HCl, pH 8.0), and twice with TE (10 mM Tris-HCl, pH 8.0, and 1 mM EDTA). Immunoprecipitated chromatin was eluted with elution buffer (1% SDS, 0.1 M NaHCO3 in 1xTE). Reverse cross-linking was performed by incubation at 65 °C for 12 h. After RNA and protein digestion, the DNA was recovered by phenol/chloroform extraction and ethanol/glycogen precipitation. DNA was suspended in water and analyzed by qPCR. The primers used in qPCR were previously reported, for the TAS-L sequences (López-Falcón et al. 2014), for light (Lu et al. 2009), and for the Het-A promoter fwd 5′ AAGGCTGCAAAATCCGTCCA 3′, and rev 5′ CGTGGTTGTCGTCAGTAGGA 3′. The % Input was calculated as reported in (Lin et al. 2012). Briefly, % Input = 100/E (dCt normalized ChIP), where E represents the efficiency of each region primers and dCt normalized ChIP = Ct sample − [Ct input − Log2 (input dilution factor)]. Three independent biological experiments were performed each with three technical replicates. P value **<0.001 indicates significant differences.
Western blot
Third instar larvae were rinsed in ice-cold PBS 1X; 20 pairs of salivary glands were dissected from each analyzed genotype and homogenized with a pestle in a 1.5-ml Eppendorff tube in 20 μl of Laemmli buffer. Samples were boiled for 15 min, and proteins were separated in a 10% acrylamide/bis-acrylamide denaturing gel. After electrophoresis, proteins were transferred to a nitrocellulose membrane (Bio-Rad). Detection of the proteins was then carried out as previously described (López-Falcón et al. 2014). An anti-actin antibody was used as a loading control (JLA-20 from DSHB).
Statistical analysis
For the frequency of chromosome aberrations, a t student test was performed using RStudio software in order to identify the genotypes with significant differences versus the number of aberrations found in the wild-type strain. P value: * <0.05, ** <0.01.
For the real-time PCR and RT-PCR, in order to determine significant differences, fold differences on transcript abundance and DNA copies among mutant genotypes and the wild-type strain were analyzed through the Mann-Whitney statistic test by using RStudio software considering real-time PCR quantifications from three independent biological assays with technical triplicates. Statistical analyses for the other experiments are mentioned at the specific section.
Results
Mutations in dadd1 and dxnp result in chromosomal aberrations
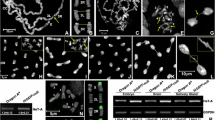
It has been previously reported that the dAdd1 and dXNP proteins cooperate with HP1a to maintain a heterochromatic state and that they act as suppressors of position effect variegation (Schneiderman et al. 2009; Emelyanov et al. 2010; Alekseyenko et al. 2014; López-Falcón et al. 2014). Maintenance of heterochromatin is important to avoid chromosomal aberrations that lead to chromosome instability. For instance, mutations in the gene Su(var)205, encoding the HP1a protein, lead to telomeric fusions, as this protein also forms part of the capping complex of Drosophila telomeres (Fanti et al. 1998; Perrini et al. 2004). Given that the dXNP and dAdd1 proteins interact physically and genetically with HP1a and that ATRX has an important role in telomeric maintenance in vertebrates, we decided to analyze whether mutations of the dadd1 or dxnp genes could phenocopy the telomeric fusions observed in Su(var)205 mutant flies. We analyzed mitotic chromosomes of third instar larvae brains from organisms carrying different mutant alleles, which affected only the dadd1 or dxnp genes or combinations between these alleles (see Materials and Methods). We found that larval brain cells from organisms carrying alleles that affect dxnp or dadd1 presented severe chromosomal aberrations. Different types of chromosomal aberrations were observed, as shown in Fig. 1a–e. These aberrations included axial decondensations, in which the chromosome arms are longer if we evaluate the length from the centromere to the telomere, (Fig. 1b) and centromeric or telomeric fusions (Fig. 1c, d).
Mutations in dxnp and dadd1 generate chromosomal aberrations. a Wild-type metaphase chromosomes: the two pairs of each chromosome (the sexual pair and chromosomes 2, 3, and 4 (dot chromosome)) can be observed. b Example of chromosomal decondensations: it can be observed that the chromosome arms were larger than the wild-type chromosome arms (length from the centromere to the telomere). c Centromeric fusions, with the arrow pointing to one of the centromeric fusions. d Telomeric fusions, with the arrow pointing to one of the telomeric fusions. e Percentage of observed chromosomal aberrations. The number of cells with aberrations/number of total mitoses is shown. Error bars represent standard deviation of aberrant mitoses of three independent experiments per genotype. Significance code: ***P < 0.001; **P < 0.01; *P < 0.05. f Cell death was evaluated using a TUNEL assay. The contours of larval brains are shown with a dotted white line. Wild-type flies did not show cell death; however, genotypes that harbored the null dadd1 2 allele and dxnp 2 /dxnp 3 showed a small amount of cell death in the brain lobes and thoracic ganglia (arrows). TUNEL signal outside the contoured white dotted lines belongs to tracheas or tissue such as the ring gland. g Quantification of apoptotic cells per larval brain. The number of TUNEL positive cells per brain for each genotype in f is shown. Error bars represent standard deviation of TUNEL positive cell number of ten brains per genotype. **P < 0.01 indicate significative differences with respect to wild type
We quantified these observed aberrations (Fig. 1e and Supp. Table 1) and found that heteroallelic or trans-heterozygous organisms had approximately twofold more aberrations than cells derived from wild-type organisms; for instance, compare the wild type (13%) versus the dxnp 2 /dxnp 3 (23%), whereas cells derived from organisms carrying the null dadd1 2 allele in a homozygous condition and the dxnp 2 allele, which affects both dXNP isoforms, in a heterozygous condition showed up to threefold more chromosomal aberrations (Fig. 1e; see the dadd1 2 /dadd1 2 ;dxnp 2/+ genotype, which reached 36%). Next, we analyzed the frequency of the different types of aberrations present in these mutant combinations to assess if there was a preponderance of one of the phenotypes observed (Supp. Table 1). We found that the aberrations observed were heterogeneous among the different alleles; we did not notice any significant enrichment of a specific phenotype, except in the case of the dadd1 2 /dadd1 2 ;dxnp 2 /+ genotype, which showed an enrichment of telomeric fusions compared with the other phenotypes (Supp. Table 1). Interestingly, the dadd1 2 /dadd1 2 ;dxnp 3/+ genotype (the dxnp 3 allele only affects the dXNPL isoform) did not show an enrichment of the telomeric fusions, indicating the importance of the dXNPs isoform in avoiding these type of aberrations (see Materials and Methods for a description of the alleles). These results suggest that the dAdd1 and dXNP proteins are involved in the maintenance of chromosomal stability.
Organisms carrying a null mutation in dadd1 in a homozygous condition were semi-viable, with approximately 63% of the flies surviving to adulthood (Supp. Table 2). A homozygous line can be properly maintained under laboratory conditions. Since we observed that the null dadd1 flies in combination with the dxnp 2 allele reach adulthood despite showing a high number of chromosomal aberrations, we decided to analyze whether these aberrations had an impact on cell survival. We performed a TUNEL assay to determine if larval brain cells of the mutant organisms displayed apoptosis. We found that there was a small amount of cell death only in brains of organisms carrying the dadd1 2 allele in a homozygous condition and in heteroallelic dxnp 2 /dxnp 3 flies; only a few cells of certain regions of the larval brain were affected (Fig. 1f, see arrows). Quantification of these data shows that the apoptotic cell number was higher than the sporadic cell death observed in wild-type brains (Fig. 1g). Nevertheless, there was a possibility that if the observed aberrations could not be resolved by apoptosis, they would accumulate during development and we would see the effects of these aberrations in a loss of heterozygosity assay (de Andrade et al. 2004). We used the mwh 1 mutant allele, which, in a homozygous condition leads to the appearance of three sensory bristles in the wing cells instead of the one observed in the wild-type condition; the combination of this allele in a heterozygous condition with other mutant alleles, which can affect chromosome stability (by deletions of segments or loss of the wild-type chromosome), generates a loss of heterozygosity. We tested the mwh 1 allele in combination with dxnp 2, dadd1 2, or trans-heterozygous combinations of these alleles and found that mutations in dxnp and dadd1 did in fact result in a loss of heterozygosity (Supp. Table 3). The LOH assay shows that the single and double mutants (dadd1 2 or dxnp 2) increased loss of heterozygosity when compared to the control. However, when we compare single and double mutant, significant differences were obtained only in the cases of dadd1 2 /dadd1 2; mwh 1 /+ vs dadd1 2 /dadd1 2; and dxnp 2 /mwh 1.
Taken together, these data indicate that although the null dadd1 flies were semi-viable, combinations between these and dxnp mutant alleles affected even more organism survival ((López-Falcón et al. 2014), (Supp. Table 2) leading to chromosome aberrations and loss of heterozygosity, revealing a cooperation between these proteins for the maintenance of chromosomal stability.
dAdd1 and dXNP maintain the silencing of Het-A and TART retrotransposons in somatic cells
An important feature of chromosome stability is the correct maintenance of silencing of retrotransposon regions and repetitive sequences (Goodier 2016). Drosophila telomeres have three main regions, the CAP (which includes the HP1a protein and others of the terminin complex), the HTT array (which is composed of three non-LTR transposons known as Het-A, TART, and TAHRE), and a TAS (telomeric-associated sequences) region rich in repetitive sequences (Silva-Sousa et al. 2012). Given the phenotypes we observed and the previously reported HP1a involvement in the regulation of transcription of the HTT array, we decided to analyze whether the dXNP and dAdd1 proteins could maintain the silenced state of the retrotransposons present in the telomeric HTT region in somatic cells (Silva-Sousa et al. 2012). The mechanism of retrotransposition is highly regulated and silenced in somatic cells. In the case of non-LTR retrotransposons, such as the ones present in the HTT array, one of the first steps in the retrotransposition mechanism involves transcription of these elements. The retrotransposition mechanism is active in germ cells, but in somatic cells, this mechanism must be silenced to maintain chromosomal stability (Biessmann and Mason 2003; Capkova Frydrychova et al. 2009). Mutations that affect Su(var)205 (the gene that encodes HP1a) result in telomeric fusions, loss of retrotransposon silencing, and the production of longer telomeres that lead to chromosomal instability (Fanti et al. 1998; Perrini et al. 2004). The vertebrate ATRX protein has been identified as an essential regulator of the silencing of retro-elements (Sadic et al. 2015; He et al. 2015). Furthermore, the dXNP and dAdd1 proteins have also been found to bind to the TAS telomeric regions (Antao et al. 2012). To assess whether dXNP and dAdd1 were capable of mediating the silencing of these retro-elements, we first evaluated the number of integrated copies of the TART and Het-A retrotransposons, in somatic cells of the different dadd1 and dxnp mutant backgrounds. The number of integrated copies was evaluated through qPCR using primers specific for each retrotransposon (see Materials and Methods); as a positive control, we used chromosomal DNA isolated from the Gaiano III (GIII) strain, which has more copies of these retrotransposons than a wild-type fly (Siriaco et al. 2002) and we used w 1118 as a wild-type control.
To evaluate whether the dAdd1 and dXNP proteins are involved in the retrotransposons transcript repression, we isolated RNA and used specific primers to amplify the sense transcript of each retrotransposon by qRT-PCR. As in the previous experiment, we used the w 1118 strain as the wild-type condition and the GIII strain as a positive control.
The retrotransposons in the GIII strain (positive control, second box on each plot, Fig. 2(a)) always had higher transcript levels and more integrated copies than the wild type (first box in all the plots, Fig. 2(a)). When we analyzed the TART and Het-A retrotransposons in the different allelic combinations, we found that most of them were similar to the wild type; however, in the dadd1 2 /dadd1 2 ;dxnp 2 /+ genotype, they present more transcripts and a higher number of integrated copies (see the last box from left to right on the TART and Het-A plots, Fig. 2(a)). Interestingly, in flies of the null dadd1 2 /dadd1 2 genotype, the TART element showed a higher number of transcripts than the wild type, but contrary to what was observed for the dadd1 2 /dadd1 2 ;dxnp 2 /+ genotype, it did not show a higher number of integrated copies (see sixth box from left to right on the TART plots, Fig. 2(a)). This result reveals that for the TART retrotransposon, dadd1 and dxnp could have different activities in transcript repression and in preventing the integration of the retrotransposon copies into the genome and that an additional mutation (in this case on dxnp) is necessary to complete the retrotransposition mechanism. The Het-A element only showed a higher number of transcripts and number of integrated copies in the dadd1 2 /dadd1 2 ;dxnp 2 /+ genotype (last box from left to right on the Het-A plots, Fig. 2(a)). We also observed this result by DNA in situ hybridization of polytene chromosomes using a probe for Het-A. When we analyzed the dadd1 2 /dadd1 2 genotype, we could see that the Het-A signal was not homogenous in all the telomeres and there were some telomeres that showed a weak Het-A signal (Fig. 2(d, d′)). We believe this result is reflected on the qPCR quantifications in which we do not see a significant increment in this retrotransposon copies versus the wild type (Fig. 2(a)); however, we have to take into account that we used polytene chromosomes for the in situ hybridization and adult organisms for the copy number quantification. In the chromosomes obtained from the dadd1 2 /dadd1 2 ;dxnp 2 /+ genotype, the probe signal was higher compared to the wild-type and similar to the GIII strain (Compare Fig. 2(b, b′ and c, c′ to e, e′); additionally, we observed telomeric associations, which were apparently mediated by DNA (Supp. Fig. 1).
(a) The dxnp and dadd1 mutants show aberrant transcription and integration of the retrotransposons of the HTT array. Boxplot of the transcript abundance and number of integrated copies of the TART and Het-A retrotransposons are shown. The genotypes are shown at the bottom of the graphs. The fold difference versus white (wild-type strain) was evaluated through a Mann-Whitney test. Significance code: ***P < 0.001; **P < 0.01; *P < 0.05. (b, c, d, e) DNA In situ hybridization of wild-type (w 1118), GIII, dadd1 null and dadd1 2 /dadd1 2 ;xnp 2 /+ polytene chromosomes, respectively, using a Het-A probe, a magnification of the chromosome tips is shown in b′, c′, d′, and e′. Note that for the GIII genotype (positive control), the signal is amplified due to the number of integrated copies
Overall, these data indicate that the dXNP and dAdd1 proteins maintain the repression of the HTT array and do so in part by inhibiting the transcription of these elements, which is the first step of the retrotransposition mechanism.
dAdd1 proteins maintain HP1a localization at telomeric regions
The HP1a protein localizes to the three telomeric regions (CAP, HTT, and TAS) (Fanti et al. 1998; Antao et al. 2012; Silva-Sousa et al. 2012), and mutations on Su(var)205 that affect the wild-type levels of the protein (Fanti et al. 1998) result in telomeric fusions and a deregulation of retrotransposon silencing, which leads to longer telomeres. Interestingly, mutations in the HP1a chromodomain do not affect HP1a localization at the telomeric regions (Biessmann and Mason 2003). It has been demonstrated that HP1a can bind directly to telomeric DNA through its hinge region, which has been proposed to be one of the mechanisms by which HP1a is targeted to the telomeric region to achieve the capping function (Perrini et al. 2004). Given these reports and the fact that the phenotypes observed in the telomeres of dxnp and dadd1 mutants (telomeric fusions and loss of retrotransposon repression) resemble HP1a mutations, we decided to analyze whether the dXNP or dAdd1 proteins were necessary to retain HP1a at the telomeric regions. We analyzed the HP1a distribution in polytene chromosomes by immunolocalization using the C1A9 antibody (James et al. 1989). HP1a localization was well defined at the chromocenter and at the tip of the chromosome arms in wild-type polytene chromosomes (Fig. 3a), and HP1a colocalized with dAdd1 proteins at this telomeric region (Fig. 4a´´). Based on a closer view of the telomeric regions at the tip of the chromosome arms, the HP1a signal was present at all of the analyzed telomeres in the wild-type strain (Fig. 3(a′)), as previously reported (Fanti et al. 1998). We then evaluated the presence of HP1a in the polytene chromosomes of heteroallelic dxnp 2 /dxnp 3 flies. The localization of HP1a at the chromocenter was maintained (Fig. 3(b)), although the chromocenter was fragile, and breaks could occasionally be observed due to the squashing technique. A magnified view of the telomeric regions (Fig. 3(b′)) reveals that the HP1a signal was retained at some of the analyzed telomeres. Next, we decided to analyze the null dadd1 2 allele in a homozygous condition, in which the chromosomes showed a defined signal at the chromocenter but a complete loss of the HP1a signal at the tip of the chromosomes (Fig. 3(c) and magnifications in Fig. 3(c′)); additionally, there was an overall decondensation that could be observed when we compared the wild-type or the dxnp 2 /dxnp 3 chromosomes with the genotypes that carry the dadd1 2 null allele (compare Fig. 3(a′ and b′ to c′ and d′)). The loss of HP1a was also observed when we analyzed the polytene chromosomes derived from organisms carrying the null dadd1 2 allele in a homozygous condition in combination with the dxnp 2 (Fig. 3(d, d′)) or dxnp 3 (Supp. Fig. 2) alleles. In these genotypes, HP1a localization was retained at the chromocenter but was lost at all of the telomeres. These results indicate that the dAdd1 proteins are indispensable for HP1a localization at the telomeric regions. Indeed, immunostaining experiments in mitotic chromosomes in dAdd1 null organisms confirm the requirement of the products of this gene in the telomeric location of HP1a, but not in the centromeric regions (Supp. Fig. 3). In contrast, the dxnp 2 /dxnp 3 heteroallelic flies (Fig. 3(b, b′)) retained HP1a binding at the telomeric regions of some chromosome arms, providing convincing evidence for the specific requirements of distinct chromatin proteins in regulating HP1a localization at different heterochromatic domains (Oikemus et al. 2004; Singh and Lakhotia 2016).
The dAdd1 proteins are essential to maintain telomeric HP1a protein localization. Polytene chromosome preparations were immunostained with the C1A9 (anti-HP1a) monoclonal antibody. Chromosomes were visualized by confocal microscopy. (a) Wild-type HP1a localization on polytene chromosomes: the signal can be observed at the chromocenter and on the telomeric regions (a′). (b) Polytene chromosome from a heteroallelic mutant dxnp fly. In these chromosomes, the HP1a signal was still localized at the chromocenter but was lost at some telomeres, as shown in b′. The chromocenter was also fragile and subject to breaks due to the squashing technique. (c) The null dadd1 chromosomes maintained the HP1a signal at the chromocenter but lost all of the HP1a telomeric signals, as shown in c′. (d) Chromosomes of the dadd1 2 /dadd1 2 ;dxnp 2 /+ genotype maintained the HP1a signal at the chromocenter but also lost the telomeric signal; furthermore, these chromosomes additionally showed decondensation of the telomeric regions, as shown in d′. In a′, b′, c′ and d′ magnification images of telomeric regions of different immunostained chromosomes are shown, only the first row of each magnification corresponds to the telomeres shown in the complete immunostained chromosome shown in a, b, c, and d. (e) HP1a signal in telomeres of wild-type X polytene chromosomes (w 1118). In the wild type condition, HP1a (red signal) is present at the tip of the chromosome. X chromosomes were marked by immunostaining with H4K16Ac (green signal). (f) In the null dadd1 condition, we can see that HP1a is lost from all X chromosomes analyzed. (g) In the dxnp 2 /dxnp 3 heteroalleic combination the HP1a signal is partially lost. (h) The HP1a protein is lost from specific telomeric regions in the dadd1 null genotype. Chromatin immunoprecipitation experiments were performed using the C1A9 antibody (anti-HP1a) and the promoter of the Het-A retrotransposon and TAS-L telomeric regions were evaluated as well as the light gene which has a pericentromeric localization. HP1a protein was present at all the analyzed sites in the wild-type condition whereas in the dadd1 null condition, it was completely lost from the Het-A promoter. Error bars represent standard deviation. **P < 0.01
The dAdd1a protein isoform is necessary to maintain HP1a localization at the telomeric region. (a) Wild-type localization of the dAdd1 (green signal) proteins at the polytene chromosomes. (a′) Wild-type localization of the HP1a (red signal) protein. (b), (b′) In the null dadd1 mutant, the dAdd1 signal was lost; however, the HP1a signal is conserved at the pericentric heterochromatin. (c) Rescue experiments performed by expressing the dAdd1a protein isoform show that dAdd1a localizes to heterochromatic regions. (c′, c″) Conditional expression using the sgs3-Gal4 driver showed that the HP1a signal was restored at these telomeres. (d) Rescue experiments performed by the conditional expression of the dAdd1b protein isoform using the de sgs3-Gal4 driver showed that the dAdd1b protein also has a heterochromatic distribution and becomes enriched at the chromocenter of polytene chromosomes. (d′, d″) HP1a signal was not restored at the telomeres in which dAdd1b was conditionally expressed; however, we can see that dAdd1b also has a telomeric location (green signal)
Since the X chromosome of male flies is hyperacetylated for dosage compensation purposes (Gelbart et al. 2009), we decided to take advantage of this phenomena and mark the X chromosome of male-derived polytene chromosomes with the H4K16Ac antibody. This would allow us to follow the X chromosome in all the mutant backgrounds and help us determine if the X chromosome arm retained or not the HP1a signal in the dxnp 2 /dxnp 3 mutant background. Additionally, this will help us determine without a doubt that the lost of HP1a signal we observe is in fact at the tip of these chromosomes and not derived from a possible chromosome breakage event. We found that in a wild-type background, all X chromosomes retained HP1a signal (Fig. 3(e)), whereas in the dadd1 2 null chromosomes, the HP1a signal was lost confirming our previous result (Fig. 3(g)). Nevertheless, in the dxnp 2 /dxnp 3 mutant background, some HP1a signal remained at the X chromosome telomeres (Fig. 3(f)) and also at the tip of other unmarked chromosomes (data not shown). We conclude that dAdd1 proteins are essential to maintain HP1a bound to the telomeric regions and that loss of dXNP proteins partially affects binding of HP1a at the telomeres.
The fact that HP1a was lost at all telomeric regions in the null dadd1 background does not explain the differences observed in the frequency of the telomeric fusions between this mutant and the previously reported Su(var)205 mutants (Fanti et al. 1998) One possible explanation was that the immunohistochemistry experiments were not sensible enough to detect low levels of HP1a in telomeric regions. Therefore, to address this question, we performed chromatin immunoprecipitation experiments in salivary glands of the wild-type and null dadd1 backgrounds to evaluate the presence of HP1a at three different heterochromatic regions, the Het-A promoter and the TAS-L region both telomeric and the light gene which is located at pericentromeric heterochromatin (López-Panadès et al.; Lu et al. 2009; Antao et al. 2012). We found that HP1a was enriched at all the three heterochromatic regions in the wild-type strain (w 1118 ), whereas in the null dadd1 background, HP1a was lost from the Het-A promoter but not entirely from the TAS-L region; furthermore, HP1a remained enriched at the pericentric light gene at similar levels as the wild-type strain in the null dadd1 background (Fig. 3(h)).
These results confirm our previous observations that showed a specific HP1a loss only at the telomeres without affecting the pericentromeric regions. Additionally, the fact that some levels of HP1a still remain bound to the TAS regions provides a plausible explanation for the lower frequency of telomeric associations found in dadd1 mutants with respect to Su(var)205 mutants. In this case, not all the telomeric regions are affected equally as in the of Su(var)205 mutants and this could eventually lead to a milder or a stronger telomeric fusions phenotype.
dAdd1a, but not dAdd1b, is required for HP1a localization at the telomeres
Given that we used a null dadd1 allele to evaluate the localization of HP1a, it is possible that only one of the isoforms encoded by this gene was responsible for the HP1a telomeric localization. The dAdd1a isoform has a conserved ADD domain at the amino-terminus and no other conserved domain. In contrast, the dAdd1b and dAdd1c isoforms also conserve the ADD domain but have extra domains at the carboxyl terminus known as MADF domains (López-Falcón et al. 2014). Hence, we decided to generate transgenic flies that conditionally expressed dAdd1a or dAdd1b isoforms; we chose dAdd1b to represent the isoforms with the MADF domains to assess which isoform could rescue HP1a telomeric localization (see Materials and Methods). In Fig. 4, the immunolocalization of the dAdd1 proteins (using our previously reported pan-dAdd1 antibody) and HP1a in the telomeres of the wild-type flies could be observed (Fig. 4(a and a′, respectively)), in which there was colocalization of both proteins at the telomeric regions (Fig. 4(a″)). In the null dadd1 flies, the dAdd1 signal disappears (Fig. 4(b)) and the HP1a signal is only observed at the chromocenter (Fig. 4(b′)) and not at the telomeric regions (Fig. 4(b″)). Next, we found that the conditional expression of the dAdd1a isoform in the salivary glands of individuals carrying the null dadd1 2 allele in homozygous condition led to a recovery of the HP1a localization signal at the telomeric region (Fig. 4(c, c′, c″)). Interestingly the dAdd1a protein isoform colocalizes at all the HP1a sites at the chromosome arms and at the chromocenter but its distribution is quite homogeneous and does not seem to be enriched at any particular site (Fig. 4(c)). When we analyze the HP1a distribution, although exogenous expression of dAdd1a restores HP1a telomeric localization, it seems to lose its normal enrichment at the chromocenter (Compare Fig. 4(a′–c′)). Thus it is possible that exogenous expression of dAdd1a in a null dadd1 2 background compromises wild type HP1a enrichment at the chromocenter. We performed a western blot to compare wild type and ectopically expressed dAdd1a levels, in the lane with protein extracts from wild type salivary glands we can see two close migrating bands between the 150 and the 100 molecular weight markers as we usually see with this antibody (López-Falcón et al. 2014) in the lane corresponding to the ectopic expression of the dAdd1a protein isoform we can see that there is a clear increase in the levels of dAdd1a, additionally we can see a band that migrates closer to the 150 Kda molecular weight marker, we believe this band represents a modified version of the dAdd1a protein isoform. (Supp. Fig. 4a see enrichment of band between the 100KDa weight marker). Perhaps the combination of higher levels of dAdd1a and lack of the other dAdd1 protein isoforms leads to a disruption of HP1a localization at the chromocenter. This can also be observed in Supp. Fig. 4 in which we present polytene chromosomes that have low levels of dAdd1a and retain HP1a localization at the chromocenter (Supp. Fig. 4b and b′), but others that have higher levels of dAdd1a in which HP1a localization is affected (Fig. 4(c′) and Supp. Fig. 4c and c′). In contrast, when we expressed the dAdd1b isoform, it also localizes to the telomeres (Fig. 4(d″)); however, the HP1a signal is not recovered at the telomeres and in this case, the enrichment of HP1a signal was conserved at the chromocenter (Fig. 4(d″, 4d′) respectively) We also evaluated whether these proteins could revert the retrotransposon copy numbers to a wild-type condition. We performed in situ hybridization of polytene chromosomes using the Het-A probe. In these chromosomes, we can see that both dAdd1a and dAdd1b conditional expressions prevent the integration of DNA copies of Het-A, even though dAdd1b fails to target HP1a to the telomeric regions (Supp. Fig. 5 a, b, c and d). We quantified the number of integrated retrotransposon copies by qPCR in salivary glands in which dAdd1a or dAdd1b was conditionally expressed and found that although dAdd1a targets HP1a to the telomeres, the qPCR results indicate that the integrated Het-A copies are very variable including samples close to wild-type and others close to the dadd1 null line. Conditional expression of dAdd1b results in integrated Het-A copies closer to the wild-type condition (Supp. Fig. 5e). These results indicate that dAdd1b protein isoform is also preventing the integration of this retrotransposon at the telomeric region and does so independently of HP1a recruitment. Taken together, these results indicate that the dAdd1a protein is responsible for the targeting and maintenance of HP1a at the telomeric regions and provide additional evidence for a role of dAdd1b in the maintenance of the telomeric chromatin domain, a role that is independent of HP1a targeting to this region.
Discussion
Recent studies have described more than 100 HP1a interactors through different methodologies (Alekseyenko et al. 2014; Ryu et al. 2014; Swenson et al. 2016); furthermore, the importance of some of these interactors in the maintenance of HP1a immunolocalization-specific patterns has been proposed and recently assayed in cultured cells (Swenson et al. 2016). HP1a was described in the late 1980s as one of the major components of heterochromatin (James et al. 1989), and since that time, many HP1a variants have been described (Abel et al. 2009; Zhang et al. 2011). The main characteristic of these proteins is the presence of a chromodomain that is capable of recognizing primarily the di/tri-methylated state of H3K9. The research done on HP1a points towards a role for the protein as part of several protein complexes, each of which likely maintains different heterochromatic domains (Smothers and Henikoff 2001; Eissenberg and Elgin 2014).
The ATRX protein has been identified as a protein involved in the maintenance of heterochromatic regions, mainly pericentric and telomeric regions rich in repetitive sequences and transposable retro-elements (Ritchie et al. 2008; De La Fuente et al. 2011; Noh et al. 2016). Vertebrate ATRX has two important domains, the SNF2 (helicase/ATPase) domain and the ADD (H3K9me3/H3K4 unmodified recognition) domain. In Drosophila, these domains are separated and encoded by two different genes: the dadd1 gene encodes three protein isoforms derived from alternative splicing events, which conserve an ADD domain, and the dxnp gene, which encodes two protein isoforms that conserve an SNF2 domain. Our group has previously demonstrated that the dAdd1 and dXNP proteins interact physically and that they co-localize with HP1a in several heterochromatic regions, including the chromocenter (López-Falcón et al. 2014). The independent study of these proteins in Drosophila can help in understanding the different roles played by these domains.
The results obtained in the present study demonstrate the cooperation between the ADD and the SNF2 domains to maintain chromosomal stability and in the targeting of the HP1a protein to telomeric domains.
The dAdd1 and dXNP proteins are HP1a interactors but clearly some of the protein isoforms may play different roles than HP1a in the regulation of the different telomeric domains. The chromosomal aberrations observed in the dxnp and dadd1 mutants are not just restricted to telomeric fusions and are more generalized than the ones reported for HP1a. Mutations in Su(var)205 often give rise to telomeric associations (in salivary glands) and telomeric fusions (in mitotic chromosomes), but mutations in this protein also affect HTT array retrotransposon expression, leading to chromosomal instability; thus, HP1a regulates both the CAP and the HTT array (Perrini et al. 2004). The phenotypes observed for the dxnp and dadd1 alleles, in addition to the telomeric fusions, also include decondensations, in which the chromosome arms are longer, and centromeric fusions. These results are consistent with reports in the literature, as these proteins have a wide genomic distribution and have been shown to mediate the suppression of position effect variegation using pericentromeric reporters (Schneiderman et al. 2009; Emelyanov et al. 2010; López-Falcón et al. 2014). An interesting issue raised by our results is the fact that there seems to be a differential requirement for the specific isoforms of these proteins in selected heterochromatic compartments and in the prevalence of certain phenotypes analyzed. For instance, the dxnp 2 allele, which affects both dXNP isoforms, in combination with a null dadd1 background, presented more telomeric fusions than any other of the genotypes analyzed (see Fig. 1e and Suppl. Table 1); the same combination, but with the dxnp 3 allele, which affects only the long dXNP isoform (dXNPL), did not show as many telomeric fusions. These data suggest that the short (dXNPs) isoform has an important role in preventing these types of chromosomal aberrations. Additionally, the frequency of telomeric fusions observed in dadd1 and dxnp mutants is lower than the previously reported frequency when different allelic combinations of Su(var)205 gene were assayed (Fanti et al. 1998). One possible explanation for the differences observed in the frequency of telomeric fusions is provided by our chromatin immunoprecipitation experiments which demonstrate that flies lacking the dadd1 gene still have some levels of HP1a at the TAS-L region, providing evidence that at the telomeric heterochromatic domain the different regions (in this case the HTT array and the TAS regions) may have differential responses to the loss of dAdd1 proteins. When we analyzed the transcripts and copy numbers of the TART and Het-A retrotransposons, dAdd1 proteins appeared to have a major role in regulating the transposition and transcription of the TART and Het-A retrotransposons, as shown in Fig. 2(a, b). These phenotypes are only evident in combinations of the null dadd1 allele and the dxnp 2 allele. However, higher transcript levels do not always reflect on higher number of integrated copies as can be seen for the TART retrotransposon in the null dadd1 background (Fig. 2(a)). The lack of correlation between integrated copies and transcript abundance of this retrotransposon show that there must be another mechanism by which these proteins are maintaining the HTT array. Homologous recombination is the second mechanism by which Drosophila telomeres are maintained; interestingly, vertebrate ATRX has been shown to inhibit homologous recombination by sequestering the MRN complex (Clynes et al. 2015). Somatic mutations in vertebrate ATRX lead to an Alternative Lengthening of Telomeres mechanism (ALT) in certain types of human cancers (Napier et al. 2015; Ramamoorthy and Smith 2015). Hence, it is possible that the Drosophila dXNP proteins could also prevent homologous recombination, and this could explain in part why an additional mutation in the null dadd1 background in this case in dxnp is required to complete the retrotransposition mechanism, whereas the dAdd1 proteins, seem to have a role in regulating the transcription of these retro-elements. Also a differential regulation of transcription for the TART and Het-A elements has been previously described (Silva-Sousa et al. 2013). The authors demonstrate that the TART retrotransposon is much more sensible to mutations on the repressors DREF, Ken and TRF2 than the Het-A element leading to higher levels of expression and copy number integration. Our results have placed the dAdd1 proteins (along with DREF, Ken and TRF2) also as negative regulators of TART expression. Interestingly, mutations in these proteins (DREF, Ken and Trf2) also lead to retrotransposon integration, we can assume then, given our previous data on the interaction of DREF and dXNP that albeit loss of dAdd1 proteins, the levels of dXNP could possibly be maintained through its interaction with DREF and this prevents the integration of the retrotransposon copies into the genome (Valadez-Graham et al. 2012). It would be interesting in the future to address the interdependence of these proteins to bind Het-A and TART regulatory regions in the different mutant backgrounds to get a better understanding of the differential regulation of these retrotransposons.
Overall, these data lead us to propose that the SNF2 (helicase/ATPase) domain is required to prevent the integration of the retrotransposons, while the ADD domain, possibly through the targeting of HP1a (and other activities), maintains a repressed transcriptional state of the retrotransposon elements in somatic cells. According to our results, the ADD-containing proteins are essential to maintain HP1a at all of the chromosomes telomeres, while dXNP appears to affect only a subset of the telomeres analyzed. As mentioned previously, the ADD domain is able to recognize the H3K9me3 histone mark when it is in combination with the H3K4 without methylation (Iwase et al. 2011; Alekseyenko et al. 2014), an interesting feature is that the ADD domain can keep binding to this mark even if the H3Ser10 is phosphorylated, whereas the chromodomain of HP1a cannot (Noh et al. 2014). There are reports in the literature that demonstrate that the Jil-1 kinase is present at the HTT telomeric domain, acting as a transcriptional activator for the expression of the Het-A retrotransposon (Silva-Sousa et al. 2012). Our results place the dAdd1 and dXNP proteins as negative regulators of HTT retrotransposon expression. When we performed the rescue experiments with the dAdd1a or dAdd1b protein isoforms in a null dadd1 background it was interesting to observe that both proteins are localized at the telomeric regions. The dAdd1a signal is homogeneous at all the observed regions. However, only dAdd1a co-localized with HP1a at all the observed sites, as previously reported (Alekseyenko et al. 2014). An interesting observation is that in the chromosomes derived from the dAdd1a exogenous expression, the wild type enrichment of HP1a at the chromocenter is disrupted in some of the preparations observed. This result indicates the importance of maintaining the correct wild type levels of dAdd1 proteins. When we analyzed the localization of dAdd1b protein isoform we could see that this protein failed to target HP1a at the telomeres and at the chromosome arms, even though it does localizes to the telomeres and other regions at the chromosome arms. Another interesting feature is that dAdd1b seems to be enriched at the chromocenter and this does not perturb HP1a localization and enrichment at this region (Fig. 4(c, c″, d, d′)).
Thus, perhaps the dAdd1a protein is required to maintain correct levels of HP1a at the telomeres in regions where there is H3Ser10 phosphorylation, thereby maintaining the balance between an active transcriptional state (mediated by Jil-1) and a silenced state required to maintain correct levels of the retrotransposons and to avoid incorrect retrotransposition mechanisms. Whereas dAdd1b could also carry silencing activities independent of HP1a.
Based on these data, we propose a model in which the dXNP and dAdd1 proteins cooperate to maintain chromosomal stability by the transcriptional silencing of the retrotransposons of the HTT array and by promoting the correct targeting of HP1a to all of the telomeres (Fig. 5).
Model for the maintenance of chromosome stability mediated by dXNP and dAdd1. In somatic wild-type cells, dXNP, together with dAdd1a, cooperate to facilitate the localization of HP1a to telomeric regions, maintaining a silenced state. An RNAi mechanism is also involved in the maintenance of the silenced state (Ghildiyal et al. 2008). In the absence of dXNP and dAdd1, the HP1a protein is lost at all the chromosomes telomeres and the transcription of the HTT array is deregulated promoting the integration of retrotransposon copies into the genome. However, HP1a is not completely lost from the TAS regions. Our data also indicate that the RNAi mechanism is probably insufficient to maintain the silenced state in the dxnp and dadd1 mutants, demonstrating the predominant role of these proteins for the maintenance of a silenced state in somatic cells
Other dXNP and dAdd1-interacting proteins have been identified also in Drosophila telomeres, including DREF (Silva-Sousa et al. 2012; Valadez-Graham et al. 2012), CG1910 (Alekseyenko et al. 2014) and dSETDB1 (Cardoso et al. 1998; Gou et al. 2010). It is interesting that dSETDB1 is also present at Drosophila telomeres, as SETDB1 has been established to participate in the retrotransposon silencing through a DNA methylation-dependent mechanism in vertebrates. Other dAdd1 interactors include Bonus (BON) (Alekseyenko et al. 2014), which has been conserved through evolution; the family of proteins in vertebrates most related to this protein are the TIF1 families, which include TIF1 alpha, beta, and gamma. One of these families includes TIF1/TRIM28, which is also known as KAP1. In vertebrates, KAP1, Setdb1, and DAXX form a complex with ATRX that helps to maintain heterochromatin at the telomeres and IAP repeats (Sadic et al. 2015; Voon and Wong 2016). It would be important in the future to address whether these proteins participate in the maintenance of the silenced state of the HTT array along with the dXNP and dAdd1 proteins.
In conclusion, we have demonstrated that the dAdd1 and dXNP proteins cooperate to maintain genomic stability through at least two different mechanisms, preventing retrotransposon transcription and integration of the retrotransposons in the HTT array, as well as targeting HP1a to telomeric regions in somatic cells. Our study has also revealed the specific roles of the different dXNP and dAdd1 protein isoforms in the maintenance of the telomeric heterochromatic domain.
References
Abel J, Eskeland R, Raffa GD, et al (2009) Drosophila HP1c is regulated by an auto-regulatory feedback loop through its binding partner Woc
Alekseyenko AA, Gorchakov AA, Zee BM et al (2014) Heterochromatin-associated interactions of Drosophila HP1a with dADD1, HIPP1, and repetitive RNAs. Genes Dev 28:1445–1460. doi:10.1101/gad.241950.114
Antao JM, Mason JM, Dejardin J, Kingston RE (2012) Protein landscape at Drosophila melanogaster telomere-associated sequence repeats. Mol Cell Biol 32:2170–2182
Bassett AR, Cooper SE, Ragab A, Travers AA (2008) The chromatin remodelling factor dATRX is involved in heterochromatin formation
Biessmann H, Mason JM (2003) Telomerase-independent mechanisms of telomere elongation. Cell Mol Life Sci 60:2325–2333
Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118:401 LP–401415
Brower-Toland B, Findley SD, Jiang L et al (2007) Drosophila PIWI associates with chromatin and interacts directly with HP1a. Genes Dev 21:2300–2311
Canzio D, Chang EY, Shankar S et al (2011) Chromodomain-mediated oligomerization of HP1 suggests a nucleosome bridging mechanism for heterochromatin assembly. Mol Cell 41:67–81. doi:10.1016/j.molcel.2010.12.016
Capkova Frydrychova R, Biessmann H, Mason JM (2009) Regulation of telomere length in Drosophila. Cytogenet Genome Res 122(3–4):356–364
Cardoso C, Timsit S, Villard L et al (1998) Specific interaction between the XNP/ATR-X gene product and the SET domain of the human EZH2 protein. Hum Mol Genet 7:679–684
Clynes D, Jelinska C, Xella B et al (2015) Suppression of the alternative lengthening of telomere pathway by the chromatin remodelling factor ATRX. Nat Commun 6:7538
de Andrade HH, Reguly ML, Lehmann M (2004) Wing somatic mutation and recombination test. Methods Mol Biol 247:389–412
De La Fuente R, Baumann C, Viveiros MM (2011) Role of ATRX in chromatin structure and function: implications for chromosome instability and human disease. Reproduction 142:221–234. doi:10.1530/REP-10-0380
Deng Y, Chang S (2007) Role of telomeres and telomerase in genomic instability, senescence and cancer. Lab Investig. doi:10.1038/labinvest.3700673
Eissenberg JC, Elgin SCR (2014) HP1a: a structural chromosomal protein regulating transcription. Trends Genet 30:103–110. doi:10.1016/j.tig.2014.01.002
Elbarbary RA, Lucas BA, Maquat LE (2016) Retrotransposons as regulators of gene expression. Science 351:aac7247. doi:10.1126/science.aac7247
Emelyanov AV, Konev AY, Vershilova E, Fyodorov DV (2010) Protein complex of Drosophila ATRX/XNP and HP1a is required for the formation of pericentric beta-heterochromatin in vivo. J Biol Chem 285:15027–15037
Fanti L, Giovinazzo G, Berloco M, Pimpinelli S (1998) The heterochromatin protein 1 prevents telomere fusions in Drosophila telomeric fragments from other chromosomes or were involved in other rearrangement breakpoints. However, terminal deletions have since been recovered in Dro. Mol Cell 2:527–538
Gelbart ME, Larschan E, Peng S et al (2009) Drosophila MSL complex globally acetylates H4K16 on the male X chromosome for dosage compensation. Nat Struct Mol Biol 16:825–832
Ghildiyal M, Seitz H, Horwich MD, Li C, Du T, Lee S, Xu J, Kittler ELW, Zapp ML, Weng Z, Zamore PD (2008) Endogenous siRNAs derived from transposons and mRNAs in Drosophila somatic cells. Science 320(5879):1077–1081
Goodier JL (2016) Restricting retrotransposons: a review. Mob DNA 7:16. doi:10.1186/s13100-016-0070-z
Gou D, Rubalcava M, Sauer S, et al (2010) SETDB1 is involved in postembryonic DNA methylation and gene silencing in Drosophila
He Q, Kim H, Huang R et al (2015) The Daxx/Atrx complex protects tandem repetitive elements during DNA hypomethylation by promoting H3K9 trimethylation. Cell Stem Cell 17:273–286
Iwase S, Xiang B, Ghosh S et al (2011) ATRX ADD domain links an atypical histone methylation recognition mechanism to human mental-retardation syndrome. Nat Struct Mol Biol 18:769–776
James TC, Eissenberg JC, Craig C et al (1989) Distribution patterns of HP1, a heterochromatin-associated nonhistone chromosomal protein of Drosophila. Eur J Cell Biol 50:170–180
Lin X, Tirichine L, Bowler C (2012) Protocol: chromatin immunoprecipitation (ChIP) methodology to investigate histone modifications in two model diatom species. Plant Methods 8:48. doi:10.1186/1746-4811-8-48
Ling D, Salvaterra PM (2011) Robust RT-qPCR data normalization: validation and selection of internal reference genes during post-experimental data analysis. PLoS One 6:e17762. doi:10.1371/journal.pone.0017762
López-Falcón B, Meyer-Nava S, Hernández-Rodríguez B et al (2014) Characterization of the Drosophila group ortholog to the amino-terminus of the alpha-thalassemia and mental retardation X-linked (ATRX) vertebrate protein. PLoS One. doi:10.1371/journal.pone.0113182
López-Panadès E, Gavis ER, Casacuberta E Specific localization of the Drosophila telomere transposon proteins and RNAs, give insight in their behavior. Control Telomere Biol Organ. doi:10.1371/journal.pone.0128573
Lu X, Wontakal SN, Emelyanov AV et al (2009) Linker histone H1 is essential for Drosophila development, the establishment of pericentric heterochromatin, and a normal polytene chromosome structure. Genes Dev 23:452–465. doi:10.1101/gad.1749309
Mathieu N, Pirzio L, Freulet-Marrière M-A et al (2004) Telomeres and chromosomal instability. Cell Mol Life Sci. doi:10.1007/s00018-003-3296-0
Napier CE, Huschtscha LI, Harvey A et al (2015) ATRX represses alternative lengthening of telomeres. Oncotarget 6:16543–16558
Noh K-M, Maze I, Zhao D et al (2014) ATRX tolerates activity-dependent histone H3 methyl/phos switching to maintain repetitive element silencing in neurons. Proc Natl Acad Sci U S A:1–8
Noh KM, Allis CD, Li H (2016) Reading between the lines: “aDD”-ing histone and DNA methylation marks toward a new epigenetic “sum.”. ACS Chem Biol 11:554–563
Oikemus SR, McGinnis N, Queiroz-Machado J et al (2004) Drosophila atm/telomere fusion is required for telomeric localization of HP1 and telomere position effect. Genes Dev 18:1850–1861
Papaconstantinou M, Pepper AN, Wu Y et al (2010) Menin links the stress response to genome stability in Drosophila melanogaster. PLoS One 5(11):e14049
Perrini B, Piacentini L, Fanti L et al (2004) HP1 controls telomere capping, telomere elongation, and telomere silencing by two different mechanisms in Drosophila. Mol Cell. doi:10.1016/j.molcel.2004.06.036
Ramamoorthy M, Smith S (2015) Loss of ATRX suppresses resolution of telomere cohesion to control recombination in ALT cancer cells. Cancer Cell 28:357–369
Ritchie K, Seah C, Moulin J et al (2008) Loss of ATRX leads to chromosome cohesion and congression defects. J Cell Biol 180:315–324
Ryu HW, Lee DH, Florens L et al (2014) Analysis of the heterochromatin protein 1 (HP1) interactome in Drosophila. J Proteome 102:137–147
Sadic D, Schmidt K, Groh S et al (2015) Atrx promotes heterochromatin formation at retrotransposons. EMBO Rep 16:836–850. doi:10.15252/embr.201439937
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108
Schneiderman JI, Sakai A, Goldstein S, Ahmad K (2009) The XNP remodeler targets dynamic chromatin in Drosophila. Proc Natl Acad Sci U S A 106:14472–14477
Scott MP, Weiner AJ, Hazelrigg TI, Polisky BA, Pirrotta V, Scalenghe F, Kaufman TC (1983) The molecular organization of the Antennapedia locus of drosophila. Cell 35(3):763–776
Silva-Sousa R, López-Panadès E, Piñeyro D, Casacuberta E (2012) The chromosomal proteins JIL-1 and Z4/Putzig regulate the telomeric chromatin in Drosophila melanogaster. PLoS Genet. doi:10.1371/journal.pgen.1003153
Silva-Sousa R, Varela MD, Casacuberta E (2013) The Putzig partners DREF, TRF2 and KEN are involved in the regulation of the Drosophila telomere retrotransposons, HeT-A and TART. Mob DNA 4:18. doi:10.1186/1759-8753-4-18
Singh AK, Lakhotia SC (2016) The hnRNP A1 homolog Hrb87F/Hrp36 is important for telomere maintenance in Drosophila melanogaster. Chromosoma 125:373–388
Siriaco GM, Cenci G, Haoudi A et al (2002) Telomere elongation (Tel), a new mutation in Drosophila melanogaster that produces long telomeres. Genetics 160:235–245
Smothers JF, Henikoff S (2001) The hinge and chromo shadow domain impart distinct targeting of HP1-like proteins. Mol Cell Biol 21:2555–2569
Sullivan W, Ashburner M, Hawley RS (2000) Drosophila protocols. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Swenson JM, Colmenares SU, Strom AR et al (2016) The composition and organization of Drosophila heterochromatin are heterogeneous and dynamic. elife 5:e16096. doi:10.7554/eLife.16096
Valadez-Graham V, Yoshioka Y, Velazquez O et al (2012) XNP/dATRX interacts with DREF in the chromatin to regulate gene expression. Nucleic Acids Res. doi:10.1093/nar/gkr865
Voon HPJ, Wong LH (2016) New players in heterochromatin silencing: histone variant H3.3 and the ATRX/DAXX chaperone. Nucleic Acids Res 44:1496–1501. doi:10.1093/nar/gkw012
Walter MF, Biessmann MR, Benitez C, Török T, Mason JM, Biessmann H (2007) Effects of telomere length in Drosophila melanogaster on life span, fecundity, and fertility. Chromosoma 116(1):41–51
Williams BC, Karr TL, Montgomery JM, Goldberg ML (1992) The Drosophila l(1)zw10 gene product, required for accurate mitotic chromosome segregation, is redistributed at anaphase onset. J Cell Biol 118:759 LP–759773
Wong LH, McGhie JD, Sim M et al (2010) ATRX interacts with H3.3 in maintaining telomere structural integrity in pluripotent embryonic stem cells. Genome Res 20:351–360. doi:10.1101/gr.101477.109
Zhang D, Wang D, Sun F (2011) Drosophila melanogaster Heterochromatin protein HP1b plays important roles in transcriptional activation and development. Chromosoma 120:97–108
Acknowledgements
We thank Dr. James Mason for providing the GIII strain. We also thank Silvia Meyer for the construction of the plasmids used. We would like to thank Benjamín Hernández for his participation at the beginning of this work. We also thank Claudia Mónica Flores Loyola for her participation in the LOH assay. J.C. was supported by a Masters degree scholarship from the Consejo Nacional de Ciencia y Tecnología (CONACyt, 404495). J.M.M-M was supported by a DGAPA-UNAM postdoctoral fellowship. We also thank Arturo Pimentel, Andrés Saralegui and Dr. Chris Wood from the LMNA for the advice on the use of the microscopes. We thank Dr. Martha Vazquez for her valuable comments on this work. This work was supported by grants from CONACyT 219673 and DGAPA UNAM number IN200315 to MZ and grant 177393 from (CONACyT) and grants IN204915 and IA200613 from (PAPIIT-UNAM) to VV-G.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Materials
ESM 1
(PDF 8954 kb)
Rights and permissions
About this article
Cite this article
Chavez, J., Murillo-Maldonado, J.M., Bahena, V. et al. dAdd1 and dXNP prevent genome instability by maintaining HP1a localization at Drosophila telomeres. Chromosoma 126, 697–712 (2017). https://doi.org/10.1007/s00412-017-0634-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-017-0634-9