Abstract
Dnmt2 is a member of the animal DNA methyltransferase family of enzymes. While the role of other Dnmt proteins has been extensively characterized, comparably little is known about Dnmt2. This is surprising because Dnmt2 is the most widely conserved Dnmt protein, with homologues in protists, plants, fungi, and animals. In this review, we discuss the evidence supporting the seemingly contradictory roles of Dnmt2 in both DNA and RNA methylation. New studies are uncovering the enzymatic mechanisms that mediate these activities and also provide first insights into the biological functions of Dnmt2. Lastly, we also discuss observations that suggest a possible role for Dnmt2 in human health and disease, which further emphasizes the importance of defining Dnmt2-modulated cellular pathways in future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Dnmt2 was originally identified in a search for de novo DNA methyltransferases. The initial characterization of Dnmt1, the major DNA methyltransferase activity of mammalian cells, had revealed a preference for hemimethylated substrates consistent with a specialized function in the maintenance of DNA methylation patterns (Goll and Bestor 2005). Because it was recognized that DNA methylation patterns need to be established on unmethylated DNA during early development, the identification of de novo DNA methyltransferases had become an important topic in the field of epigenetics. Dnmt2 was identified through the analysis of expressed sequence tags that showed homologies with bacterial DNA methyltransferases (Okano et al. 1998; Yoder and Bestor 1998). But even though the strict sequence conservation of catalytic motifs clearly predicted a DNA methyltransferase activity, no such activity could be found in initial biochemical assays, and no DNA methylation changes could be observed in experiments with Dnmt2 mutant mouse ES cells (Okano et al. 1998; Yoder and Bestor 1998). However, the extensive conservation of Dnmt2 as a single copy gene in eukaryotes with homologues in dozens of protist, plant, fungal, and animal genomes (Fig. 1) is indicative of an important role for Dnmt2 proteins.
Evolutionary conservation of Dnmt2 proteins in protists, plants, fungi, and animals. A BLAST (Basic Local Alignment Search Tool) search with the human Dnmt2 protein sequence retrieved 108 protein sequences with extensive similarities. These sequences represent Dnmt2 orthologs in 65 different species
Dnmt2 as a DNA methyltransferase
The conservation of the catalytic (cytosine-5) DNA methyltransferase motifs strongly suggests a DNA methyltransferase activity for Dnmt2 proteins. This conservation was first recognized in the pmt1 gene product from Schizosaccharomyces pombe (Wilkinson et al. 1995). However, no catalytic DNA methyltransferase activity could be detected for this protein, which was attributed to the insertion of a serine residue into a critical proline-cysteine dipeptide that is essential for DNA methyltransferase activity in other enzymes. A later study indicated that significant site-specific DNA methyltransferase activity could be restored by removal of the inserted serine residue (Pinarbasi et al. 1996). However, these results have not been confirmed by independent studies.
Of note, the strong similarities between Dnmt2 and DNA methyltransferases also extend to the structural level since human DNMT2 has been shown to have substantial structural similarity with the bacterial DNA methyltransferase M.HhaI (Dong et al. 2001). The same study showed that Dnmt2 can form denaturant-resistant protein-DNA complexes, which suggested that Dnmt2 can bind to DNA. As a consequence, the DNA methyltransferase activity of Dnmt2 has now been analyzed in various experimental settings and model organisms. Shotgun bisulfite sequencing of Drosophila genomic DNA suggested that Dnmt2 methylates isolated cytosine residues without any recognizable target sequence specificity (Lyko et al. 2000). Similarly, purified recombinant human Dnmt2 methylated DNA substrates at about one out of 250 cytosine residues in a nonprocessive manner and with little or no sequence specificity (Hermann et al. 2003) To explain the low DNA methyltransferase activity and processivity, the requirement for a specific cofactor, as well as the presence of a sterically unfavorable tyrosine residue in the target recognition domain of Dnmt2 have been discussed (Goll and Bestor 2005). However, more recent bisulfite sequencing analyses have now suggested a prominent locus-specific DNA methyltransferase activity of Dnmt2 on selected retroelements in Dictyostelium and Drosophila (Kuhlmann et al. 2005; Phalke et al. 2009).
To resolve the question whether Dnmt2 distributively methylates isolated cytosine residues or processively methylates specific target sequences, robust in vivo assays for the reproducible detection of Dnmt2-dependent DNA methylation will have to be established. In addition, genomic DNA methylation patterns need to be characterized in “Dnmt2 only” model systems, like Drosophila and Dictyostelium that contain a Dnmt2 homologue, but no homologue of the classical DNA methyltransferases Dnmt1 or Dnmt3. In this context, it is important to notice that the presence of a Dnmt2 homologue does not necessarily predict the presence of DNA methylation. The genomes of nematodes, for example, are generally considered to be unmethylated, and Caenorhabditis elegans belongs to a small group of organisms that does not contain any Dnmt-like gene. However, a Dnmt2 gene is present in the satellite nematode model Pristionchus pacificus, but no indications for DNA methylation could be observed (Gutierrez and Sommer 2004). This suggests that Dnmt2 has additional functions not related to DNA methylation.
The tRNA methyltransferase activity of Dnmt2
The possibility that Dnmt2 may have additional enzymatic activities has now been confirmed experimentally. The use of recombinant Dnmt2 protein and RNA preparations from various model systems uncovered a prominent transfer RNA (tRNA) methyltransferase activity of the enzyme (Goll et al. 2006). This was an important finding because it demonstrated Dnmt2 activity on a specific substrate, cytosine 38 of transfer RNAAsp. However, the study also suggested that Dnmt2 activity might be limited to this substrate, which represented the core argument for the conclusion that Dnmt2 is a highly specific RNA methyltransferase rather than a DNA methyltransferase (Goll et al. 2006). Importantly, more recent results (see below) indicate that the enzymatic specificity of Dnmt2 is probably not as high as initially concluded.
It was initially suggested that in vitro transcripts of tRNAAsp would not be methylated by Dnmt2, presumably, because they lack other tRNA modifications required for Dnmt2 activity (Goll et al. 2006). This notion has now been rejected by two studies demonstrating detectable methylation of in vitro transcribed tRNAAsp substrates (Hengesbach et al. 2008; Jurkowski et al. 2008). Several other tRNAs are known to be methylated at C38 by unknown RNA 5mC methyltransferases (Sprinzl and Vassilenko 2005), which raises the possibility that Dnmt2 activity is not limited to tRNAAsp. Indeed, recent experiments in Drosophila suggest that Dnmt2 is responsible for C38 methylation at additional tRNAs (M.S. and F.L., unpublished data). Transfer RNA methylation has been implied in the regulation of tRNA folding and stability (Alexandrov et al. 2006; Helm 2006). Misfolded and/or less stable tRNAs might, thus, impact on the rate and fidelity of protein synthesis, especially under stress conditions or during aging. In agreement with wider substrate specificity, the analysis of tRNA preparations from wild type and Dnmt2 mutant Arabidopsis and zebrafish also revealed Dnmt2-dependent methylation of RNA molecules that appeared to be smaller than tRNA (Goll et al. 2006; Rai et al. 2007). It will be extremely interesting to explore the possibility whether Dnmt2 methylates other classes of RNA in vivo, and the recent development of RNA bisulfite sequencing for the sequence-specific methylation analysis of RNAs (Schaefer et al. 2009b) should facilitate the identification of novel Dnmt2 substrate RNAs.
Dnmt2 utilizes a DNA methyltransferase mechanism for RNA methylation
In light of the data discussed above it is clear that Dnmt2 family members represent unusual enzymes. Based on the presence of catalytic sequence motifs, the protein sequence predicts a DNA methyltransferase activity for Dnmt2. On the other hand, the tRNA methyltransferase activity of Dnmt2 is very robust and easily detectable both in vitro and in vivo (Goll and Bestor 2005; Hengesbach et al. 2008; Jurkowski et al. 2008; Schaefer et al. 2009b). Remarkably, however, Dnmt2 is not a canonical RNA methyltransferase since it does not share any significant sequence similarity with known RNA methyltransferases. Elucidating the catalytic mechanism(s) used by Dnmt2 will, therefore, be critical for understanding the substrate specificity and the biological role of this enzyme. Importantly, the robust RNA methyltransferase activity of Dnmt2 also raises the question whether other DNA methyltransferases have the ability to methylate RNAs. A prominent observation for further investigation is the cytoplasmic form of the maintenance DNA methyltransferase Dnmt1 in postmitotic neurons (Inano et al. 2000). Furthermore, it has also been shown that an oocyte form of Dnmt1 localizes to the cytoplasm during early stages of mouse embryonic development (Howell et al. 2001; Mertineit et al. 1998). It will be interesting to identify Dnmt1-associated RNAs and to test whether these RNAs can be methylated by Dnmt1.
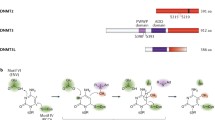
Dnmt2 has been shown to methylate both DNA and tRNAAsp at the cytosine-5 position (Goll et al. 2006; Hermann et al. 2003). Both modifications depend on a common methyl-group donor (S-adenosyl-L-methionine), but are normally catalyzed by different enzyme families with different enzymatic mechanisms. Most notably, different amino acid side chains are used for the protonation of the substrate cytosine ring (aspartic acid for RNA methylation and glutamic acid for DNA methylation; Fig. 2). In order to identify the catalytic amino acid for the protonation of C38 in tRNAAsp, a recent study has examined the effects of a number of cysteine point mutations in the human Dnmt2 enzyme (Jurkowski et al. 2008). The results showed that mutation of C24, C140, C287, or C292 did not abolish the tRNAAsp methylation activity. E119, however, the glutamic acid residue required for the protonation of cytosine rings in DNA substrates, was found to be essential for the methylation of tRNAAsp (Fig. 2). These results strongly suggested that Dnmt2 utilizes a DNA methyltransferase mechanism for the methylation of tRNAAsp and support the notion that Dnmt2 represents a noncanonical enzyme of the Dnmt family, rather than a canonical DNA or RNA methyltransferase (Jeltsch et al. 2006).
Enzymatic mechanisms of RNA methylation, DNA methylation, and Dnmt2-mediated methylation of tRNAAsp. While canonical 5mC:RNA methyltransferases use the DAPC peptide in motif IV for protonation and the TCS peptide in motif VI for nucleophilic attack on C-6, 5mC:DNA methyltransferases use the ENV peptide in motif VI for protonation and the PCQ peptide in motif IV for catalysis. Mutational analysis (Jurkowski et al. 2008) has shown that Dnmt2 utilizes a DNA methyltransferase-like mechanism involving the ENV and PCQ motifs for the methylation of tRNAAsp. The function of the Dnmt2-specific CFT motif has not been established yet
The subcellular localization pattern of Dnmt2 suggests diverse biological functions
The subcellular localization of an enzyme can be an important indicator for its biological function. DNA methyltransferases, for example, are predominantly nuclear enzymes, consistent with their role in the modification of a nuclear substrate (Bachman et al. 2001; Easwaran et al. 2004). The localization of RNA molecules, however, is more dynamic and differs between specific RNAs and even between individual maturation steps. Also, tRNA maturation has been shown to involve a variety of steps in the nucleolus, the nucleoplasm and in the cytoplasm, and the localization of the modifying enzymes appears to be as complex as that of the RNAs themselves (Hopper and Phizicky 2003).
Like for other highly conserved proteins, it has been notoriously difficult to obtain high-quality antibodies against Dnmt2. Nevertheless, evidence has been provided for both a nuclear and a cytoplasmic distribution of Dnmt2 in various model systems. The results have been interpreted differently, depending on the study context. Earlier reports that focused on the DNA methyltransferase activities of Dnmt2 have underscored the nuclear localization of the enzyme (Fisher et al. 2004; Kuhlmann et al. 2005; Kunert et al. 2003), while later reports that focused on the RNA methyltransferase activity of Dnmt2 have highlighted the cytoplasmic localization of the protein (Goll et al. 2006; Rai et al. 2007). Remarkably, the experimental data look similar in all studies and can be interpreted as both nuclear and cytoplasmic localization. This notion was later confirmed by biochemical fractionation of protein extracts from Drosophila embryos that revealed Dnmt2 protein both in nuclear and in cytoplasmic fractions (Schaefer et al. 2008). The same study also indicated that nuclear Dnmt2 is associated with the nuclear matrix, which had also been observed for Entamoeba Dnmt2 (Banerjee et al. 2005). The function of the nuclear matrix association is presently unknown, but the association might explain the difficulties in biochemically isolating DNA (or RNA) substrates of Dnmt2, since matrix associated nucleic acids are commonly lost during standard nucleic acid purification procedures.
The biological functions of Dnmt2
Various laboratories have established Dnmt2 mutants in order to facilitate the functional characterization of this enzyme (Table 1). Pmt1 mutant fission yeast were reported to be indistinguishable from wild type strains, which was considered to be consistent with the notion that the serine insertion into the catalytic PC dipeptide renders the protein enzymatically inactive (Wilkinson et al. 1995). A targeted deletion of Dnmt2 in mouse ES cells failed to reveal any detectable phenotypes in these cells (Okano et al. 1998). Similarly, a double-stranded RNA knockdown of Dnmt2 in Drosophila embryos did not cause any detectable defects (Kunert et al. 2003), which was later confirmed by the analysis of flies with a genetically mutated Dnmt2 gene (Goll et al. 2006). The same study reported that Dnmt2 mutant mice and plants are viable and fertile (Goll et al. 2006). On the other hand, Dictyostelium strains, lacking Dnmt2, showed minor developmental phenotypes (Katoh et al. 2006). Surprisingly, a detailed analysis of Dnmt2 mutant zebrafish that had been generated by the injection of antisense morpholino oligonucleotides uncovered lethal differentiation defects in the retina, liver, and brain (Rai et al. 2007). These phenotypes could be rescued by transgenic expression of cytoplasmic Dnmt2, but not by transgenic expression of nuclear Dnmt2, thus, suggesting that the observed defects were caused by effects of Dnmt2 on cytoplasmic substrates. While these studies suggest that mutations of Dnmt2 are not detrimental to the viability and fertility of most laboratory organisms (Tab. 1), it is important to notice that the mutant phenotypes of Dnmt2 flies and mice have not been analyzed in detail yet. This will be an important aspect of future research, in order to identify the cellular pathways that are modulated by Dnmt2.
The specific role of Dnmt2 in epigenetic silencing pathways has recently been analyzed in Drosophila (Phalke et al. 2009). The authors used variegated P-element insertions across the Drosophila genome to show that Dnmt2 is required for epigenetic silencing of retrotransposons and subtelomeric repeats. In principle, these observations can be explained by effects of Dnmt2 on DNA or on RNA, but RNA-mediated effects have not been investigated by the authors. Results obtained with a modified bisulfite sequencing protocol using a short deamination time of only 1 h suggested that the majority of cytosine residues in the long terminal repeats of Invader4 retrotransposons are substantially methylated in wild type embryos, but almost completely unmethylated in Dnmt2 mutant embryos (Phalke et al. 2009). Even though the study did not provide any rescue data, the results argue for a role of Dnmt2 in the DNA methylation of Invader4 during early embryogenesis. Importantly, the authors also offered a mechanistic explanation for how epigenetic silencing of Invader4 elements can be maintained in the absence of DNA methylation. The presented genetic data imply the Suv4-20/Hmt4-20 histone H4 lysine 20 methyltransferase (Sakaguchi et al. 2008) in the maintenance of retroelement silencing initiated by Dnmt2-dependent DNA methylation. In this context, another striking finding is the strong reduction of histone H4 lysine 20 trimethylation in Dnmt2 mutants during all stages analyzed suggesting that at least in Drosophila, the bulk of this histone modification is depending on Dnmt2 function. These findings are intriguing because they clearly imply Dnmt2 in the epigenetic silencing of one retroelement in Drosophila. It remains to be shown, however, whether the DNA of other retrotransposons is similarly modified by Dnmt2 and whether Dnmt2-mediated DNA methylation of retroelements can be confirmed in other model organisms.
It should be noted that other studies have suggested a role of Dnmt2 in fundamentally different cellular pathways (Fig. 3). The aforementioned morpholino-induced knock-out phenotypes in zebrafish (Rai et al. 2007) point to a tissue- or organ-specific role of cytoplasmic Dnmt2 during fish development. It will be of great importance to identify the RNAs affected by Dnmt2 depletion in specific zebrafish tissues in order to explore the possibility that small regulatory RNAs can be modulated by Dnmt2 dependent methylation. In addition, overexpresison of Dnmt2 in Drosophila has been shown to extend the lifespan of flies, and the underlying mechanism has been linked to oxidative stress resistance (Lin et al. 2005). Similarly, overexpression of Dnmt2 in Entamoeba increased the resistance to hydrogen peroxide and induced the expression of heat shock proteins (Fisher et al. 2006). The use of proteomic methods for the identification of differentially expressed proteins also indicated that several metabolic enzymes became upregulated in Dnmt2-overexpressing amoebas (Fisher et al. 2006). The latter findings are more consistent with a role of Dnmt2 in the regulation of metabolic pathways through RNA methylation than with a role in epigenetic regulation by DNA methylation. Additional studies are required to further define the biological pathway(s) regulated by Dnmt2.
A role for Dnmt2 in human health and disease?
Both DNA methylation and RNA methylation play important roles in human health and disease. DNA methylation is a critical factor in epigenetic gene regulation and in the etiology of human tumors (Esteller 2008). Transfer RNA methylation has been linked to the regulation of tRNA functionality (Helm 2006) and by implication, to human metabolic disorders. In addition, the substantial evolutionary conservation of Dnmt2 also suggests that the enzyme modulates cellular pathways with important biological functions. However, the absence of strong mutant phenotypes in all but one model organism has so far hindered the identification of Dnmt2-associated pathways. Whether the tissue differentiation defects observed in zebrafish (Rai et al. 2007) are caused by the disruption of tRNA associated pathways, by effects on other RNAs or by the deregulation of nucleic acid-independent pathways will, therefore, be important for characterizing the pathobiological relevance of Dnmt2.
In an alternative approach, a recent study has made use of the fact that Dnmt2 can be inhibited by the established DNMT inhibitor and FDA-approved anticancer drug azacytidine (Schaefer et al. 2009a). It was shown that azacytidine causes demethylation of tRNAAsp in human cancer cells and that the drug decreases the metabolic activity of cancer cells. As such, monitoring the Dnmt2 activity by tRNAAsp methylation analysis represents a promising candidate biomarker for azacytidine therapy. In addition, the results also suggest that inhibition of RNA methylation might have an antiproliferative effect in cancer cells and thus, indicate an association of Dnmt2 with cancer relevant pathways. More detailed analyses of the Dnmt2 mutant phenotype in mice will be critically important for understanding the role of Dnmt2 in human cancers.
References
Alexandrov A, Chernyakov I, Gu W, Hiley SL, Hughes TR, Grayhack EJ, Phizicky EM (2006) Rapid tRNA decay can result from lack of nonessential modifications. Mol Cell 21:87–96
Bachman KE, Rountree MR, Baylin SB (2001) Dnmt3a and Dnmt3b are transcriptional repressors that exhibit unique localization properties to heterochromatin. J Biol Chem 276:32282–32287
Banerjee S, Fisher O, Lohia A, Ankri S (2005) Entamoeba histolytica DNA methyltransferase (Ehmeth) is a nuclear matrix protein that binds EhMRS2, a DNA that includes a scaffold/matrix attachment region (S/MAR). Mol Biochem Parasitol 139:91–97
Dong A, Yoder JA, Zhang X, Zhou L, Bestor TH, Cheng X (2001) Structure of human DNMT2, an enigmatic DNA methyltransferase homolog that displays denaturant-resistant binding to DNA. Nucleic Acids Res 29:439–448
Easwaran HP, Schermelleh L, Leonhardt H, Cardoso MC (2004) Replication-independent chromatin loading of Dnmt1 during G2 and M phases. EMBO Rep 5:1181–1186
Esteller M (2008) Epigenetics in cancer. N Engl J Med 358:1148–1159
Fisher O, Siman-Tov R, Ankri S (2004) Characterization of cytosine methylated regions and 5-cytosine DNA methyltransferase (Ehmeth) in the protozoan parasite Entamoeba histolytica. Nucleic Acids Res 32:287–297
Fisher O, Siman-Tov R, Ankri S (2006) Pleiotropic phenotype in Entamoeba histolytica overexpressing DNA methyltransferase (Ehmeth). Mol Biochem Parasitol 147:48–54
Goll MG, Bestor TH (2005) Eukaryotic cytosine methyltransferases. Annu Rev Biochem 74:481–514
Goll MG, Kirpekar F, Maggert KA, Yoder JA, Hsieh CL, Zhang X, Golic KG, Jacobsen SE, Bestor TH (2006) Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science 311:395–398
Gutierrez A, Sommer RJ (2004) Evolution of dnmt-2 and mbd-2-like genes in the free-living nematodes Pristionchus pacificus, Caenorhabditis elegans and Caenorhabditis briggsae. Nucleic Acids Res 32:6388–6396
Helm M (2006) Post-transcriptional nucleotide modification and alternative folding of RNA. Nucleic Acids Res 34:721–733
Hengesbach M, Meusburger M, Lyko F, Helm M (2008) Use of DNAzymes for site-specific analysis of ribonucleotide modifications. RNA 14:180–187
Hermann A, Schmitt S, Jeltsch A (2003) The human Dnmt2 has residual DNA-(cytosine-C5) methyltransferase activity. J Biol Chem 278:31717–31721
Hopper AK, Phizicky EM (2003) tRNA transfers to the limelight. Genes Dev 17:162–180
Howell CY, Bestor TH, Ding F, Latham KE, Mertineit C, Trasler JM, Chaillet JR (2001) Genomic imprinting disrupted by a maternal effect mutation in the Dnmt1 gene. Cell 104:829–838
Inano K, Suetake I, Ueda T, Miyake Y, Nakamura M, Okada M, Tajima S (2000) Maintenance-type DNA methyltransferase is highly expressed in post-mitotic neurons and localized in the cytoplasmic compartment. J Biochem 128:315–321
Jeltsch A, Nellen W, Lyko F (2006) Two substrates are better than one: dual specificities for Dnmt2 methyltransferases. Trends Biochem Sci 31:306–308
Jurkowski TP, Meusburger M, Phalke S, Helm M, Nellen W, Reuter G, Jeltsch A (2008) Human DNMT2 methylates tRNAAsp molecules using a DNA methyltransferase-like catalytic mechanism. RNA 14:1663–1670
Katoh M, Curk T, Xu Q, Zupan B, Kuspa A, Shaulsky G (2006) Developmentally regulated DNA methylation in Dictyostelium discoideum. Eukaryot Cell 5:18–25
Kuhlmann M, Borisova BE, Kaller M, Larsson P, Stach D, Na J, Eichinger L, Lyko F, Ambros V, Soderbom F, Hammann C, Nellen W (2005) Silencing of retrotransposons in Dictyostelium by DNA methylation and RNAi. Nucleic Acids Res 33:6405–6417
Kunert N, Marhold J, Stanke J, Stach D, Lyko F (2003) A Dnmt2-like protein mediates DNA methylation in Drosophila. Development 130:5083–5090
Lin MJ, Tang LY, Reddy MN, Shen CK (2005) DNA methyltransferase gene dDnmt2 and longevity of Drosophila. J Biol Chem 280:861–864
Lyko F, Ramsahoye BH, Jaenisch R (2000) DNA methylation in Drosophila melanogaster. Nature 408:538–540
Mertineit C, Yoder JA, Taketo T, Laird DW, Trasler JM, Bestor TH (1998) Sex-specific exons control DNA methyltransferase in mammalian germ cells. Development 125:889–897
Okano M, Xie S, Li E (1998) Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat Genet 19:219–220
Phalke S, Nickel O, Walluscheck D, Hortig F, Reuter G (2009) Epigenetic control of retrotransposon silencing and telomere integrity in somatic cells of Drosophila depends on the cytosine 5 methyltransferase DNMT2. Nat Genet 41:696–702
Pinarbasi E, Elliott J, Hornby DP (1996) Activation of a yeast pseudo DNA methyltransferase by deletion of a single amino acid. J Mol Biol 257:804–813
Rai K, Chidester S, Zavala CV, Manos EJ, James SR, Karpf AR, Jones DA, Cairns BR (2007) Dnmt2 functions in the cytoplasm to promote liver, brain, and retina development in zebrafish. Genes Dev 21:261–266
Sakaguchi A, Karachentsev D, Seth-Pasricha M, Druzhinina M, Steward R (2008) Functional characterization of the Drosophila Hmt4–20/Suv4–20 histone methyltransferase. Genetics 179:317–322
Schaefer M, Hagemann S, Hanna K, Lyko F (2009a) Azacytidine inhibits RNA methylation at DNMT2 target sites in human cancer cell lines. Cancer Res:in press
Schaefer M, Pollex T, Hanna K, Lyko F (2009b) RNA cytosine methylation analysis by bisulfite sequencing. Nucleic Acids Res 37:e12
Schaefer M, Steringer JP, Lyko F (2008) The Drosophila cytosine-5 methyltransferase Dnmt2 is associated with the nuclear matrix and can access DNA during mitosis. PLoS ONE 3:e1414
Sprinzl M, Vassilenko KS (2005) Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res 33:D139–140
Wilkinson CR, Bartlett R, Nurse P, Bird AP (1995) The fission yeast gene pmt1+ encodes a DNA methyltransferase homologue. Nucleic Acids Res 23:203–210
Yoder JA, Bestor TH (1998) A candidate mammalian DNA methyltransferase related to pmt1p of fission yeast. Hum Mol Genet 7:279–284
Acknowledgments
The authors would like to acknowledge their support from the Deutsche Forschungsgemeinschaft (SPP1129 and FOR1082).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E.A. Nigg
Rights and permissions
About this article
Cite this article
Schaefer, M., Lyko, F. Solving the Dnmt2 enigma. Chromosoma 119, 35–40 (2010). https://doi.org/10.1007/s00412-009-0240-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-009-0240-6