Abstract
Centromeric protein F (CENP-F) is a 367-kDa human kinetochore protein that was identified a decade ago, but its function was only recently revealed by studies that used small interfering RNA to deplete the protein from cells. All studies showed that CENP-F is important for chromosome alignment, but these studies differed as to whether CENP-F is important to the mitotic checkpoint. We report here that CENP-F is essential for cells to sustain a prolonged mitotic delay in response to unattached kinetochores. Cells depleted of CENP-F exit mitosis in the presence of defective kinetochore attachments resulting from treatment with nocodazole, or the depletion of kinetochore proteins CENP-E and hSgo1. Kinetochores depleted of CENP-F exhibited a reduction in the amounts of the mitotic checkpoint proteins Mad1, Mad2, hBUBR1, hBUB1, and hMps1. We postulate that CENP-F is not an essential component of the mitotic checkpoint but facilitates the duration of the mitotic delay. Separately, we show that CENP-F is a novel microtubule-binding protein that possesses two microtubule-binding domains at opposite ends of the molecule. The C-terminal microtubule-binding domain was found to stimulate microtubule polymerization in vitro. These activities provide a biochemical explanation for how CENP-F contributes to kinetochore attachments in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Centromeric protein F (CENP-F) is a 367-kDa kinetochore protein that was originally identified through the use of a human autoimmune serum (Casiano et al. 1993; Liao et al. 1995; Rattner et al. 1993) and independently isolated as Mitosin in a screen for proteins that interacted with the retinoblastoma tumor suppressor (Zhu et al. 1995b). CENP-F exhibits a very dynamic localization pattern whereby it undergoes transitions from the nuclear matrix to the nuclear rim and then to kinetochores during the G2 phase of the cell cycle (Liao et al. 1995; Rattner et al. 1993). Kinetochore localization persists until the onset of anaphase when it relocalizes to the spindle midzone. Immuno-electron microscopy studies showed that CENP-F is concentrated at the outer kinetochore and may extend into the fibrous corona (Rattner et al. 1993; Zhu et al. 1995a). The temporal pattern of CENP-F localization provided the earliest molecular evidence that mammalian kinetochores are assembled in a step-wise fashion that initiates prior to the onset of mitosis (Liao et al. 1995).
Structure and function studies revealed that the kinetochore-targeting domain of CENP-F is located near the C terminus (Zhu et al. 1995a), and its localization is sensitive to farneslytransferase inhibitors. This sensitivity is consistent with the fact that CENP-F is farnesylated and that mutation of its C-terminal farnesylation site blocks kinetochore targeting (Ashar et al. 2000; Hussein and Taylor 2002). In addition to the importance of farnesylation, the assembly of CENP-F onto kinetochores has been shown to depend on Bub1 kinase (Johnson et al. 2004), CENP-I (Liu et al. 2003), Nup358/RanBP2 (Joseph et al. 2004; Salina et al. 2003), and Sgt1 (Steensgaard et al. 2004). Even though cells depleted of these proteins exhibited defective kinetochore functions, it was not possible to ascribe them solely to CENP-F, as the localization of other kinetochore proteins was also affected.
Recent studies have shown that CENP-F is a target of the FoxM1 transcription factor (Laoukili et al. 2005). Cells (the human osteosarcoma cell line, U2OS) depleted of FoxM1 exhibited mitotic defects that were due partly to the loss of CENP-F, as direct depletion of CENP-F also led to similar defects in chromosome segregation and in the mitotic checkpoint. A separate study using Hela cells also showed that loss of CENP-F disrupted kinetochore attachments (Yang et al. 2005). Kinetochores depleted of CENP-F were shown to have reduced levels of CENP-E and p150 glued, microtubule-binding proteins that contribute to proper kinetochore attachments (Echeverri et al. 1996; McEwen et al. 2001). Contrary to a previous study that showed that CENP-F is important for the mitotic checkpoint, this study showed that cells depleted of CENP-F were delayed in mitosis for approximately 2 h and then died without exiting mitosis (Yang et al. 2005). More recent studies (Holt et al. 2005; Bomont et al. 2005), reported that CENP-F was not essential for the mitotic checkpoint in Hela cells. As with earlier studies, these studies also concluded that CENP-F was important for establishing proper kinetochore attachments. In addition, Holt et al. reported that a small number of cells depleted of CENP-F contained separated sister chromatids. On the other hand, Bomont et al. demonstrated that there are two phenotypes for cells depleted of CENP-F. A minority of CENP-F-depleted cells showed a defect in kinetochore assembly, provoking massive chromosome mis-segregation. In contrast, the majority of CENP-F-deficient cells exhibit a strong mitotic delay with reduced tension between kinetochores of aligned chromatids and decreased stability of kinetochore microtubules. The delay was mediated by the mitotic checkpoints, as some of the CENP-F-depleted kinetochores continuously recruited Mad1.
To further our mechanistic understanding by which CENP-F contributes to kinetochore attachments, as well as to clarify its role in the mitotic checkpoint, we have characterized its biochemical properties and analyzed its importance during mitosis. We report here that CENP-F is a novel microtubule-binding protein that contains two microtubule-binding domains. The C-terminal domain stimulated microtubule polymerization in vitro. These activities can explain the importance of CENP-F to kinetochore attachments in vivo. Despite their importance to kinetochore attachments, cells depleted of CENP-F were only transiently delayed in mitosis before exiting with lagging chromosomes. We found that CENP-F is not an essential component of the mitotic checkpoint but is required by cells to sustain a prolonged mitotic delay in response to nocodazole or depletion of the kinetochore proteins hSgo1 and CENP-E. Quantitative analysis showed that kinetochores depleted of CENP-F exhibited a moderate reduction in the levels of checkpoint proteins Mad2, Bub1, BubR1, and hMPS1. Thus, kinetochores depleted of CENP-F may not be capable of generating the normal levels of the “wait for anaphase” signal that are required for sustaining a mitotic block.
Materials and methods
DNA constructs and RNA interference
A nested polymerase chain reaction (PCR) (Z-taq, Takara) was used to clone different CENP-F fragments from a human Hela cell complementary DNA library (Clontech). PCR products were cloned into pENTR vectors (Gateway, Invitrogen) and recombined in vitro into different expression vectors. Small interfering RNA (siRNAs) were obtained from commercial sources (Qiagen and Dharmacon) and were delivered into Hela cells by Oligofectamine (Invitrogen), according to the manufacturer’s instructions. All the siRNAs worked equally effectively. Data presented in this paper were obtained with the following siRNAs.
CENP-F siRNAs:
-
r(GAAUCUUAGUAGUCAAGUA)d(TT),
-
r(GGUUAUUGUCUGCCUUGAA)d(TT).
-
CENP-F SMART pool.
CENP-E siRNA:
-
r(CGAUACUGUUAACAUGAAU)d(TT)
hSgo1 siRNA:
-
r(ACAGUAGAACCUGCUCAGA)d(TT)
Immunofluorescence and video microscopy
Cells were fixed for 7 min in freshly prepared 3.5% paraformaldehyde/phosphate-buffered saline pH 7.0, permeabilized in KB (20 mM Tris–HCl pH 7.5, 150 mM NaCl, and 0.1% bovine serum albumin) plus 0.2% Triton X-100 for 4 min at room temperature (R.T.), and rinsed in KB for 5 min. Primary and secondary antibodies were diluted in KB and added to coverslips for 30 min at 37°C in a humidified chamber. Human anti-centromere autoimmune antibody (ACA) was a gift from J.B. Rattner (University of Calgary, Alberta, Canada). Mouse anti-tubulin antibodies (Sigma) and affinity-purified antibodies to CENP-E, Mad2, Bub1, BubR1, and hMps1 were used at concentrations between 0.5 and 1 μg/ml. Secondary antibodies conjugated to Alexafluor 488, Alexafluor 555, and Alexafluor 647 (Molecular Probes) were used at 0.5–1 μg/ml. To test the stability of microtubules to cold, cells in medium were chilled at 4°C for 10 min, and then fixed, extracted, and stained.
For video microscopy, Hela cells stably transfected with gfp:H2B (generated in our lab) were observed with a Nikon TE300 microscope that was equipped with epifluorescence optics and enclosed in a temperature-controlled incubator. Images were captured with a 20× objective using a Retiga EX (QImaging) camera controlled with ImagePro Plus (Mediacybernetics). High-resolution analysis was conducted with an UltraView spinning disk confocal microscope using a 60× objective and a Hamamatsu Orca camera. Immunofluorescence images were obtained with a TE2000 microscope that is equipped with epifluorescence optics. Images were visualized using a 100× numerical aperture 1.4 objective and captured with a Cascade 512F camera (Roper) using Metavue software. Signal intensities were quantified on maximum projections of z-stacks (0.5-μM slices) using ImagePro Plus (MediaCybernetics). Deconvolution was performed on image stacks using AutodeBlur (AutoQuant). Quantitative analysis of staining intensities at kinetochores was as described (Hoffman et al. 2001). Briefly, two squares, a 9×9-pixel square that circumscribed the brightest signal and a 13×13-pixel square that included the surrounding background signal, were drawn around each kinetochore. Kinetochore fluorescence was calculated by the following formula: \(F_{{{\rm{kinetochore}}}} = F_{{\rm{I}}} - F_{{{\rm{background}}}}\). F background was calculated as follows: \(F_{{{\rm{background}}}} = {\left( {F_{{\rm{O}}} - F_{{\rm{I}}} } \right)}{\left( {81/88} \right)}\), where F O stands for integrated fluorescence of the outer square and F I is the integrated fluorescence of the inner square.
Microtubule binding and in vitro translation
S100 extracts prepared from Hela cells were incubated with taxol (10 μM) at 37°C for 30 min and centrifuged (80,000×g at R.T.) to pellet microtubules and associated proteins. Supernatant and pellet fractions were then probed for tubulin and CENP-F through Western blots. Microtubules were also prepared from purified bovine brain tubulin (Cytoskeleton) and used in pelleting assays with in vitro translated fragments of CENP-F according to the manufacturer’s instructions (Cytoskeleton). TNT T7 Quick for PCR DNA was used to translate CENP-F fragments in vitro (Promega). Kinetic analysis of microtubule polymerization was monitored with a spectrophotometer that maintained the temperature at 37°C.
Results
Cells depleted of CENP-F exhibit an attenuated mitotic checkpoint response
To study the functions of human CENP-F during mitosis, Hela cells were transfected with CENP-F siRNAs, fixed 48 h later, and stained with CENP-F and ACA antisera. Quantification of CENP-F staining of prometaphase and metaphase cells showed a 20 to 50-fold reduction (>95%) in fluorescence intensity (after normalizing to ACA intensity) between control and CENP-F-depleted cells (Fig. 1a,c). Quantitative immunoblotting showed a >25-fold depletion (>95%) of CENP-F protein in cells transfected with CENP-F siRNA, as compared to control. The depletion was specific, as the level of BubR1, a protein that also localizes to kinetochores, was unaltered (Fig. 1b).
CENP-F depletion impairs the mitotic checkpoint in Hela cells. a Immunofluorescence images of mitotic cells following transfection with luciferase (a negative control) or CENP-F siRNA and stained with CENP-F and ACA antisera. Scale bar: 10 μm. b Immunoblots of lysates from cells transfected with control and CENP-F siRNAs probed with CENP-F and hBUBR1 antibodies. c Quantitation of CENP-F in lysates from control and CENP-F siRNA-transfected cells by immunoblotting (red bars) or on kinetochores (n=65) by immunofluorescence staining (green bars). Error bars show standard error of the mean (s.e.m.) from multiple cells (n=10). The CENP-F intensity was normalized to ACA and corrected for background noise. d Mitotic index of control and CENP-F-depleted cells treated with 12 ng/ml or 120 ng/ml nocodazole for 16 h. Error bars represent s.e.m. from three independent experiments. e, f Plots of the frequency at which siRNA-transfected cells entered anaphase as determined by videomicroscopy. NEBD=T 0. Note that CENP-F depletion reduces the times of anaphase onset for cells that were also depleted of CENP-E (e) or hSgo1 (f)
Our first functional test was to determine if CENP-F is an important component of the mitotic checkpoint by analyzing the fates of cells after exposure to nocodazole. Cells transfected with control or CENP-F siRNA’s were exposed to a low dose of nocodazole (12 ng/ml) that suppressed microtubule dynamics but did not disrupt bipolar spindle formation. After 16 h in the presence of the drug, ∼49% of the control cells were blocked in mitosis, while 12% of cells transfected with the CENP-F siRNA were in mitosis. At higher concentrations of nocodazole (120 ng/ml) that blocked spindle formation, control cells and cells depleted of CENP-F exhibited mitotic indices (MIs) of 55% and 33%, respectively. By staining for CENP-F, we confirmed that the mitotic cells we scored were indeed depleted of the proteins after transfection with the siRNA. Control cells and CENP-F-depleted cells not exposed to the drug exhibited comparable mitotic indices of 8% and 3%, respectively. Thus, after overnight treatment with low and high concentrations of nocodazole, cells depleted of CENP-F accumulated fewer mitotic cells than those seen for control cells (Fig. 1d). Nevertheless, the increased mitotic indices (15% and 33% vs 3%) over non-drug-treated cells show that cells depleted of CENP-F are capable of activating their checkpoint response. However, cells depleted of CENP-F may not be able to sustain a prolonged delay, as in control Hela cells.
To further test the importance of CENP-F in the mitotic checkpoint, we examined how cells depleted of CENP-F responded to the inactivation of the kinetochore proteins CENP-E and hSgo1. Mitotic progression was monitored by filming Hela(H2B:GFP) cells (n=150) following transfection with different combinations of siRNAs. The times of anaphase onset [nuclear envelope breakdown (NEBD) was set as T=0 min] for individual cells were determined. When cells were cotransfected with control (luciferase) and CENP-E siRNAs, ∼35% of the cells exited mitosis within 3 h after NEBD (Fig. 1e), while >65% of the cells that entered mitosis during filming were delayed in mitosis for >3 h. In contrast, 75% of the cells cotransfected with CENP-F and CENP-E siRNAs entered anaphase within 3 h after NEBD (Fig. 1e). The average time of anaphase onset for cells that were simultaneously depleted of CENP-E and CENP-F was approximately 87 min, as compared to 50 min for cells depleted of just CENP-F or >3 h for cells depleted of just CENP-E. Examination of cells cotransfected with control and Sgo1 siRNAs showed that only 5% had entered anaphase within 3 h of NEBD while 60% of cells cotransfected with CENP-F and Sgo1 siRNAs entered anaphase within 3 h of NEBD (Fig. 1f). The average time of anaphase onset for cells depleted of both hSgo1 and CENP-F was approximately 100 min, as compared to >5 h for cells depleted of hSgo1 alone. In both cases, the duration of the mitotic delay resulting from the depletion of either CENP-E or hSgo1 was reduced when CENP-F was depleted.
We next examined how the loss of CENP-F affected the localization of mitotic checkpoint proteins at kinetochores. Mitotic cells that exhibited >95% depletion of CENP-F were chosen for this analysis. Using this criteria, there was an across-the-board reduction in the amounts of checkpoint proteins relative to controls. Quantitative analysis revealed that kinetochores depleted of CENP-F retained 81, 66, 64, 76, and 65% of the normal amounts of Mad1, Mad2, hBubR1, hBub1, and hMPS1, respectively (Supplemental Figure 1). The reduction in the levels of checkpoint proteins might therefore account for the attenuated checkpoint in cells depleted of CENP-F.
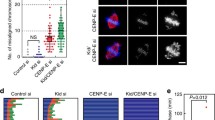
Time-lapse studies showed that Hela(Gfp:H2B) cells depleted of CENP-F failed to align properly at the spindle equator and then prematurely entered anaphase with lagging chromosomes (Fig. 2a). CENP-F-depleted cells were found to enter anaphase approximately 52 min after NEBD, while the time to anaphase onset was 40 min for control cells (Fig. 2b). Nearly 80% of the cells transfected with CENP-F siRNA had visible segregation defects in anaphase, as determined by the presence of lagging chromosomes and chromatin bridges (Fig. 2a,c). These defects were also seen for cells depleted of the hBub1 mitotic checkpoint kinase (Fig. 2c). This contrasts with controls, where >90% of the cells appear to segregate their chromosomes normally (Fig. 2a,c). The presence of lagging chromosomes in the anaphase of CENP-F-depleted cells suggests they are exiting mitosis in the presence of improperly aligned chromosomes.
Cells depleted of CENP-F exit mitosis with improperly aligned chromosomes. a Hela (gfp:H2B) cells were transfected with luciferase (top) and CENP-F siRNA (middle and bottom) and monitored by time-lapse videomicroscopy. Images were collected using an UltraView spinning disc confocal at 5-min intervals. b Comparison of the times of anaphase onset for control and CENP-F siRNA-treated cells. Successive frames from videos, as shown in a, were analyzed. Data were collected from 10 (control) to 18 (CENP-F-depleted) cells. Tm denotes the peak time of the distribution. Scale bar: 10 μm. c Analysis of anaphase cells transfected with control, Bub1, and CENP-F siRNAs. Orange denotes morphologically normal chromosome segregation; green, lagging chromosomes; blue, multinucleated and abnormally shaped nuclear masses; purple, mitotic delay at T=120 min. Error bars represent the standard error of the mean from four independent experiments
CENP-F is essential for maintaining stable kinetochore attachments
We next characterized the kinetochore attachments in cells depleted of CENP-F. To reduce complications contributed by defects in the mitotic checkpoint, studies were conducted in the presence of a proteosome inhibitor that blocked cells from exiting mitosis. In the presence of MG132, control cells arrest in metaphase and their kinetochores are saturated with microtubules, as is evident from the lack of detectable Mad1 staining (Fig. 3a). Of the metaphases that were examined, 20% exhibited a single Mad1-positive kinetochore (Fig. 3b). The remaining control metaphases had no detectable Mad1. Examination of the interkinetochore distance between kinetochores that lacked Mad1 staining (and thus, were fully saturated with microtubules) showed that the kinetochores were under tension (Figs. 3a,c). Cells depleted of CENP-E characteristically accumulated monopolar chromosomes in addition to bipolar attached chromosomes. In the presence of MG132, the bipolar-attached kinetochores appeared to be saturated with microtubules, as they did not exhibit detectable Mad1 staining (Fig. 3a). Furthermore, tension between these kinetochores was reduced when compared with the controls (Fig. 3c). As previously shown, strong Mad1 staining was detected at all of the unattached kinetochores near the poles (Fig. 3a). On average, a cell depleted of CENP-E contained 8.7 Mad1-positive kinetochores, the majority of which were associated with the unattached kinetochores near the poles (Fig. 3c). In cells depleted of CENP-F, the presence of Mad1-positive kinetochores indicated that there were defective attachments. On average, there were 8.3 Mad1-positive kinetochores per cell. Furthermore, kinetochore tension was significantly reduced and was comparable to that seen for the unattached kinetochores in the CENP-E-depleted cells (Fig. 3c).
Kinetochores depleted of CENP-F lack tension and retain Mad1. Hela cells were transfected with siRNA as indicated and treated with MG132 for 1 h before fixing. a Immunofluorescence images of cells stained for 4′,6-diamidino-2-phenylindole DAPI, CENP-F (not shown) or CENP-E (not shown), Mad1, and ACA. For each sample, a Z-series was taken with a depth 0.25 μm. A single optical section is shown in c, d, e, h, i, j, m, n, and o. Scale bar: 10 μm. b Comparison of the frequency of mitotic cells with Mad1-positive kinetochores. Numbers in each bar indicate the average number of Mad1-positive kinetochores per cell (n=25 cells). Error bars show the standard error of the mean. c Kinetochore tension was indirectly determined by measuring the center-to-center distance between pairs of ACA foci, as marked by arrows in a. Bars indicate the interkinetochore distance. The histogram was derived from measurements from over 40 kinetochores in more than eight cells
An analysis of the breast carcinoma cell line MCF7 showed a similar response to loss of CENP-E and CENP-F (Fig. 4a). MCF7 cells depleted of CENP-E accumulated monopolar chromosomes whose kinetochores were Mad1-positive and thus depleted of microtubule attachment (Fig. 4a,e,f). Mad1 staining was not detected at kinetochores that established bipolar attachments (Fig. 4a,g,h). In cells depleted of CENP-F, the chromosomes were less organized than seen in Hela cells, as they were scattered throughout the spindle. The shape of the bipolar spindle was abnormal, and microtubules did not establish end-on connections with kinetochores (Fig. 4a,n,p). Instead, we observed kinetochores associated with the sides of microtubules (Fig. 4a,p) or lacking detectable attachments. The interactions between microtubules and kinetochores were also examined in Hela cells that were depleted of CENP-F (Fig. 4b). Control metaphase cells contained symmetrical bipolar spindles. As with MCF7 cells, the spindles in Hela cells that were depleted of CENP-F was malformed such that it was often twisted out of the plane of focus, and its microtubules were less organized than those of controls. To test the stability of kinetochore–microtubule attachments, Hela cells were exposed to cold temperatures to depolymerize unstable microtubules. Control cells treated with cold retained virtually all of their kinetochore microtubules, as shown by the fact that all of the kinetochore pairs maintained end-on microtubule attachments (Fig. 4b). In contrast, we observed a variety of defects in cells depleted of CENP-F (see insets in bottom right panel of Fig. 4b). We identified kinetochores that were not attached to microtubules (top inset) and kinetochores with merotelic (middle inset), where a single kinetochore is attached to microtubules from opposite poles, as well as syntelic (bottom inset) attachments (a kinetochore pair attached to microtubules from the same pole).
Kinetochores depleted of CENP-F fail to establish stable microtubule attachments. MCF7 or Hela cells were transfected with the indicated siRNAs and then treated for 1 h with MG132 before fixing. a Immunofluorescence of mitotic MCF7 cells depleted of CENP-E (top) and CENP-F (bottom) stained with Mad1, tubulin, ACA, and 4′,6-diamidino-2-phenylindole (DAPI). Scale bar: 10 μm. b Hela cells transfected with control and CENP-F siRNAs were exposed to the cold before fixing and staining for tubulin, ACA, and DAPI. Consecutive optical sections from a z-stack are shown
The defects in microtubule attachment by kinetochores depleted of CENP-F have been attributed to the reduction in CENP-E and p150glued (Yang et al. 2005). We quantified and compared the level of CENP-E staining (normalized to ACA) between control and CENP-F-depleted kinetochores and found no difference (Fig. 5). The normalized amount of CENP-E can vary by more than tenfold in the CENP-F-depleted cells. This variation may be attributed to the fact that the amount of CENP-E at kinetochores is sensitive to microtubule attachments (Hoffman et al. 2001). The kinetochores exhibiting the highest level of CENP-E are likely those that have lost their microtubule attachments. We treated cells depleted of CENP-F with nocodazole to eliminate microtubule attachments and found comparable levels of CENP-E at all the kinetochores (data not shown). Thus, CENP-F is not required by CENP-E to localize to kinetochores. In addition to CENP-E, we found that the localization of other proteins (the hZW10/ROD complex, hSgo1, and the HEC1/hNdc80 complex) that are important for microtubule attachments were not altered by the loss of CENP-F (data not shown).
CENP-F is a novel microtubule-binding protein of the kinetochore
The possibility that CENP-F might be a microtubule-binding protein was discovered when we were attempting to disrupt CENP-Fs function by overexpressing a fragment that contained its kinetochore-targeting domain. We observed that the gfp:CENP-F1756-3114 colocalized with microtubules in transfected cells (Fig. 6a). If cells were fixed before permeabilization, CENP-F was found to colocalize with microtubules near the periphery of the cell (Fig. 6b). In both cases, the vast majority of the endogenous and transfected proteins were in the nucleus. In support of the cytological data, CENP-F cosedimented with microtubules that were polymerized from taxol-treated Hela cell lysates (Fig. 6c). When different segments of CENP-F were transfected into the cells, the amino- and carboxy-terminal 979 and 1358 residues, respectively, were found to cosediment with microtubules. Next, we expressed a series of fragments that spanned the entirety of CENP-F by in vitro translation and tested for their ability to bind to microtubules that were polymerized from purified tubulin. The results confirmed the transfection data, in that both the amino- and carboxy-terminal fragments cosedimented with microtubules (Fig. 6d). Further mapping experiments localized the microtubule-binding domains to lie in a 385-amino-acid amino-terminal fragment and in a 187-amino-acid C-terminal fragment. (Fig. 6e).
CENP-F contains two microtubule-binding domains that bind microtubules in vivo and in vitro. a Hela cells transfected with gfp:CENP-F1756-3114 were stained with tubulin. b Endogenous CENP-F can be detected near the periphery of the interphase microtubule array in cells that were fixed before permeabilization. c S100 lysates prepared from Hela cells were treated with taxol, and after centrifugation, the supernatant (s) and microtubule pellet (p) were probed for CENP-F and tubulin. d Three overlapping fragments that spanned full-length CENP-F were expressed in Hela cells and then tested for their ability to cosediment with microtubules polymerized from S100 lysates. e A schematic depicting the various fragments expressed by in vitro translation and tested for microtubule binding in vitro by microtubule pellet assay using purified tubulin. Bold lines indicate the smallest fragments that bind microtubules. Sodium dodecyl sulfate polyacrylamide gel electrophoreses of supernatants (50% of the fraction) and pellets (100% of the fraction) from the various microtubule pelleting assays
We succeeded to express the C-terminal microtubule-binding domain in bacteria (Fig. 7a) and used it to confirm that it directly binds to microtubules (data not shown). Next, we tested the effect of this domain on microtubule polymerization in vitro. The assay was conducted at a concentration of tubulin that was below that required for self-polymerization (Fig. 7b). Polymerization was stimulated when the reaction was supplemented with a substochiometric amount of the C-terminal domain (1:25 CENP-F to tubulin). No stimulatory activity was detected if a similar concentration of a non-microtubule-binding protein (PIN1) was added to the reaction. The combined data show that CENP-F is a novel microtubule-binding protein and that its C-terminal microtubule-binding domain can stimulate microtubule polymerization in vitro.
The C-terminal microtubule-binding domain of CENP-F promotes microtubule polymerization in vitro. a Coomassie stained gel displaying different amounts of recombinant human Pin1, C-terminal 187 residues of CENP-F, and purified bovine brain tubulin that were used for in vitro studies. b Kinetics of tubulin polymerization under different reaction conditions were monitored by change in absorbance at 37°C
Discussion
Our finding that CENP-F is important for kinetochore attachments is in general agreement with recent reports (Holt et al. 2005; Laoukili et al. 2005; Yang et al. 2005). Our time-lapse studies of Hela cells showed that depletion of CENP-F did not lead to an accumulation of monopolar chromosomes, as chromosomes were able to congress towards the center of the cell. However, the chromosomes failed to achieve metaphase alignment before the cells exited mitosis with lagging chromosomes. If mitotic exit was blocked with a proteosome inhibitor, the chromosomes did appear aligned in the center of the spindle, as reported by Holt et al. Thus, kinetochores depleted of CENP-F are capable of establishing bipolar connections that promote congression. Nevertheless, many of these kinetochores lacked proper attachments, as they retained Mad1 staining, lacked tension, and exhibited sensitivity to cold. Chromosomes in MCF7 cells appeared more sensitive to the loss of CENP-F, as they were more widely distributed throughout the spindle even when mitotic exit was blocked by MG132. We do not understand the reasons for the difference but can only suggest that CENP-F plays a more crucial role in maintaining bipolar attachments in MCF7 cells. Given this possibility, the variable results that have been reported in the literature may be attributed to inherent differences (spindle dynamics, volume of the cell, and number of chromosomes) by which different cell lines capture and align their chromosomes.
The importance of CENP-F to kinetochore attachments can now be understood from the standpoint that CENP-F is a microtubule-binding protein. Regardless of whether CENP-F affects the assembly of other proteins that are important for attachments, its direct contribution to maintaining microtubule attachments at kinetochores must be considered. The significance of its two microtubule-binding domains remains unclear, and future studies are required to test their functional importance in vivo. The ability of the C-terminal domain to stimulate microtubule polymerization in vitro suggests that it may contribute to subunit addition to microtubules that are attached to the kinetochore. Even though depletion of CENP-F did not affect other proteins that are important for kinetochore attachments (CENP-E, p150, hZW10/hROD, and hNuf2/Ndc80), it is interesting that these proteins cannot fulfill the functions of CENP-F. This suggests that stable microtubule attachments depend on the cooperative actions of multiple microtubule-binding proteins at the kinetochore. Each type of microtubule-binding protein is likely to contribute differently to how kinetochores interact stably with microtubules.
Our studies confirm reports that cells depleted of CENP-F cannot sustain a mitotic delay in response to kinetochore attachment defects. We found that cells depleted of CENP-F were delayed from exiting mitosis for only 12 min, despite the presence of defective bipolar attachments. As a consequence, all anaphase cells contained lagging chromosomes that resulted in the accumulation of multinucleated (aneuploid) cells. Our studies suggest that CENP-F is not an essential component of the mitotic checkpoint, as cells depleted of CENP-F were able to delay mitosis when exposed to nocodazole. Nevertheless, the reduction in the mitotic index (MI) relative to controls suggests that the duration of this delay must be reduced when CENP-F is depleted. In addition, we found that cells depleted of CENP-F responded differently to different doses of nocodazole. When cells were treated with the drug at a low dose that dampened microtubule dynamics but not spindle formation, the MI was only 15%. Cells treated with a dose of nocodazole that blocked spindle formation exhibited a MI of 33%. As the MI of control cells treated at the two concentrations of drugs were similar (49% vs 55%), the difference in the MI (15% vs 33%) exhibited by cells depleted of CENP-F at the different doses of drugs may indicate how CENP-F contributes to the mitotic checkpoint. An obvious difference is that the number of unattached kinetochores is far fewer in cells treated with a low dose of the drug. If the magnitude of the “wait for anaphase” output is reduced as a result of the loss of CENP-F, the total output from the few unattached kinetochores may not be enough to sustain a block that is achieved when all of the kinetochores in the cells are unattached.
The difference in the numbers of unattached kinetochores when CENP-F is depleted from cells may explain the apparent discrepancy in the literature on the importance of CENP-F in the mitotic checkpoint. It is clear that if there is an accumulation of unattached kinetochores in cells depleted of CENP-F; mitosis is delayed for 2 to 3 h before they die (Yang et al. 2005) or divide with unaligned chromosomes (Holt et al. 2005). Regardless of the fates of these cells, the mitotic checkpoint was active but was unable to delay mitotic exit for a prolonged period. This is fully consistent with our finding that the mitotic delay exhibited by cells that were depleted of CENP-E or hSgo1 was significantly reduced when CENP-F was also depleted. However, these cells were still able to delay mitosis, as they did not enter anaphase until 87 and 100 min after NEBD, while control cells entered anaphase at ∼40 min after NEBD. We conclude that kinetochores depleted of CENP-F are unable to generate sufficient amounts of the “wait for anaphase” signal to maintain a prolonged mitotic arrest. In our hands, the very brief mitotic delay (∼12 min) exhibited by CENP-F-depleted cells not treated with nocodazole is likely due to the low number of unattached kinetochores that failed to generate a threshold level of the “wait for anaphase” signal to maintain a prolonged mitotic delay.
Our analysis revealed a modest across-the-board reduction (∼30 to 40%) in the amounts of checkpoint proteins Mad1, Mad2, hBUBR1, hBUB1, and hMPS1, as reported by Bomont et al. If these proteins are thought to be directly involved in generating the “wait for anaphase” signal, their reduction would then result in a decreased output of this inhibitory signal. We therefore conclude that CENP-F is not an essential component of the mitotic checkpoint. Nevertheless, it does contribute in a significant way to the mitotic checkpoint pathway, perhaps by moderating the efficiency by which checkpoint proteins generate the “wait for anaphase” signal. For instance, CENP-F might interact directly with checkpoint proteins at the kinetochore or might be important for the integrity of the proteins’ binding sites.
References
Ashar HR, James L, Gray K, Carr D, Black S, Armstrong L, Bishop WR, Kirschmeier P (2000) Farnesyl transferase inhibitors block the farnesylation of CENP-E and CENP-F and alter the association of CENP-E with the microtubules. J Biol Chem 275:30451–30457
Bomont P, Maddox P, Shah JV, Desai AB, Cleveland DW (2005) Unstable microtubule capture at kinetochores depleted of the centromere-associated protein CENP-F. EMBO J 24:3927–3939
Casiano CA, Landberg G, Ochs RL, Tan EM (1993) Autoantibodies to a novel cell cycle-regulated protein that accumulates in the nuclear matrix during S phase and is localized in the kinetochores and spindle midzone during mitosis. J Cell Sci 106(4):1045–1056
Echeverri CJ, Paschal BM, Vaughan KT, Vallee RB (1996) Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol 132:617–633
Hoffman DB, Pearson CG, Yen TJ, Howell BJ, Salmon ED (2001) Microtubule-dependent changes in assembly of microtubule motor proteins and mitotic spindle checkpoint proteins at PtK1 kinetochores. Mol Biol Cell 12:1995–2009
Holt SV, Vergnolle MA, Hussein D, Wozniak MJ, Allan VJ, Taylor SS (2005) Silencing Cenp-F weakens centromeric cohesion, prevents chromosome alignment and activates the spindle checkpoint. J Cell Sci 118:4889–900
Hussein D, Taylor SS (2002) Farnesylation of Cenp-F is required for G2/M progression and degradation after mitosis. J Cell Sci 115:3403–3414
Johnson VL, Scott MI, Holt SV, Hussein D, Taylor SS (2004) Bub1 is required for kinetochore localization of BubR1, Cenp-E, Cenp-F and Mad2, and chromosome congression. J Cell Sci 117:1577–1589
Joseph J, Liu ST, Jablonski SA, Yen TJ, Dasso M (2004) The RanGAP1–RanBP2 complex is essential for microtubule–kinetochore interactions in vivo. Curr Biol 14:611–617
Laoukili J, Kooistra MR, Bras A, Kauw J, Kerkhoven RM, Morrison A, Clevers H, Medema RH (2005) FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol 7:126–136
Liao H, Winkfein RJ, Mack G, Rattner JB, Yen TJ (1995) CENP-F is a protein of the nuclear matrix that assembles onto kinetochores at late G2 and is rapidly degraded after mitosis. J Cell Biol 130:507–518
Liu ST, Hittle JC, Jablonski SA, Campbell MS, Yoda K, Yen TJ (2003) Human CENP-I specifies localization of CENP-F, MAD1 and MAD2 to kinetochores and is essential for mitosis. Nat Cell Biol 5:341–345
McEwen BF, Chan GK, Zubrowski B, Savoian MS, Sauer MT, Yen TJ (2001) CENP-E is essential for reliable bioriented spindle attachment, but chromosome alignment can be achieved via redundant mechanisms in mammalian cells. Mol Biol Cell 12:2776–2789
Rattner JB, Rao A, Fritzler MJ, Valencia DW, Yen TJ (1993) CENP-F is a .ca 400 kDa kinetochore protein that exhibits a cell-cycle dependent localization. Cell Motil Cytoskeleton 26:214–226
Salina D, Enarson P, Rattner JB, Burke B (2003) Nup358 integrates nuclear envelope breakdown with kinetochore assembly. J Cell Biol 162:991–1001
Steensgaard P, Garre M, Muradore I, Transidico P, Nigg EA, Kitagawa K, Earnshaw WC, Faretta M, Musacchio A (2004) Sgt1 is required for human kinetochore assembly. EMBO Rep 5:626–631
Yang Z, Guo J, Chen Q, Ding C, Du J, Zhu X (2005) Silencing mitosin induces misaligned chromosomes, premature chromosome decondensation before anaphase onset, and mitotic cell death. Mol Cell Biol 25:4062–4074
Zhu X, Chang KH, He D, Mancini MA, Brinkley WR, Lee WH (1995a) The C terminus of mitosin is essential for its nuclear localization, centromere/kinetochore targeting, and dimerization. J Biol Chem 270:19545–19550
Zhu X, Mancini M, Chang K, Liu C, Chen C, Shan B, Jones D, Yang-Feng T, Lee W (1995b) Characterization of a novel 350-kilodalton nuclear phosphoprotein that is specifically involved in mitotic-phase progression. Mol Cell Biol 15:5017–5029
Acknowledgements
We acknowledge the excellent technical support by J. Hittle and B. Conner, and advice from S.T. Liu and other members of the Yen lab. We give special thanks to J. Peterson for the use of equipment. This work is supported by the National Institute of Health GM44762, core grants CA06927 CA75138, and an appropriation from the Commonwealth of Pennsylvania.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E.A. Nigg
Electronic supplementary material
Below is the image and a link to the electronic supplementary material
Fig8
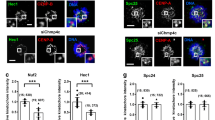
CENP-F is not essential for checkpoint proteins to assemble onto kinetochores during prometaphase. Hela cells transfected with control and CENP-F siRNAs were fixed and stained for CENP-F and Bub1, BubR1, MPS1, Mad1 or Mad2. Scale bar: 10 μm. Cells in prometaphase were selected and only those whose kinetochores were depleted of CENP-F by >25-fold were chosen for analysis. The strongest signal detected at a control kinetochore was chosen as 100%. Approximately 150 kinetochores were quantified for each antibody. Error bars represent s.e.m
Rights and permissions
About this article
Cite this article
Feng, J., Huang, H. & Yen, T.J. CENP-F is a novel microtubule-binding protein that is essential for kinetochore attachments and affects the duration of the mitotic checkpoint delay. Chromosoma 115, 320–329 (2006). https://doi.org/10.1007/s00412-006-0049-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-006-0049-5