Abstract
Analysis of chromosome localization of three molecular markers, 18S-5.8S-28S rDNA, 5S rDNA and a 180 bp satDNA, showed that B chromosomes in the grasshopper Eyprepocnemis plorans originated independently in Eastern (Caucasus) and Western (Spain and Morocco) populations. Eastern B chromosomes are most likely derived from the smallest autosome, which is the only A chromosome carrying the three markers, in coincidence with Caucasian B chromosomes. Western B chromosomes, however, lack 5S rDNA and are most likely derived from the X chromosome, which is the only A chromosome carrying the two remaining markers, always in the same order with respect to the centromere, as the B chromosome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
B chromosomes are extra chromosomes present in some individuals of many eukaryotic species. In most cases, B chromosomes behave as genome parasites that are maintained in natural populations because of specific accumulation mechanisms, despite being detrimental to the host. Most of them are heterochromatic chromosomes composed of repetitive DNA, and the general view is that they contain genetically inert DNA (see Camacho et al. 2000).
B chromosomes most likely derive from the host genome, albeit intra- or interspecifically. Although the intraspecific hypothesis likely applies to many, perhaps most, B chromosomes, there is sound evidence that some of them have arisen through interspecific hybridization (see McAllister and Werren 1997; Perfectti and Werren 2001). In every case, one of the most difficult questions to address is the identification of the ancestor A chromosome from which the B was derived. The rapid molecular evolution of the newly formed B chromosomes and their enrichment in repetitive DNAs (Camacho et al. 2000) makes such identification difficult. For instance, in the plant Crepis capillaris, all B DNA sequences isolated by microdissection were also found to be present in the A chromosomes, but it was not possible to assign a B origin to a specific A chromosome (Jamilena et al. 1994). In rye, likewise, B chromosomes are mostly composed of DNA sequences shared with A chromosomes (Wilkes et al. 1995; Houben et al. 1996), suggesting their intraspecific origin (Puertas 2002). Langdon et al. (2000) have reported the interesting de novo formation of satellite DNA repeats from complex euchromatic sequences in rye. In maize, many DNA sequences in the B chromosomes are highly repetitive and shared with the A chromosomes (Alfenito and Birchler 1993), which also suggests an intraspecific origin for this B (Stark et al. 1996). But, in this species, a DNA repeat has been isolated from the B centromere (Alfenito and Birchler 1993) that is related to a centric repeat in chromosome 4, suggesting a possible common origin for both chromosomes (Page et al. 2001). Recently, Dhar et al. (2002) have reported the origin of a heterochromatic B chromosome from an A chromosome, in the plant Plantago lagopus, by means of a sequence of processes such as aneuploidy, the formation of a ring chromosome, chromosome fragmentation, massive amplification of 5S rDNA and centromere misdivision with chromatid nondisjunction to generate the isochromosome. This work thus illuminates possible pathways for B chromosome origin that might have occurred in other species.
The B chromosome system of the grasshopper Eyprepocnemis plorans has revealed many details of B chromosome evolution (Camacho et al. 1997). López-León et al. (1994) have hypothesised that the B2 chromosome most likely derived from the X chromosome because it was the only A chromosome showing the same order, with respect to the centromere, for a 180 bp satellite DNA (satDNA) and 18S-5.8S-28S ribosomal DNA (rDNA), i.e. centromere-satDNA-rDNA. This order is conserved in the above-mentioned principal B chromosomes, e.g. B1, B2, B5 and B24 (Cabrero et al. 1999). In the present study, we analyzed the chromosome location of these two repetitive DNAs in specimens from Morocco and the Caucasus, and added a new molecular marker to the analysis, i.e. the 5S rDNA. These new data suggest that B chromosomes from the Caucasus and those from the western Mediterranean region arose independently and most likely from different A chromosomes.
Materials and methods
Adult males of E. plorans were collected at several localities in the Spanish provinces of Granada, Málaga and Albacete, as well as several populations from Morocco and another from Daghestan (North Caucasus, Russia).
Chromosome preparations were made from embryo cells, obtained following the technique described in Camacho et al. (1991), or from spermatocytes by the technique described in Cabrero et al. (1999). In order to facilitate probe accessibility for fluorescence in situ hybridization (FISH), each spermatocyte preparation was incubated in 150 μl of pepsin (50 μg/ml in 0.01 N HCl) at 37°C in a humid chamber for 5 to 15 min. After three washes in distilled water, the slides were dehydrated in a series of 70%, 90% and 100% ethanol for 3, 3, and 5 min, respectively, and then air-dried. Slides were stored at 60°C overnight before in situ hybridization.
Three different DNA probes were used: (1) pTa71, which contains a 9 kb Eco RI repeat unit of 18S-5.8S-26S rDNA isolated from Triticum aestivum (Gerlach and Bedbrook 1979) kindly provided by R.B. Flavell and M. O’Dell; (2) pEpD15 with a 180 bp Dra I fragment of tandem repetitive DNA from E. plorans (López-León et al. 1994, 1995), and (3) a 5S rDNA probe that was obtained through polymerase chain reaction (PCR) amplification of genomic DNA from a 0B E. plorans male, collected in Salobreña (Granada, Spain), using the primers: 5S-nRNA.F-1 (5′-AACGACCATACCACGCTGAA-3′) and 5S-nRNA.R-1 (5′-AAGCGGTCCCCCATCTAAGT-3′), designed from the Drosophila melanogaster 5S rDNA. The single PCR product obtained (92 bp long) was directly sequenced and showed high homology with the 5S rDNA of many organisms in the Genbank.
All DNA probes were labeled by nick translation with FluoroGreen (fluorescein-11-dUTP) (18S-5.8S-28S rDNA and 5S rDNA) or FluoroRed (rhodamine-11-dUTP) (pEpD15 and 5S rDNA), using standard techniques.
Single or double FISH was performed following the technique described in López-León et al. (1994). In brief, DNA probes were mixed to a final concentration of 5 ng/μl in a solution containing 40% formamide, 10% dextran sulfate, 0.1% SDS (sodium dodecyl sulphate) in 1×SSC and 70 ng/μl sperm salmon. Chromosomal DNA was denatured, along with the hybridization mixture (30 μl), on a hot plate at 80°C for 8 min. Hybridization was performed at 37°C overnight. After two washes in 2×SSC at 37°C, one in 2×SSC at room temperature and one in 4×SSC, Tween 20 (5 min each), slides were counterstained with 2 μg/ml of 4′,6 diamidino-2-phenylindole (DAPI) and mounted in antifade solution (Vectashield H-1000). Photographs were taken on Fujichrome 400 Provia color film. Slides were digitized with a Hewlett Packard Photo Smart scanner and the figures were composed with Adobe Photoshop.
Results and discussion
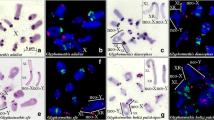
Consistent with what was observed in the Spanish specimens (López-León et al. 1994; Cabrero et al. 2003a), FISH analysis in the Moroccan specimens revealed that the largest clusters of 18S-5.8S-28S rDNA are located in the X, S9, S10, S11 and B chromosomes, in addition to very small clusters in most remaining chromosomes (Fig. 1a) (see also Bakkali et al. 2001). The satDNA was proximally located on all chromosomes except M6, M8 and S10 (Fig. 1b). The order of the two repetitive DNAs relative to the centromere was, on the chromosomes carrying both DNA sequences, centromere–18S-5.8S-28S rDNA–satDNA in all chromosomes except the X and S11 chromosomes where it was centromere–satDNA–18S-5.8S-28S rDNA (Fig. 1c). B chromosomes in Spanish and Moroccan samples are mainly composed of these two repetitive DNAs (Cabrero et al. 1999).
Fluorescence in situ hybridization (FISH) for two DNA probes in the grasshopper Eyprepocnemis plorans. Bars represent 5 μm in all panels. a rDNA in a metaphase I spermatocyte from a male collected in SO.DE.A (Morocco). b Merging of 4′,6-diamidino-2-phenylindole (DAPI) fluorescence and FISH for the 180 bp satDNA in a diplotene cell from a male collected at Smir (Morocco). c Double FISH for rDNA (green) and the 180 bp satDNA (red) in a mitotic metaphase of an embryo produced by a female from Smir (Morocco). Note the heterozygosity for the proximal satDNA in the S11 chromosome, and the coincident order of the two probes in the X, B and S11 chromosomes, but not in the S9 chromosome. d FISH for rDNA in a diplotene cell from a male collected at Dhagestan (Russian Caucasus) and double FISH for a B chromosome from this same population (inset)
In Caucasusian specimens, the satDNA was found to be located in the paracentromeric region of the X chromosome and eight A chromosomes (excluding M8, S9 and S10). In sharp contrast to Spanish and Moroccan specimens, rDNA in Caucasian specimens was limited to two chromosomes only (S9 and S11) (Cabrero et al. 2003b). B chromosomes in Caucasian E. plorans are composed of these two repetitive DNAs but, in this case, B chromosomes are mostly made of rDNA (which is remarkably absent from the X chromosome), the amount of satDNA being small and limited to the centromere region (Fig. 1d).
Localization of the 5S rRNA genes was multichromosomal in all populations analyzed (Figs. 2, 3). All B chromosome variants analyzed from Spanish and Moroccan populations lack 5S rDNA (Fig. 2) but, remarkably, B chromosomes from the Caucasus carry a large cluster of this DNA sequence (Fig. 3).
Fluorescence in situ hybridization (FISH) for 5S rDNA in the grasshopper Eyprepocnemis plorans. showing 4′,6-diamidino-2-phenylindole staining (above) and FISH (below) on the same embryo cell. Note the absence of 5S rDNA in a the B1 and B2 chromosomes from Salobreña (Granada, Spain), b B24 from Torrox (Málaga, Spain) and c B5 fromFuengirola (Málaga, Spain). Bars represent 5 μm in all panels
Whereas B chromosomes from specimens from Spain and Morocco are very similar in many respects, including DNA content (large amounts of satDNA and 18S-5.8S-28S rDNA but absence of 5S rDNA), those from the Caucasus differ in carrying lower amounts of satDNA but larger amounts of 18S-5.8S-28S rDNA and, most importantly, in the presence of 5S rDNA, which was absent in occidental B chromosomes. This suggests that Eastern (Caucasus) and Western (Spain and Morocco) B chromosomes might have originated independently. An independent origin of B chromosomes in geographically distant populations of the same species has been postulated, for instance, in the grasshopper Myrmeleotettix maculatus, for B chromosomes in Britain and the Island of Öland (Southern Sweden) (Hewitt 1973), and the chive Allium schoenoprassum for British and French populations (Stevens and Bougourd 1994).
As deduced from Table 1, Western B chromosomes most likely are derived from the paracentromeric region of the X chromosome because: (1) both X and B chromosomes in specimens from Spain and Morocco show the presence of satDNA and 18S-5.8S-28S rDNA in exactly the same order relative to the centromere, and (2) both X and B lack 5S rDNA. This second property is of great help in ruling out the S11 as the possible B origin since, although S11 may also bear the satDNA and 18S-5.8S-28S rDNA in the same order as the X and B chromosomes (in fact this order in the S11 is polymorphic, see Cabrero et al. 2003a), the S11 harbors, in the same paracentromeric region where the two other markers are placed, a conspicuous cluster of 5S rDNA in all populations analyzed. Therefore, unless this DNA had been lost from the B chromosomes, the most likely explanation is that the B arose from the X chromosome, which was our initial hypothesis (López-León et al. 1994).
B chromosomes from Eastern specimens, however, are most likely derived from the S11 autosome because it is the only A chromosome carrying the three molecular markers analyzed, which are present in the B chromosome (Table 1). The other autosome carrying 18S-5.8S-28S rDNA, i.e. S9, lacks satDNA in the Caucasian specimens (Cabrero et al. 2003b), which rules out this chromosome as a candidate for B origin. Since the X chromosome lacked the 18S-5.8S-28S rDNA but the B chromosome is mainly composed of this kind of DNA (see Fig. 1d) the hypothesis of origin of B from the X chromosome is scarcely plausible, although the possibility actually exists that the B arose from the X and later acquired the 18S-5.8S-28S rDNA. As Hewitt (1973) suggested, X and small autosomes are the most likely sources of B chromosomes because they are best tolerated as polysomic elements.
Although the multiregional origin of B chromosomes in E. plorans is reasonably deduced from the available data, the possibility still remains that with more information on DNA sequences and B types, the evolutionary pathway of these B chromosomes in E. plorans is more complex than it appears. In any case, our present results suggest that B chromosomes may arise recurrently, as A-chromosome byproducts, in different populations.
References
Alfenito MR, Birchler JA (1993) Molecular characterization of a maize B chromosome centric sequence. Genetics 135:589–597
Bakkali M, Cabrero J, López-León MD, Perfectti F, Camacho JPM (2001) Population differences in the expression of nucleolus organizer regions in the grasshopper Eyprepocnemis plorans. Protoplasma 217:185–190
Cabrero J, López-León MD, Bakkali M, Camacho JPM (1999) Common origin of B chromosome variants in the grasshopper Eyprepocnemis plorans. Heredity 83:435–439
Cabrero J, Perfectti F, Gómez R, Camacho JPM, López-León MD (2003a) Population variation in the A chromosome distribution of satellite DNA and ribosomal DNA in the grasshopper Eyprepocnemis plorans. Chromosome Res 11:375–381
Cabrero J, Bugrov A, Warchałowska-Śliwa E, López-León MD, Perfectti F, Camacho JPM (2003b) Comparative FISH analysis in five species of Eyprepocnemidine grasshoppers. Heredity 90:377–381
Camacho JPM, Cabrero J, Viseras E, López-León MD, Navas-Castillo J, Alché JD (1991) G banding in two species of grasshopper and its relationship to C, N and fluorescent banding techniques. Genome 34:638–643
Camacho JPM, Shaw MW, López–León MD, Pardo MC, Cabrero J (1997) Population dynamics of a selfish B chromosome neutralized by the standard genome in the grasshopper Eyprepocnemis plorans. Am Nat 149:1030–1050
Camacho JPM, Sharbel TF, Beukeboon LW (2000) B-chromosome evolution. Philos Trans R Soc Lond B Biol Sci 355:163–178
Dhar MK, Friebe B, Koul AK, Gill BS (2002) Origin of an apparent B chromosome by mutation, chromosome fragmentation and specific DNA sequence amplification. Chromosoma 111:332–340
Gerlach WL, Bedbrook JR (1979) Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Res 7:1869–1885
Hewitt GM (1973) The integration of supernumerary chromosomes into the Orthopteran genome. Cold Spring Harbor Symp Quant Biol 38:183–194
Houben A, Kynast RG, Heim U, Hermann H, Jones RN, Forster JW (1996) Molecular cytogenetic characterisation of the terminal heterochromatic segment of the B-chromosome of rye ( Secale cereale). Chromosoma 105:97–103
Jamilena M, Ruiz-Rejón C, Ruiz-Rejón M (1994) A molecular analysis of the origin of the Crepis capillaris B chromosome. J Cell Sci 107:703–708
Langdon T, Seago C, Jones RN, Ougham H, Thomas H, Forster JW, Jenkins G (2000) De novo evolution of satellite DNA on the rye B chromosome. Genetics 154:869–884
López-León MD, Neves N, Schwarzacher T, Heslop-Harrison JS, Hewitt GM, Camacho JPM (1994) Possible origin of a B chromosome deduced from its DNA composition using double FISH technique. Chromosome Res 2:87–92
López-León MD, Vázquez P, Hewitt GM, Camacho JPM (1995) Cloning and sequence analysis of an extremely homogeneous tandemly repeated DNA in the grasshopper Eyprepocnemis plorans. Heredity 75:370–375
McAllister BF, Werren JH (1997) Hybrid origin of a B chromosome (PSR) in the parasitic wasp Nasonia vitripennis. Chromosoma 106:243–253
Page BT, Wanous MK, Birchler JA (2001) Characterization of a maize chromosome 4 centromeric sequence: evidence for an evolutionary relationship with the B chromosome centromere. Genetics 159:291–302
Perfectti F, Werren JH (2001) The interspecific origin of B chromosomes: experimental evidence. Evolution 55:1069–1073
Puertas MJ (2002) Nature and evolution of B chromosomes in plants: a non-coding but information-rich part of plant genomes. Cytogenet Genome Res 96:198–205
Stark EA, Connerton I, Bennet ST, Barnes SR, Parker JS, Forster JW (1996) Molecular analysis of the structure of the maize B chromosome. Chromosome Res 4:15–23
Stevens JP, Bougourd SM (1994) Unstable B-chromosomes in a European population of Allium schoenoprasum L. (Liliaceae). Biol J Linn Soc 52:357–363
Wilkes TM, Francki MG, Langridge P, Karp A, Jones RN, Forster JW (1995) Analysis of rye B chromosome structure using fluorescent in situ hybridization (FISH). Chromosome Res 3:466–472
Acknowledgements
This study was supported by grants from the Spanish Ministerio de Ciencia y Tecnología (BOS2000-1521) and Plan Andaluz de Investigación, Grupo no. CVI-165.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E.R. Schmidt
Rights and permissions
About this article
Cite this article
Cabrero, J., Bakkali, M., Bugrov, A. et al. Multiregional origin of B chromosomes in the grasshopper Eyprepocnemis plorans . Chromosoma 112, 207–211 (2003). https://doi.org/10.1007/s00412-003-0264-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-003-0264-2