Abstract
The purpose of this study was to evaluate the effect of 0.05% sodium fluoride and 0.12% chlorhexidine mouthwashes on the micro-hardness of tooth enamel and cementum that was exposed to therapeutic doses of gamma radiation. Sixty extracted human teeth were divided into two groups, one was irradiated, the other was not irradiated. The two groups were further subdivided into three subgroups, which were each treated either with 0.05% sodium fluoride or with 0.12% chlorhexidine; the third subgroup served as a control. After demineralization–remineralization cycling, teeth from the irradiated groups showed a significantly lower micro-hardness when compared to those from the non-irradiated groups. Both in the irradiated and non-irradiated groups, teeth from the control subgroups showed a significantly lower micro-hardness, as compared to teeth treated with sodium fluoride and chlorhexidine. For non-irradiated enamel samples, those treated with chlorhexidine showed a significantly less micro-hardness compared to those treated with sodium fluoride. In contrast, irradiated enamel showed no significant difference in micro-hardness, whatever treatment (chlorhexidine or sodium fluoride) was applied. For cementum, treatment with chlorhexidine resulted in a significantly lower micro-hardness compared to sodium fluoride, both for the irradiated and non-irradiated groups. It is concluded that gamma irradiation with therapeutic doses typically used for head and neck carcinoma treatment has a direct effect in reducing micro-hardness of tooth enamel and cementum. Mouthwash protocols including, for example, application of 0.05% sodium fluoride or 0.12% chlorhexidine three times per day for 6 weeks, can protect enamel and cementum against the reduction in hardness and demineralization caused by gamma irradiation. Sodium fluoride offers more protection compared to chlorhexidine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, a relatively high incidence of head and neck carcinoma including those of the oral cavity has been reported (Filion et al. 2010). Gamma irradiation is an important treatment regimen, either applied solely or as an adjuvant to surgery and chemotherapy (Wilken et al. 2011). Unfortunately, radiation therapy may also have devastating dento-oral effects. One of the most common late complications of head and neck radiotherapy is rampant caries affecting the entire dentition. This is commonly referred to as radiation caries (Konjhodzic-Prcici et al. 2010).
Radiation caries is a rapidly progressing, and a highly destructive type of dental caries (Vissink et al. 2003). While it was assumed that this process is the result of xerostomia caused by gamma irradiation on salivary secretion, other disorders that cause xerostomia do not produce rampant caries that is nearly as destructive as the caries caused by radiotherapy. This observation lead to the debate, as to whether radiation caries is caused by a direct or an indirect effect to the teeth, or possibly by a combination of both (Konjhodzic-Prcici et al. 2010; Verna et al. 2010). In vitro studies on irradiated teeth showed that after irradiation the mechanical properties of teeth including compressive, tensile strengths, and hardness become altered by the radiolysis of water that generates free radicals, which in turn oxidize and denature the organic components of dental structure (Kielbassa et al. 2006; Soares et al. 2010a; Verna et al. 2010).
The pattern of caries most commonly seen after radiotherapy is circumferential at the cervical part of the tooth, affecting smooth enamel surfaces that are normally resistant to decay, as well as exposed cementum surfaces. The progression of caries may be further accelerated by poor oral hygiene of these patients due to oral discomfort and trismus. Such carious lesions represent a challenge to the restorative dentist, because access to the cervical lesions is often restricted, the excavation of caries might be incomplete, the cavity preparation margins can be difficult to define and the preparations might provide little mechanical retention for the restorations (Hu et al. 2005). In addition, the restoration becomes compromised by the detrimental effect of gamma irradiation on bond strength to human enamel and dentin when the adhesive restorative procedure is carried out after radiotherapy (Naves et al. 2012). Radiation caries often results in fracture of the clinical crown of the tooth, and therefore mastication of food and esthetics become severely compromised (Andrews et al. 2001; Franzel et al. 2006).
Implementation of oral care protocols before and after radiation therapy and frequent assessment of lesions during therapy can prevent or, at least, decrease the incidence and severity of these complications, which can improve quality of life for head and neck cancer patients (Dheeraj et al. 2011; Soares et al. 2010b). Other preventive schemes include mouthwashes of chlorhexidine gluconate (0.12%), sodium fluoride (0.05%, aqueous solution), and sodium iodide (2%) in hydrogen peroxide (10 v/v) (Tolentino et al. 2011). However, there is no definitive protocol yet to prevent or reduce radiation caries in head and neck cancer patients.
Fluoride rinses have been shown to increase the re-hardening effect on softened enamel surfaces through enhancement of remineralization. Furthermore, the successful use of topically applied fluorides to prevent dental caries in patients suffering from radiation-induced hypo-salivation has been reported (Souza et al. 2009). Mouth rinses including fluoride and chlorhexidine rinses are easy to use for patients, and unlike foam and varnish, rinses do not need professional application. Therefore, they represent a suitable option for delivering a protective agent to patients who are suffering from head and neck cancer. The present study aims at investigating the role of fluoride and chlorhexidine mouthwashes in preserving hardness and mineralization of human enamel and cementum after gamma irradiation.
Materials and methods
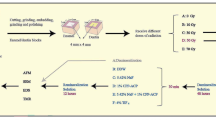
A total of 60 human posterior teeth were used in the present study. None of the teeth had any caries, restorations, surface defects, or enamel cracks. The teeth were extracted for periodontal reasons from individuals of both genders with an age range between 30 and 45 years, after receiving approval from the ethical committee at Al Azhar University. Before commencing the study, the teeth were thoroughly washed, scraped, and ultrasonically scaled, to remove plaque and calculus, then sterilized in ethylene oxide gas chamber for 8 h and stored in deionized water with thymol. The teeth were randomly divided into two main groups; Group A1: Irradiated (N = 30) and Group A2: non-irradiated (N = 30). Then, each group was further divided into three subgroups: B1, B2, and B3 (each =10 teeth) according to the immersion protocol received after gamma irradiation: 0.12% chlorhexidine mouthwash, 0.05% sodium fluoride mouthwash, and deionized water (control group).
Irradiation procedure
Group A1 samples were transferred to a cobalt-60 source (National Center for Research Radiation & Technology—Atomic Energy Authority) and exposed to γ-radiation. The dimensions of the irradiation chamber were: diameter 16 cm and height 20 cm. The teeth were positioned with their long axis perpendicular to the floor of the chamber and parallel to the cobalt source. Each tooth sample was irradiated with the same dose (60 Gy) which is typical for the therapeutic dose for treatment of carcinoma of the head and neck. The dose rate was in the range of 1.0431 KGy/hour at the time of the experiment (Maghraby et al. 2005).
Mouthwash simulation and demineralization–remineralization cycling
Two mouthwash protocols were used after irradiation. The mouthwash simulation was done using a mechanical stirrer (Aibote, Henan, China) three times/day for 1 min each, and each sample was rinsed with deionized water before and after the mouthwash simulation. The mouthwash simulation was performed for 6 weeks corresponding to a typical radiotherapy period, resulting in a total 180 min for the contact of the investigated teeth with mouthwash (Soares et al. 2010b). This procedure was followed by demineralization–remineralization cycling: all groups were subjected to five demineralization–remineralization cycles at 37 °C, using the model described by Rodrigues and co-workers (Rodrigues et al. 2004). Each cycle consisted of a 3-h immersion in demineralizing solution, followed by a 21-h immersion in re-mineralizing solution. The demineralizing solution was composed of 0.75 mM acetate buffer, containing 2.2 mM calcium (CaCl2), 2.2 mM phosphate (NaH2PO4), and 0.03 µg F/ml. The pH of the solution was 4.3. The chemical composition of the re-mineralizing solution was 1.5 mM calcium, 0.9 mM phosphate, 0.15 M KCl, 0.05 µg F/ml, and 20 mM cacodylate buffer, with a pH of 7.0. Both solutions contained thymol crystals to avoid microbial growth.
Micro-hardness testing
Each tooth was longitudinally sectioned to have a stable flat surface lingually. The buccal surface was flattened using a double-sided fine grit diamond disc (0.20 mm × 22 mm) operated at low speed. For this, an NSK Dental E-type low speed straight hand-piece was used at 18,000 rpm. The sample was then polished using an Enhance Densply rubber polishing cup and Densply polishing paste. Surface hardness of the specimens was determined using a digital display Vickers micro-hardness tester (model: Shimadzu HMV). The Vickers method is based on an optical measurement system. A load of 200 g was applied to the buccal surface of the specimens for 20 s using the Vickers diamond indentor. Three indentations were equally placed over a circle of 1 mm diameter at the middle third of the buccal surface of the enamel and the cervical third of the buccal surface of the cementum of the specimens. The indentations were carefully observed under the microscope at magnification 20×. Image analysis software allowed accurate digital measurements of their diagonals, which was then converted to micro-hardness values (MHV) using Eq. 1:
where P is the load in kg and d is the length of the diagonals in mm. Enamel and cementum hardness were determined for each tooth at baseline (after gamma irradiation) and after demineralization–remineralization cycling.
Statistical analyses
After demineralization–remineralization cycling, there was a decrease in enamel and cementum micro-hardness for all groups. This decrease was calculated for every subgroup and is presented as mean and standard deviation (SD) values. Student’s t test was used to compare between irradiated and non-irradiated groups. One-way analysis of variance (ANOVA) was used for comparison between mouthwashes. Tukey’s post-hoc test was used for pairwise comparison between the groups when ANOVA test was significant. The significance level was set at p ≤ 0.05. Statistical analysis was performed with IBM® SPSS® Statistics Version 20 for Windows.
Results
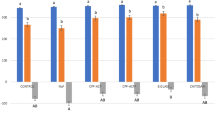
Results show that the decrease in micro-hardness of irradiated tooth enamel and cementum was significantly higher when compared to that of non-irradiated tooth enamel and cementum, regardless of the mouthwash protocol used (Tables 1, 2). Both irradiated and non-irradiated groups showed a significantly higher decrease in mean enamel and cementum micro-hardness for the control subgroups compared to the subgroups treated with sodium fluoride and chlorhexidine mouthwash (Tables 1, 2). Chlorhexidine showed a significantly higher decrease in micro-hardness compared to sodium fluoride when the enamel was not irradiated. In contrast, irradiated enamel showed no significant difference in the decrease of micro-hardness when comparing chlorhexidine with sodium fluoride mouthwashes (Table 1). For cementum, chlorhexidine mouthwash resulted in a significantly higher decrease in micro-hardness compared to sodium fluoride mouthwash. This was true for both irradiated and non-irradiated groups (Table 2).
Discussion
The results of this study provide evidence that 0.05% sodium fluoride mouthwash has a protective effect on enamel and cementum exposed to gamma radiation, by preserving their hardness and mineralization. Gamma irradiation with a dose typical for the treatment of head and neck cancer causes deterioration of the mechanical properties of dental hard tissues. The results of the present study and those of previous in vitro studies provide evidence of that effect. This can be explained by the fact that the organic matrix of enamel interacts with mineral apatite crystals via calcium ions from electrostatic binding of side chain carboxylate and surface mineral phosphate groups. Irradiation promotes side chain decarboxylation and a loss of acidic phosphate groups with the formation of new calcium ion-bridged phosphate groups. The mineral-organic interaction between apatite and organic constituents of enamel is reduced which induces micro cracks in the hydroxyapatite mineral. The cementum when exposed to gamma radiation suffers a reduction of the hydration level in the root matrices, resulting in alternations in the tissue extra cellular matrix. This process is always associated with the scission of the main chain of collagen macromolecules (Soares et al. 2010b).
Few studies demonstrated the histological changes of dental hard tissues exposed to gamma radiation. Scanning electron microscopy showed that enamel suffered surface cracks after irradiation that could easily be observed due to localized erosive areas as well as morphologic alterations and increased gaps with separation of prisms from the inter-prismatic substance. The regular structure of enamel was completely destroyed and the internal enamel structure appeared amorphous and porous. The cementum showed surface irregularities with wide cracks and a decline in Sharpey’s fiber sites, as well as erosive areas with variable depths on the surface (El-Faramawy et al. 2011). Light microscopy showed that irradiated cellular cementum had irregular concavities and dispersed bay-like resorption concavities with adjacent multinucleated cells, similar to the appearance of cementoclasts howship’s lacunae. The predominance of resorption sites related to cementum after irradiation was explained by an enhanced radio-resistance of resorptive cells compared to synthetic cells, which leads to an increase in lytic activity (El-Faramay et al. 2013).
Results of a few studies appear to be in contradiction to the findings described in the present paper. Kielbassa and co-workers (Kielbassa et al. 1999, 2000) reported that gamma irradiation did not cause a decrease in micro-hardness or mineralization of enamel. This seeming contradiction can be explained by the fact that the specimens used by Kielbassa and co-workers were kept wet during irradiation, whereas in the present study, irradiation took place in vacuum. It is known that the apatite crystals of tooth enamel include some sodium, carbonate, and magnesium by entrapment during their formation. On irradiation, these point defects could be mobilized from the surface layer of the crystals, thereby removing the entrapped ions. Wet conditions can stabilize the surface layers of the apatite crystals of enamel, therefore, reducing the dissolution rate (Jansma et al. 1990). In contrast, when the irradiation takes place under vacuum, the structural changes that have occurred in enamel might be irreversible. Most patients undergoing head and neck radiotherapy suffer from severe xerostomia during their illness and treatment (Chambers et al. 2007). This is mainly due to the destructive effect of radiation on salivary glands. The oral cavity becomes very dry and the teeth are deprived from the protective effects of saliva. Thus, irradiating the teeth under wet conditions may not represent an ideal condition for investigating the effect of gamma irradiation on tooth structure.
Clinical data suggest that the sensitivity of the dental pulp is impaired in patients undergoing head and neck radiotherapy. In contrast, histological studies that compared morphological aspects of the microvasculature and innervation of dental pulps obtained from teeth of patients who underwent head and neck radiotherapy and control dental pulps showed that there were no evident differences in the morphology of blood vessels and nerve bundles, the presence of odontoblasts, the patterns of inflammatory infiltration, and the amount of calcification or fibrosis. These data indicate that direct effects of radiotherapy are not able to generate morphologic changes in the microvasculature, innervation, and extracellular matrix components of the dental pulp in head and neck cancer patients (Faria et al. 2014).
Sodium fluoride mouthwash had a significant effect in this study, in minimizing loss of hardness of enamel, when used three times daily for 1 min, for a total period of 6 weeks, which corresponds to the timeframe of radiotherapy treatment for head and neck cancers. This effect is due to the ability of fluoride ions to substitute for hydroxyl ions forming fluoroapatite, which is harder and less susceptible to dissolution. This is the primary reason underlying the beneficial use of fluoride for enamel undergoing radiotherapy: Incorporation of fluoroapatite decreases the space filled by the organic matrix, which is the component most affected by gamma irradiation (Soares et al. 2010b). This explanation is in agreement with (Meyerowitz et al. 1991), who reported that application of 0.05% sodium fluoride mouthwash twice a day for a period of 28 days increased hardness of demineralized enamel, prevented demineralization and enhanced remineralization of enamel subjected to therapeutic radiotherapy dose. Furthermore, Markitziu and co-workers (Markitziu et al. 1991) reported a decrease in enamel solubility of irradiated enamel when treated with fluoride. The results of the present study show a protective effect by sodium fluoride rinse on the cementum of irradiated and non-irradiated teeth similar to that found on enamel. This finding is in agreement with those of other studies in the literature. For example, Almqvist and Lagerlof reported that fluoride concentration attained in cementum after rinses with of 0.025, 0.2, and 1.0% sodium fluoride are highly effective, in inhibiting root hard-tissue demineralization and enhancing remineralization (Almqvist and Lagerlof 1993). The protective effect of fluoride on cementum was also found to depend on fluoride concentration (Heilman et al. 1997).
When comparing the decrease in micro-hardness of enamel and cementum subjected to 0.12% chlorhexidine mouthwash with that of control groups which received deionized water, chlorhexidine showed a protective effect for both irradiated and non-irradiated groups. Chlorhexidine was found to maintain morphological properties of tooth structure and prevents the degradation of collagen. Attin and co-workers showed that over a period of only 7 days, 41–42% of a chlorhexidine solution was absorbed by the tooth surface (Attin et al. 2008). Lim and Choi demonstrated that enamel treated with chlorhexidine showed statistically significantly higher Vickers micro-hardness values compared to that of a control group treated with saline (Lim and Choi 1998). Several other studies showed that chlorhexidine mouthwash, twice a day for a period of four weeks protected dental hard tissues from demineralization when compared to saline solution mouth wash. For example, Katz and co-workers (1982) and Joysten-Bechal and co-workers (1992) recommended the use of chlorhexidine rinse before, during and after radiotherapy as it stopped radiation caries and caused arrest of pre-existing enamel lesions. Bizhang and co-workers (2007) found that chlorhexidine varnish resulted in significantly decreased mineral loss and lesion depth when compared to a control group for teeth exposed to 60 Gy of radiation. Chlorhexidine rinse is retained in the oral cavity for almost four hours after rinsing and remains active in spite of radiation-induced changes in the oral cavity and salivary glands (Toljanic et al. 1992). The protective effect of chlorhexidine on cementum can be explained by the fact that chlorhexidine can bind to the collagen fibrils and maintains the structure of the network of collagen fibers (Soares et al. 2010b).
It is interesting to note that fluoride mouthwash showed a more significant protective effect than chlorhexidine mouthwash when the enamel was not irradiated. In contrast, irradiated enamel showed no significant difference in the decrease of micro-hardness when comparing chlorhexidine with sodium fluoride subgroups (Table 1). This may be explained by the fact that the irradiated enamel suffered a higher loss in hardness compared to the non-irradiated enamel as well as alteration in mechanical properties and prismatic structure and thus may respond differently to mouthwash treatment. It is possible that chlorhexidine was absorbed better in irradiated enamel due to the presence of cracks and erosive areas, caused by irradiation (El-Faramawy et al. 2011). Further research would be needed to explore this finding.
Conclusion
Gamma irradiation with typical therapeutic doses for head and neck carcinoma has a direct effect in reducing the hardness of tooth enamel and cementum. Mouthwash regimens, such as 0.05% sodium fluoride and 0.12% chlorhexidine used three times daily for 6 weeks, can significantly protect enamel and cementum against the reduction in hardness and demineralization caused by gamma irradiation. Sodium fluoride offers more protection compared to chlorhexidine.
References
Almqvist H, Lagerlof F (1993) Effect of intermittent delivery of fluoride to Solution on root hard-tissue de- and remineralization measured by absorptiometry. J Dent Res 72:1593–1598
Altenburgera MJ, Klasserc M, Schirrmeistera JF, Hellwiga E (2006) Remineralization of carious enamel lesions after application of a CHX/F-mouthrinse compared with sole CHX- and placebo-application. Oral Health Prev Dent 4:255–263
Andrews N, Griffits C (2001) Dental complications of head and neck radiotherapy. Aus Dent J 46:174–182
Attin T, Abouassi T, Becker K, Wiegand A, Roos M, Attin R (2008) A new method for chlorhexidine determination: CHX release after application of differently concentrated CHX-containing preparations on artificial fissures. Clin Oral Investig 12:189–196
Bizhang M, Seemann R, Römhild G, Chun YH, Umland N, Lang H, Zimmer S (2007) Effect of a 40% chlorhexidine varnish on demineralization of dentin surfaces in situ. Am J Dent 20:193–197
Brooks SL, Miles DA (1993) Advances in diagnostic imaging in dentistry. Dent Clin North Am 37:575–590
Chambers MS, Rosenthal DI, Weber RS (2007) Radiation-induced xerostomia. Head Neck 29:58–63
Dheeraj K, Namrataa R, Sudhir K, Amit S (2011) Oral complications and Its management during radiotherapy. Indian J Dent Sci 4:50–53
El-Faramawy N, Ameen R, El-Haddad K, Maghraby A, El-Zainy M (2011) Effects of gamma radiation on hard dental tissues of albino rats using scanning electron microscope—Part 1. Radiat Eff Defect S 166: 927–934
El-Faramawy N, Ameen R, El-Haddad K, El-Zainy M (2013) Effects of gamma radiation on hard dental tissues of albino rats: investigation by light microscopy. Radiat Environ Biophys 52:375–387
Faria KM, Brandao TB, Ribeiro ACP, Vasconcellos AFG, Thiago de Carvalho I, Freire de Arruda F, Junior GC, Gross VC, Almeida OP, Lopes MA, Santos-Silva AR (2014) Micromorphology of the dental pulp is highly preserved in cancer patients who underwent head and neck radiotherapy. J Endod 40:1553–1559
Filion EJ, McClure LA, Huang D, Seng K, Kaplan MJ, Colevas AD, Gomez SL, Chang ET, Le QT (2010) Higher incidence of head and neck cancers among Vietnamese American men in California. Head Neck 32:1336–1344
Fränzel W, Gerlach R, Hein HJ, Schaller HG (2006) Effect of tumor therapeutic irradiation on the mechanical properties of teeth tissue. Z Med Phys 16:148–154
Heilman JR, Jordan TH, Warwick R, Wefel JS (1997) Remineralization of root surfaces demineralized in solutions of differing fluoride levels. Caries Res 31:423–428
Hu JU, Chen XC, Li YQ, Smales RJ, Yip KH (2005) Radiation-induced root surface caries restored with glass ionomer cement placed in conventional and ART cavity preparations: results at two years. Aus Dent J 50:186–190
Jansma J, Borggreven JMPM, Driessens FCM, Gravenmade EJ (1990) Effect of X-ray irradiation on the permeability of bovine dental enamel. Caries Res 24:164–168
Joyston-Bechal S, Hayes K, Davenport ES, Hardie JM (1992) Caries incidence, mutans streptococci and lactobacilli in irradiated patients during a 12-month preventive program using chlorhexidine and fluoride. Caries Res 26:384–390
Katz S (1982) The use of fluoride and chlorhexidine for the prevention of radiation caries. J Am Dent Assoc 104:164–170
Kielbassa AM, Wrbas KT, Schulte-Mönting J, Hellwig E (1999) Correlation of transversal microradiography and microhardness on in situ-induced demineralization in irradiated and non-irradiated human dental enamel. Arch Oral Biol 44:243–251
Kielbassa AM, Schendera A, Schulte-Mönting J (2000) Microradiographic and microscopic studies on in situ induced initial caries in irradiated and non-irradiated dental enamel. Caries Res 34:41–47
Kielbassa AM, Hellwig E, Meyer-Lueckel H (2006) Effects of Irradiation on in situ remineralization of human and bovine enamel demineralized in vitro. Caries Res 40:130–135
Konjhodzic-Prcici A, Keros J, Ajanovic M, Smajkic N, Hasic-Brankovic L (2010) Incidence of radiation caries in patients undergoing radiation therapy in the head and neck region. Pesq Bras Odontoped Clin Integr João Pessoa 10:489–492
Lim EK, Choi YC (1998) The preventive effect of chlorhexidine varnish on enamel demineralization. J Korean Acad. Pediatr Dent 25:825–836
Maghraby E, Badr N, Mahmoud E (2005) Effect of γ-radiation on selected mechanical properties of tooth-colored restorative materials. Egypt Dent J 51:805–818
Markitziu A, Weshler Z, Avital M, Gedalia I (1991) Modulatory effect of fluoride and irradiation on rat molar rate of wear. Bull Group Int Rech Sci Stomatol Odontol 34:139–144
Meyerowitz C, Featherstone JD, Billings RJ, Eisenberg AD, Fu J, Shariati M, Zero DT (1991) Use of an intra-oral model to evaluate 0.05% sodium fluoride mouth rinse in radiation-induced hypo salivation. J Dent Res 70:894–898
Naves LZ, Novais VR, Armstrong SR, Correr-Sobrinho L, Soares CJ (2012) Effect of gamma radiation on bonding to human enamel and dentin. Support Care Cancer 20:2873–2878
Rodrigues LKA, Jaime AC, Marines N (2004) The effect of gamma radiation on enamel hardness and its resistance to demineralization in vitro. J Oral Sci 46:215–220
Soares CJ, Moura CCG, Soars PB, Naves LZ (2010a) Scanning electric microscopy used to analyze the effect of gamma irradiation on enamel and dentin. Microscopy: science, technology, applications and education microscopy book series. Volume 1. Badajoz, Spain, pp 372–378
Soares CJ, Castro CG, Neiva NA, Soares PV, Santos-Filho PCF, Naves LZ (2010b) Effect of gamma irradiation on ultimate tensile strength of enamel and dentin. J Dent Res 89:159–164
Souza FRN, Mont LM, Ciesielskil FIN, De Castro AL, Pace G, Gaetti-Jardim E (2009) Influence of preventive protocols on side effects of radiotherapy for treatment of head and neck cancer. Int J Odontostomatol 3:167–172
Tolentino E, Centurion BS, Ferreira LHC, De Souza AP, Damante JH, Rubira-Bullen IRF (2011) Oral adverse effects of head and neck radiotherapy: literature review and suggestion of a clinical oral care guideline for irradiated patients. J Appl Oral Sci 19:448–454
Toljanic JA, Hagen JC, Takahashi Y, Shapiro RD (1992) Evaluation of the substantivity of a chlorhexidine oral rinse in irradiated head and neck cancer patients. J Oral Maxillofac Surg 50:1055–1059
Van Strijp AJ, Gerardu VA, Buijs MJ, Van Loveren C, Ten Cate JM (2008) Chlorhexidine efficacy in preventing lesion formation in enamel and dentine An in situ Study. Caries Res 42:460–465
Verna A, Botta SB, Seino PY, Ana PA, Mathor MB, Matos AB, Oda M (2010) Microhardness evaluation of bovine teeth after gamma radiation sterilization. Dissertation, School of Dentistry University of São Paulo
Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP (2003) Oral sequele of head and neck radiotherapy. Crit Rev Oral Biol Med 14:199–212
Wilken R, Veena MS, Wang MB, Srivatsan ES (2011) Curcumin: a review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer 10:1–19
Yokota ET, Miles DA, Newton CW, Brown CE (1994) Interpretation of periapical lesions using RadioVisioGraphy. J Endodon 20:490–494
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdalla, R., Niazy, M.A., Jamil, W.E. et al. The role of fluoride and chlorhexidine in preserving hardness and mineralization of enamel and cementum after gamma irradiation. Radiat Environ Biophys 56, 187–192 (2017). https://doi.org/10.1007/s00411-017-0690-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-017-0690-9