Abstract
Ionizing radiation can induce a wide range of DNA damage that leads to chromosomal aberrations. Some of those aberrations (dicentrics and micronuclei) are applied in biodosimetry. Biological dosimetry assumes similar radiosensitivity of each donor, but it does not exclude inter-individual variations in radiation susceptibility. Therefore, for biological reasons, it is always challenging to investigate inter-individual variability in response to radiation. For mechanistic reasons, it is also interesting to investigate the correlation between dicentric and micronuclei formation in response to radiation. In this experiment, irradiated blood specimens from 14 healthy male and female donors have been used to evaluate inter-individual variability in response to the genotoxic effects of X-ray radiation, as well as the dose–response relationship and test sensitivity using two endpoints (dicentrics and micronuclei). The results showed similar patterns of cytogenetic biomarker distribution between donors, but differences in the response of some donors at some doses. Data also showed that responses of male donors were better detected using the dicentric test, while for females, micronucleus frequencies were higher in response to the same dose of radiation. No influence of smoking status or age on specific responses was observed. Group variability in response to radiation was evaluated using coefficient of variation for each group of individuals irradiated with the same doses; as the dose increases, group variability becomes substantially lower. Despite sporadic inter-individual variability, trend of radiation-induced changes was similar. Produced calibration curves for both types of damage revealed dicentrics as genetic damage more typical for radiation than micronuclei.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ionizing radiation (IR) is a potent genotoxic agent that can induce a wide range of DNA lesions, including damage to nitrogenous bases, DNA–DNA and DNA–protein cross-linking, and single-strand DNA breaks (SSBs) and double-strand DNA breaks (DSBs). It is generally agreed, however, that the formation of DSBs is the critical radiation-induced type of damage that leads to chromosomal aberrations, such as formation of dicentrics, reciprocal translocations and rings, which involve the interaction of DSBs with each other (Rodrigues et al. 2005).
As a clastogenic agent, IR can also lead to micronuclei (MN) formation. Micronuclei induced by IR arise from acentric chromosome fragments and whole chromosomes that are unable to interact with the spindle. They lag behind at anaphase, so are not included in the main daughter nuclei (IAEA 2011).
Since their formation is induced almost exclusively by IR, those aberrations are successfully applied in biodosimetry, a methodology based on the investigation of radio-induced biological effects in order to correlate them with the dose of radiation (Silva-Barbosa et al. 2005). Biodosimetry is a valuable dose assessment method, especially when there are difficulties in interpreting the data given by physical dosimetry, in cases of radiation exposure where there are no physical dosimetry data, or in cases of overexposure during an individual’s working lifetime (IAEA 2011). Biological dosimetry using chromosome damage biomarkers is particularly important because it assumes similar radiosensitivity of each donor, but it does not exclude inter-individual variations in radiation susceptibility. Therefore, for biological reasons, it is always challenging to investigate inter-individual variability in response to powerful genotoxic agents such as IR, especially at low doses.
For mechanistic reasons, it might also be interesting to investigate the correlation between dicentric formation in response to IR measured in individual cells by the dicentric assay and the frequencies of MN found under the same experimental conditions. This comparison should allow estimation of the degree of variability observed by both methods and the test capabilities in response to various doses of radiation.
The aims of this study were to evaluate the inter-individual variability in response to the genotoxic effects of X-ray radiation, as well as the dose–response relationship and test sensitivity using two endpoints (dicentrics and micronuclei).
Materials and methods
Blood sampling and irradiation
The study included 14 healthy donors (6 males and 8 females), aged between 27 and 48 years (mean age 38 ± 6.5). Detailed information on the occupational and medical history of all subjects was obtained by completion of a targeted questionnaire including demographic data, smoking history, diet, alcohol intake, use of medication and exposure to radiation for medical purposes. Data on demographic and lifestyle factors of the participants covered by the questionnaire are presented in Table 2.
Eight peripheral venous blood samples were collected from each individual by cubital venepunction in Li-heparin vacutainers. Equal samples were collected shortly before the start of irradiation (IAEA 2011). One of them was left unirradiated (control), and the other seven were treated with one of the following doses of 6-MeV X-ray radiation: 0.1, 0.2, 0.4, 0.8, 1, 1.5 and 2 Gy, respectively.
Using a CLINAC 600EX machine, samples were irradiated at 100 cm distance from the source, at 2 cm depth, in a PMMA phantom of suitable dimensions, over an area of 15 × 15 cm2, at the room temperature.
The study fulfilled all ethical standards according to the Declaration of Helsinki.
Cell culture
Dicentric test
Li-heparin vacutainers were irradiated, and then the blood was held at 37 °C for 2 h to provide a time interval in which DNA reparation could occur. Unirradiated vacutainers were prepared in the same manner as irradiated ones.
Whole-blood microcultures were prepared using RPMI 1640 medium supplemented with 10 % of fetal bovine serum (Life Technologies, Carlsbad, Calif.). Lymphocytes were stimulated with phytohemagglutinin (PHA, PAA) at 5 µg/ml for 48 h at 37 °C. During the last 2 h of incubation, colchicine (0.05 µg/ml; Sigma-Aldrich, St. Louis, MO) was added to the medium. The cells were exposed to hypotonic solution (20 min) by stepwise addition of 0.075 M KCl followed by fixation (3 × 20 min) with cold methanol/acetic acid (3:1). Fixed cells were spread on slides and dried over a flame. The slides were aged for the next 5 days. Giemsa-stained slides were coded and scored blind under a light microscope. No FPG staining was used to discriminate between M1 and M2 cells. One thousand well-spread metaphases per subject per dose (including unirradiated samples) were screened for chromosome damage (dicentric chromosomes). Chromosomal aberrations in peripheral blood lymphocytes were analyzed according to the standard protocol (IAEA 2011).
CBMN test
For the CBMN test, irradiated as well as unirradiated lymphocytes were cultivated by the same routine as for the classical cytogenetic analysis (IAEA 2011). After incubation for 44 h, 0.1 ml of cytochalasin B (Sigma-Aldrich) solution in DMSO (Sigma-Aldrich) (final concentration of 3 μg/ml) was added to the cultures, and cultivation was continued for another 24 h. The cultures were then treated with 0.9 % NaCl solution, followed by a cold hypotonic solution (0.075 M KCl) to lyse red blood cells. The supernatant was removed and replaced with fixative consisting of methanol/acetic acid (3:1) with 1 % formaldehyde. After incubation for 20 min at room temperature, the cells were washed with fixative for four times without formaldehyde. The cells were then re-suspended gently, and the suspension dropped onto clean glass slides and allowed to dry. Cells were stained using 10 % Giemsa in distilled water.
One thousand binucleate cells per subjects per dose were analyzed, and the total number of MN found and their distribution recorded.
Statistical analysis
Dicentric and MN frequencies were calculated for controls and each radiation dose. Data distribution was examined by Kolmogorov–Smirnov’s test. The difference between dose-dependent values of the examined parameters was accessed by the paired samples t test and Mann–Whitney U test, depending on the data distribution. Inter-individual variation in dose-responsive dicentric and MN frequencies was evaluated by the one-sample Chi-squared test. Coefficient of variation (CV) was used as the measure of group variability in response to IR for both dicentric and MN frequencies. Groups defined for this purpose consisted of all individuals subjected to the same dose of IR. Dose–response curves, which display dicentric and MN frequencies, were fitted by nonlinear regression using a linear-square equation Y = c + αD + βD 2. H-response ratio was calculated as ratio of the number of higher than expected (H) values of observed cytogenetic changes and the count of measurements.
The analyses were performed using the Statistical Package for Social Sciences, SPSS 17.0 statistics (SPSS Inc, IBM, USA) and dose estimation software DoseEstimate v.5.
Results
An increase in chromosome damage was demonstrated, i.e., dicentric and MN frequency displayed dose-dependent increases.
Mean values for parameters of chromosome damage (dicentrics and micronuclei) for all donors at all doses are given in Fig. 1. There was a highly significant difference (p < 0.001) in both dicentric and MN frequencies when compared with non-irradiated blood samples. The apparent dose-dependent increase in the examined parameters can be observed in both histograms. Furthermore, for irradiated samples, a highly significant difference (p < 0.001) was noted when dicentric frequencies were compared between all dose points. For micronuclei, significant differences were found between the following consecutive dose points: 0.4–0.8, 0.8–1, 1–1.5 and 1.5–2 Gy.
Looking at the data more closely, it can be seen that some donors exhibit higher or lower responses at some doses than the average. Inter-individual variabilities in dicentric and MN frequencies are shown in Fig. 2. It can be observed that, regardless of the dose, different donors had similar distributions of the observed values.
Table 1 shows each donor’s response: higher response than the average (denoted with H) or lower than the average (denoted with L). Comparing the results for dicentric and CBMN test, it can be observed that some donors tended to have a higher response than the average value, regardless of the dose or method. In order to determine how the response differs among donors and methods, we calculated the H-response ratio for each donor, between the number of H values and the count of measurements.
For example, donor 1 had higher values than the average 8 out of 8 times for dicentric analysis, so his H-response ratio would be 1. The values of the H-response ratio of all donors are given in Table 4 sorted by overall H-response ratio. Some additional information about the donors (age, gender and smoking habit) is also shown in Table 4.
Donor 1 exhibited a higher response in most cases (overall H-response ratio 93.75 %), while donors 9 and 14 generally had lower responses (overall H-response ratio 18.75 %). Their results differed from those of other donors whose H-response ratios were not so extreme. There were also differences in H-response ratio between tests, i.e., the response of some donors was better detected using the dicentric test, and for some of them, micronucleus frequencies were higher in response to the same dose of IR. Moreover, females had higher H-response ratios for MN than males in almost all cases (Table 2).
We further analyzed the data to see whether there was a statistically significant difference between the proportions of lower and higher responses among smokers and non-smokers. Figure 3 shows the counts for lower and higher responses for both groups and both methods.
Detailed proportions are given in Tables 3 and 4. Both tables show the influence of smoking status on overall response at all applied doses of radiation measured by dicentric (Table 3) and micronuclei frequency (Table 4). As can be seen in Table 3, there were 33.9 % overall lower and 23.2 % overall higher responses among non-smokers and 21.4 % overall lower and 21.4 % overall higher responses among smokers, measured by dicentric analysis. Table 4 shows 33.9 % overall lower and 23.2 % overall higher micronuclei responses among non-smokers and 19.6 % overall lower and 23.2 % overall higher micronuclei responses among smokers.
The proportions were analyzed by the Chi-squared test, which did not show any statistically significant differences between the responses of smokers and non-smokers in the observed group of donors, either for dicentric analysis (p = 0.411) or for MN analysis (p = 0.155).
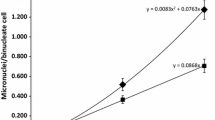
Group variability in response to IR was evaluated using CV for each group of individuals irradiated with the same dose. The results for both tests are presented in Fig. 4. For both tests, group variability in response to IR exponentially decreased as the doses were increased. According to the coefficients of determination shown in Fig. 4, it is clear that group variability to IR reflected in dicentric frequency is considerably lower and more homogenous than it is reflected in the MN response. CVs for both examined parameters have one common characteristic; as the dose increases, group variability becomes substantially lower.
Relationship between dose–response curves for dicentric and micronucleus induction
All the donors showed similar trends of changes in radiation-induced dicentrics and micronuclei. Therefore, one type of damage for all donors was combined in a single pool and compared to the other.
The linear-squared parameters of the curve for the dicentric test are α ± SE (0.0526 ± 0.0097) and β ± SE (0.0591 ± 0.0073) and, for the micronucleus test, α ± SE (0.0715 ± 0.0143) and β ± SE (0.0089 ± 0.0087). The dose–response curves are presented in Fig. 5. At low-dose points, it was observed that both curves follow similar patterns as indicated by similar α values. However, at higher doses, a large difference between β coefficients was observed, with a higher β value for dicentrics.
Discussion
Radiation-induced chromosomal changes can be detected and measured by the classical chromosomal aberration technique, based on scoring unstable chromosomal aberrations (dicentric chromosomes), a change in chromosome structure essentially unique for IR (IAEA 2011). Due to very low background levels of dicentrics (1–2 dicentrics per 1000 examined metaphase spreads), the test is sensitive enough to estimate biological effects at low doses (IAEA 2011).
However, MN background levels are generally higher than dicentric chromosomes and, unlike dicentrics, MN are not specific for radiation (IAEA 2011), although the overall impression, based on conducted studies of exposure to genotoxic agents in vitro and in vivo (Kocisova and Sram 1990; Maki-Paakinen et al. 1991; Migliore et al. 1991), is that the CBMN assay may be relatively insensitive to exposure to chemicals and agents that are either S-phase dependent or mainly induce covalent adducts on DNA (Fenech 1993).
Both tests applied in this study are conventionally used biomonitoring tools for analysis of human populations exposed to various genotoxic agents, including IR (Paul et al. 1997), as well as in biodosimetry (IAEA 2011). Furthermore, both assays are established cytogenetic techniques in the evaluation of intrinsic cellular radiosensitivity in both tumor cells and lymphocytes (Di Giorgio et al. 2010).
In this study, peripheral blood samples from 14 healthy donors were irradiated with seven doses, ranging from 0.1 to 2 Gy, in order to evaluate individual radiosensitivity, measured by the difference in response to radiation, as well as the ability of the tests to express this response. For the dicentric test, no FPG staining of metaphases was used to discriminate between M1 and M2 cells. Instead, cultures were incubated for 48 h, and during the last 2 h of incubation, colchicine was added to the medium. Based on previous experiments in our laboratory, this protocol is expected to give less than 5 % of cells in the second cell cycle. Radiation-induced chromosomal damage in peripheral blood lymphocytes was measured by each technique separately, and the results compared.
As anticipated, the control frequencies of dicentrics and micronuclei from unirradiated cells were close to 1 dicentric per 1000 metaphases and 10 micronuclei per 1000 binuclear lymphocytes and a clear dose-dependent increase in frequency of both endpoints was observed (Stephan and Pressl 1999; IAEA 2001, 2011).
Data showed similar patterns of cytogenetic biomarker distribution between donors, but differences in the response of some donors at some doses (higher or lower responses than the average). In order to determine how the response differs among donors and methods, the H-response ratio was calculated for each donor, as the ratio between the number of H values and the count of measurements. There were two extremes—donor 1, with an overall H-response ratio of 93.75 %, as an over-reactor, and donors 9 and 14, who had lower responses in most cases (overall H-response ratio of 18.75 %). All of the allocated donors were male and non-smokers, and the low responders were older than the over-reactor, showing that individual radiosensitivity, as an inherent characteristic associated with an increased reaction to IR, can probably be explained by differences in DNA repair capacity, due to specific mutations or polymorphisms in DNA repair genes (Parshad and Sanford 2001; Smart et al. 2003), rather than by age or lifestyle factors. Further analysis of differences in proportions of lower and higher responses and smoking status confirmed this. H-response ratios for other donors were not so extreme.
The results also showed differences in the H-response ratio between the tests. Thus, responses of male donors were better detected using the dicentric test, while for females, micronucleus frequencies were higher in response to the same dose of IR. No influence of smoking status or age on specific responses was observed. This finding could be expected for dicentrics since their formation is caused almost exclusively by ionizing radiation (Ainsbury et al. 2011) as confirmed in cytogenetic study of healthy unexposed persons, which reported that age, sex and smoking habit were not significant predictors of chromosomal aberrations (Stephan and Pressl 1999). Regarding micronuclei, it is well known that the micronucleus frequency is affected by age, gender, diet and other lifestyle factors (Fenech et al. 2011; Nefic and Handzic 2013; Antunes et al. 2014). Regarding the influence of smoking on micronuclei formation, study results are inconsistent. Some authors (Fenech 1993; Bonassi et al. 2005) showed that significant increases were seen in those who smoked more than 30 cigarettes/day, while most laboratories included in HUMN project (Bonassi et al. 2003) showed no significant differences between smokers and non-smokers. These literature data are in compliance with the results of this experiment: Our volunteers smoked maximum of 20 cigarettes/day, so no significant influence of smoking on micronuclei formation could be expected.
The higher responses of micronuclei in female donors can be explained by the fact that women have higher basic values of MN than men (Fenech et al. 1994; Parshad and Sanford 2001). In an international study involving nearly 7000 subjects, the baseline of micronucleus frequency in woman was 19 % higher than in men (Bonassi et al. 2001; Fenech and Bonassi 2011). These results suggest that an additional mechanism, possibly the loss of X chromosomes, contributes to the micronucleus frequency in females. The importance of sex as a variable should be taken into consideration when interpreting data from cross-sectional studies utilizing the cytokinesis-block micronucleus assay as a biomarker of chromosome damage.
Inter-individual variability of the response to IR depends on various factors, among which intrinsic factors are of the greatest significance (Parshad and Sanford 2001; Smart et al. 2003). These include different genetic predispositions in the efficiency of DNA repair mechanisms, cellular redox status and overall quantitative features of each individual (Bolegenova et al. 2009; Ginsberg et al. 2011; Mutlu-Türkoğlu et al. 2003). In this study, we observed that when low doses of IR are applied, these intrinsic differences between individuals become visible; some persons will react with greater genotoxic damage, while the others will exhibit lower genotoxic damage that can be observed via chromosome aberrations and CBMN testing. However, high doses of IR are characterized by the emission of large amounts of energy which causes significant damage to the genome. This extent of damage comes close to the individual’s adaptation maximum, and the intrinsic characteristics become a factor of very poor influence on radiation susceptibility and dose-dependent variability. However, the group variability to different doses displayed the same pattern for both tests used.
The chromosome aberration test using dicentrics as cytogenetic endpoint was shown to be a more accurate biomarker with lower inter-individual variability in predicting radiation-induced damage than the CBMN test, as previously stated by other authors (Ainsbury et al. 2011; IAEA 2011). This suggests that micronuclei development is much more dependent on intrinsic factors than the formation of dicentric chromosomes, which is in compliance with results of related research (Fenech and Morley 1986; Fenech 2007; Ceppi et al. 2011). This finding could be used for establishing more accurate confidence intervals for baseline MN frequencies and for a fuller explanation of the heterogeneity of MN values obtained in unexposed populations.
As all the donors showed similar trends of changes in radiation-induced chromosomal aberrations (dicentrics) and micronuclei, one type of damage from all donors was combined in a single pool and compared to the other in order to produce dose–response calibration curves for both kinds of chromosomal changes. A linear relationship between dose and chromosomal changes was noted at low doses, but at higher-dose points, a quadratic relationship was observed. For low LET radiation (e.g., X- and γ-rays), there is a very strong evidence that the yields of chromosome aberrations or micronuclei (Y) are related to dose (D) by the linear quadratic equation: Y = C + αD + βD 2, where C is the background frequency of chromosome aberrations/micronuclei, α is the coefficient for exchange aberrations produced by a single electron track, and β is the coefficient for aberrations produced as a consequence of two electron tracks (IAEA 2011).
The linear quadratic curves shown in Fig. 5 do not differ at low radiation doses. The linear components of the two linear quadratic curves, which define the relationship between damage and radiation dose at low-dose points, were similar, i.e., less than 30 % difference (α = 0.0526 for dicentrics and α = 0.0715 for micronuclei). The quadratic components of the curves at high doses were different (β = 0.0591 for dicentrics and β = 0.0089 for micronuclei), with an almost sevenfold higher value of the β coefficient for dicentrics. This high value results from the increase in dicentric frequencies with increasing radiation dose. Therefore, dicentrics can be considered as more typical cytogenetic change for radiation than MN. These results are in accordance with other published data, regardless of the type of radiation (IAEA 1986, 2001; Paul et al. 1997; Vencatachalam et al. 2001; Wojcik et al. 2003).
Conclusion
Individuals show marked differences in radiation sensitivity, which can be important in both radiation protection and radiation therapy fields. At the moment, current dose limits for occupational exposure, as well as protocols for radiation treatments are based on the assumption that the human population is homogeneous in its radiosensitivity; thus, individuals with high radiation sensitivity would be at increased risk of both deterministic and stochastic effects. The identification of such subgroups, as well as establishing the best method for that purpose, is relevant in all fields of application of IR.
References
Ainsbury EA, Bakhanova E, Barquinero JF, Brai M, Chumak V, Correcher V et al (2011) Review of retrospective dosimetry techniques for external ionising radiation exposures. Radiat Prot Dosimetry 147(4):573–592
Antunes AC, Martins V, Cardoso J, Santos L, Monteiro Gil O (2014) The cytokinesis-blocked micronucleus assay: dose estimation and inter-individual differences in the response to γ radiation. Mutat Res 760:17–22
Bolegenova NK, Bekmanov BO, Djansugurova LB, Bersimbaev RI, Salama SA, Au WW (2009) Genetic polymorphisms and expression of minisatellite mutations in a 3 generation population around the Semipalatinsk nuclear explosion test site, Kazakhstan. Int J Hyg Environ Health 212(2009):654–660
Bonassi S, Fenech M, Lando C, Lin Y, Ceppi M, Chang WP et al (2001) Human MicroNucleus project: international database comparison for results with the cytokinesis block micronucleus assay in human lymphocytes: I. Effect of laboratory protocol, scoring criteria, and host factors on the frequency of micronuclei. Environ Mol Mutagen 37:31–45
Bonassi S, Neri M, Lando C et al (2003) Effect of smoking habit on the frequency of micronuclei in human lymphocytes: results from the Human MicroNucleus project. Mutat Res 543:155–166
Bonassi S, Ugolini D, Kirsch-Volders M, Stromberg U, Vermeulen R, Tucker D (2005) Human population studies with cytogenetic biomarkers: review of the literature and future prospective. Environ Mol Mutagen 45:258–270
Ceppi M, Gallo F, Bonassi S (2011) Study design and statistical analysis of data in human population studies with the micronucleus assay. Mutagenesis 26:247–252
Di Giorgio M, Sardi M, Busto E, Vallerga M, Taja M, Mairal L (2010) Assessment of individual radiosensitivity in human lymphocytes using micronucleus and microgel electrophoresis “Comet” assays. In: Proceedings of third European IRPA congress June 14 − 18, Helsinki, Finland
Fenech M (1993) The cytokinesis-block micronucleus technique and its application to genotoxicity studies in human populations. Environ Health Perspect Suppl 101:101–107
Fenech M (2007) Cytokinesis-block micronucleus cytome assay. Protocol. Nat Protoc 2:1084–1104
Fenech M, Bonassi S (2011) The effect of age, gender, diet and lifestyle on DNA damage measured using micronucleus frequency in human peripheral blood lymphocytes. Mutagenesis 26:43–49
Fenech M, Morley AA (1986) Cytokinesis-block micronucleus method in human lymphocytes: effect of in vivo ageing and low-dose x-irradiation. Mutat Res 161:193–198
Fenech M, Neville S, Rinaldi J (1994) Sex is important variable affecting spontaneous micronucleus frequency in cytokinesis-blocked lymphocytes. Mutat Res 313:203–207
Fenech M, Holland N, Zeiger E, Chang WP, Burgaz S, Thomas P et al (2011) The HUMN and HUMNxL international collaboration projects on human micronucleus assays in lymphocytes and buccal cells-past, present and future. Mutagenesis 26:239–245
Ginsberg G, Angle K, Guyton K, Sonawane B (2011) Polymorphism in the DNA repair enzyme XRCC1: utility of current database and implications for human health risk assessment. Mutat Res 727:1–15
IAEA, WHO (2011) EPR-biodosimetry cytogenetic dosimetry: applications in preparedness for and response to radiation emergencies. International Atomic Energy Agency, Vienna International Centre, Vienna
INTERNATIONAL ATOMIC ENERGY AGENCY (1986) Biological dosimetry: chromosomal aberration analysis for dose assessment. Technical reports series No. 260, IAEA, Vienna
INTERNATIONAL ATOMIC ENERGY AGENCY (2001) Cytogenetic analysis for radiation dose assessment. International atomic agency technical report series 405. IAEA, Vienna
Kocisova J, Sram RJ (1990) Mutagenicity studies on paracetamol on human volunteers. Cytokinesis-block micronucleus method. Mutat Res 244:27–30
Maki-Paakinen J, Walles S, Osterman-Golkr S, Norrpa H (1991) Single-strand breaks, chromosome aberrations, sister chromatid exchanges and micronuclei in lymphocytes of workers exposed to styrene during the production of reinforced plastics. Environ Mol Mutagen 17:27–31
Migliore L, Parrini M, Sbrana I, Biagini C, Battaglia A, Loprieno N (1991) Micronucleated lymphocytes in people occupationally exposed to potential environmental contaminants: the age effect. Mutat Res 256:13–20
Mutlu-Türkoğlu Ü, Ilhan E, Öztecan S, Kuru A, Aykaç-Toker G, Uysal M (2003) Age-related increases in plasma malondialdehyde and protein carbonyl levels and lymphocyte DNA damage in elderly subjects. Clin Biochem 36:397–400
Nefic H, Handzic I (2013) The effect of age, sex, and lifestyle factors on micronucleus frequency in peripheral blood lymphocytes of the Bosnian population. Mutat Res 753:1–11
Parshad R, Sanford KK (2001) Radiation induced chromatid breaks and deficient DNA repair in cancer predisposition. Crit Rev Oncol Hematol 37:87–96
Paul SF, Venkatachalam P, Jeevanram RK (1997) Analysis of radiation dose response curve obtained with cytokinesis block micronucleus assay. Nucl Med Biol 25:413–416
Rodrigues AS, Oliveira NG, Monteiro Gil O, Léonard A, Rueff J (2005) Use of cytogenetic indicators in radiobiology. Radiat Prot Dosimetry 115:455–460
Silva-Barbosa I, Pereira-Magnata S, Amaral A, Sotero G, Cavalcanti Melo H (2005) Dose assessment by quantification of chromosome aberrations and micronuclei in peripheral blood lymphocytes from patients exposed to gamma radiation. Genet Mol Biol 28:452–457
Smart V, Curwen GB, Whitehouse CA, Edwards A, Tawn EJ (2003) Chromosomal radiosensitivity: a study of the chromosomal G2 assay in human blood lymphocytes indicating significant inter-individual variability. Mutat Res 528:105–110
Stephan G, Pressl S (1999) Chromosomal aberrations in peripheral lymphocytes from healthy subjects as detected in The first cell division. Mutat Res 446:231–237
Vencatachalam P, Pauu Solomon FD, Karthiyeka Prabhu B, Mohankumar Mary N, Gjendiran N, Jeevanram RK (2001) Estimation of dose in cancer patients treated with fractionated radiotherapy using translocations, dicentrics and micronuclei frequency in peripheral blood lymphocytes. Mutat Res 429:1–12
Wojcik A, Stephan G, Sommer S, Buraczewska I, Kuszewski T, Wieczorek A, Gózdz S (2003) Chromosomal aberrations and micronuclei in lymphocytes of breast cancer patients after an accident during radiotherapy with 8 MeV electrons. Radiat Res 160:677–683
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pajic, J., Rakic, B., Rovcanin, B. et al. Inter-individual variability in the response of human peripheral blood lymphocytes to ionizing radiation: comparison of the dicentric and micronucleus assays. Radiat Environ Biophys 54, 317–325 (2015). https://doi.org/10.1007/s00411-015-0596-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-015-0596-3