Abstract
In the present work, radiosensitivity and dosimetric potential of solid trisodium citrate dihydrate (SC) were explored through a detailed electron spin resonance (ESR) study performed at various temperatures. Irradiated SC was observed to exhibit an ESR spectrum consisting of many intense and weak resonance lines spread over a magnetic field range of 7 mT and centered at g = 2.0039. An evaluation technique based on the variations of the characteristic resonance line intensities and the spectrum area under different experimental conditions was adopted, to determine the spectroscopic, kinetic and dosimetric features of radical species responsible for the observed experimental ESR spectrum. Radicals exhibiting similar ESR characteristics to those reported in the literature for irradiated tricarboxilic acids and their organic compounds were shown to be also produced in gamma-irradiated SC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trisodium citrate dihydrate (SC) or trisodium 2-hydroxypropane-1,2,3-tricarboxylate is a sodium salt of citric acid. SC has a saline, mildly tart flavor. It is used mainly in foods as a food additive, usually for flavor or as a preservative in beverages, and in various technical applications mainly as buffering, sequestering or emulsifying agent. It is also used in blood collection tubes and for the preservation of blood in blood banks, because citrate ions cheat calcium ions in the blood and disrupt blood clotting (Bayés et al. 1999; Sakhaee et al. 1983; Salant and Wise 1916; Schubert and Wallace 1950; Oöpik et al. 2003). SC crystallizes as monoclinic dihydrate with space group C2/c including eight molecules in the unit cell (Fischer and Palladino 2003).
ESR spectroscopic features of the radicals produced in gamma- and X-ray irradiation of trisodium citrate pentahydrate and pentadeuterate, citric acid single crystals and UV irradiation of aqueous solutions of citric acid have been reported previously (Finch et al. 1979; Russell 1965; Zeldes and Livingston 1971), but no attempt has been made so far to determine the kinetic features of the produced radicals, and the radiosensitivity and possible dosimetric potential of SC.
Note, however, that information about the latter points is important for radiosterilization of food containing SC as an additive. The radicals \( {\text{COOHCH}}_{2} \mathop {\text{C}}\limits^{ \bullet } {\text{OHCH}}_{2} {\text{COOH}} \) and \( {\text{COOHCH}}_{2} {\text{COHCOOH}}\mathop {\text{C}}\limits^{ \bullet } {\text{HCOOH}} \) that are denoted as species I and III by Finch et al. (1979), respectively, have been shown in ESR spectra at room temperature to be produced and to be the predominant species after gamma irradiation of sodium citrate pentahydrate and pentadeuterate, X-ray irradiation of single crystals of citric acid and UV irradiation of aqueous solutions of sodium citrate (Finch et al. 1979; Russell 1965; Zeldes and Livingston 1971). Radical III, which is formed by loss of a methylene H atom, shows large hyperfine splitting due to an α proton (Finch et al. 1979). Radical I is proposed to be the oxidation species produced by decarboxylation of the central carboxyl group with hyperfine coupling due to the two (Russell 1965) or four (Finch et al. 1979) β-hydrogens. Another radical of unknown structure and weaker concentration than radicals I and III has also been proposed to exist in citric acid after X-ray irradiation (Finch et al. 1979).
Because SC is used as flavor or preservative in foods, radiodegradation of SC is important for controlling its additive and protective functions in irradiated foods. Thus, the aim of the present work is to study the radiosensitivity and the potential of solid SC for retrospective dosimetry, by investigating its radiation yield and the temperature-dependent kinetic features of the radicals produced in it after gamma irradiation at different doses, by ESR spectroscopy. This work is in line with previous work where we have studied the characteristics of some gamma-irradiated pharmaceuticals and food additives by ESR spectroscopy (Tuner and Korkmaz 2009; Ustundag and Korkmaz 2009; Tuner and Korkmaz 2007a, b).
Materials and methods
Sample irradiation
White polycrystalline SC powder samples were provided by GMT Food Ingredients Company (Istanbul) and stored at room temperature in a closed container protected against light and change in humidity. No further purification was performed. Samples of particle sizes smaller than 100 μm were used. All irradiations were performed at room temperature (290 K) using a 60Co-γ source at a dose rate of 1.41 kGy/h. The dose rate at the sample site was measured by a Fricke dosimeter. Investigations were performed on samples irradiated in the dose range of 1–25 kGy. Samples were irradiated at doses of 1.0, 2.0, 3.0, 5.0, 7.0, 10.0, 15.0 and 25.0 kGy, to produce the dose–response curves. Samples irradiated at a dose of 10 kGy were used to investigate room and high-temperature stability, and the kinetic features of the radiation-induced radicals. Annealing studies were performed at four different temperatures (370, 380, 390 and 400 K) by heating samples inside a microwave cavity, to avoid any drawback originating from sample positioning in the microwave cavity. Samples heated up to predetermined temperatures were kept at these temperatures for predetermined times, and ESR spectra were recorded in regular time intervals.
ESR measurements
ESR measurements were carried out in standard ESR quartz tubes of 4 mm inner diameter using a Bruker EMX-131 X-band ESR spectrometer operating at 9.8 GHz and including a high-sensitive cylindrical cavity (conditions of operation; central field—351.2 mT; scan range—11 mT; microwave power—0.25 mW; microwave frequency—9.856 GHz; receiver gain—2.52 × 103; modulation frequency—100 kHz; modulation amplitude—0.1 mT; time constant—327.68 ms; sweep time—83.89 s). Signal intensities were measured directly from recorded first-derivative spectra, and the spectrum area under the absorption curves was calculated by double integration over the total scan range of the recorded ESR spectra using the Bruker WINEPR program. Sample temperature inside the microwave cavity was monitored with a digital temperature control unit (Bruker ER 411-VT). This unit provided the opportunity of measuring the temperature with an accuracy of ±0.5 K at the site of the sample. A cooling, heating and subsequent cooling cycle was adopted, to monitor any changes in the signal intensities and spectrum area with temperature. Sample temperature was first decreased to 130 K starting from room temperature with an increment of 20 K and then increased to 400 K and finally decreased again to room temperature. The spectroscopic information derived from the ESR spectra that were obtained as the means of three successive scans was used to evaluate the experimental results.
Results and discussion
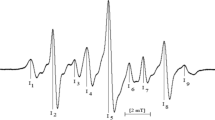
While unirradiated SC showed no ESR signal, irradiated polycrystalline SC showed an ESR spectrum consisting of many unresolved strong and weak resonance lines (Fig. 1), making it more complicated than the spectra reported in the literature for citric acid and its sodium salts (Russell 1965; Finch et al. 1979; Zeldes and Livingston 1971). Room-temperature spectra of SC were calculated to spread over a magnetic field range of 7 mT and to be centered at about g = 2.0039. Overlapping ESR signals originating from contributing species created difficulties to directly determine the features of each radical from the experimental spectra. To overcome this difficulty, the spectrum area was calculated by the double integration technique, and the variations of the assigned line intensities Y 1, Y 2, Y 3 and Y 4 (Fig. 1b) with microwave power, storage time and temperature were investigated.
Variations of signal intensities with microwave power
Samples irradiated at a dose of 10 kGy were used to determine the saturation features of the associated radical species with microwave power. Variations of the assigned line intensities with microwave power were investigated both at room temperature (290 K) and at 130 K, in the ranges of 0.004–20 mW and 0.001–0.4 mW, respectively, to determine the characteristic behavior of these lines and classify them into subgroups if possible. No pattern change occurred over the applied microwave-power ranges neither at 290 K nor at 130 K, although some intensity changes were observed. The microwave saturation behavior of each line was observed to be similar at both temperatures (290 and 130 K). Results for the saturation of the monitored Y 1, Y 2, Y 3, and Y 4 intensities (Fig. 1b) at 130 K are given in Fig. 2. It is observed that the line intensities did not exhibit the same saturation behavior (Fig. 2). While the Y 2 and Y 4 intensities, which are believed to be the two components of a doublet (due to a radical of type III), show the features of homogeneously broadened resonance lines, the Y 1 intensity, which could be seen as the weakest component of a pentate arising from the hyperfine interactions of the unpaired electron with four equivalent methylene protons (due to a radical of type I) exhibits the characteristics of an inhomogeneously broadened resonance lines. As for Y 3, its intensity does not saturate even at the highest microwave power (Fig. 2). Observed differences in the saturation behavior of the monitored intensities suggest the presence of another radical of unknown structure which is denoted as radical of type IV in the present work, in addition to the radicals I and III assigned by Finch et al. (1979) in their work on irradiated citric acid single crystals. Note that the production of a radical of unknown structure has also been suggested by Finch and coworkers in the same work.
Variable-temperature studies
Variations of the monitored intensities with temperature were also investigated using a sample irradiated at a dose of 10 kGy in the temperature range of 130–400 K. Before this experiment was carried out, the microwave power was chosen in a way that saturation even at the lowest temperature (130 K) used in the present work was avoided. Thus, a microwave power as low as 0.0025 mW was adopted throughout the temperature studies. The sample was first cooled down to 130 K starting from room temperature (290 K) with a decrement of 20 K. Then, the temperature was increased up to 400 K with the same increment and decreased again to room temperature. No pattern changes in the ESR spectra were observed below and above room temperature except reversible or irreversible changes in the intensities. Nevertheless, the increase rates of the Y 1, Y 2, Y 3 and Y 4 intensities below room temperature were different, due to differences in the saturation characteristics and contribution rates of the individual radical species. This was well observed for the Y 3 intensity which is supposed to be an overlapping hyperfine line produced by radicals I and IV that exhibit different saturation characteristics. Heating samples above room temperature caused irreversible decreases in the intensities (Fig. 3). The obvious differences in the temperature-dependent characteristics of the monitored intensities suggest once more that the Y 2 and Y 4 intensities are associated with a species other than those giving rise to the Y 1 and Y 3 intensities.
Variations of signal intensities with temperature for a sample irradiated at a dose of 10 kGy. Cooling (solid line and black symbols): 290–130 K and 400–290 K; heating (dashed line and open symbols): 130–400 K [filled square (Y 1), filled triangle (Y 2), right pointing filled triangle (Y 3) and down pointing filled triangle (Y 4)]
Long-term decay of signal intensity at room temperature
Long-term stability of radicals at room temperature is one of the most important parameters in ESR dosimetry, because it gives the possibility to discriminate between irradiated and unirradiated samples. Furthermore, long-term variations of the intensities can help in determining radical types. A sample irradiated at a dose of 10 kGy was stored at room temperature open to air, and its spectra were recorded in regular time intervals over a storage period of 64 days without changing the position of the sample in the microwave cavity. Decay data were collected for the relevant resonance lines shown in Fig. 1b, and the spectrum area obtained is presented in Fig. 4 as a function of time. As is seen, all resonance lines and the spectrum area show a fast decay at the beginning of the storage period but exhibit different rates. In agreement with literature findings on irradiated citric acid and sodium citrate (Finch et al. 1979; Russell 1965; Zeldes and Livingston 1971) and with the results of microwave saturation and variable-temperature studies reported in the present work, the experiment on long-term decay also suggests three radicals (denoted as I, III and IV) likely of different decay characteristics to take part in the formation of the experimental ESR spectrum, after irradiation of SC with gamma radiation. More specifically, radicals I and III give rise to the formation of the resonance lines of intensities Y 1, Y 2 and Y 4, while radicals I and IV are believed for the line of intensity Y 3. That is, the Y 3 intensity originates from the overlap of the central line of the pentate (due to radical I) and of the narrow singlet (due to radical IV). Thus, the decay data obtained for the monitored line intensities and spectrum area at room temperature were fitted to functions including exponential decay with time and allowing for different weights and different decay constants (k’s) representing the contributions of the involved radical species. A mathematical function presenting the characteristics of the first-order decay kinetics which has the form of \( Y_{\left( t \right)} = Y_{10} \cdot {\text{e}}^{{ - k_{1} t}} + Y_{20} \cdot {\text{e}}^{{ - k_{2} t}} + Y_{30} \cdot {\text{e}}^{{ - k_{3} t}} \) was found to describe the experimental decay data best. The parameters k 1, k 2 and k 3 represent the decay constants, while Y 10, Y 20 and Y 30 are constants proportional to the initial concentrations of the involved radicals. The results obtained for the spectrum area are given in Table 1. Decay curves calculated using the parameter values given in this table are also presented in Fig. 4 with their experimental counterparts.
Annealing studies at high temperatures
Variations of the line intensities and spectrum area above room temperature were also studied, to get information about high-temperature decay kinetics of the radicals produced in SC exposed to gamma radiation. That is, annealing studies were performed at temperatures 370, 380, 390 and 400 K, respectively, for an annealing period of 60 min. Spectrum area and signal intensities measured from recorded spectra of samples placed in heated nitrogen gas flowing through the microwave cavity were used for this purpose. The calculated area and the measured intensities were normalized to their initial values, which were obtained from the first spectrum recorded at 5 min after positioning the sample in the microwave cavity. A time of 5 min was chosen to establish initial thermal equilibrium. The high-temperature decay constants (k’s) of the radical species were calculated by fitting the high-temperature signal intensity and spectrum area data together to the same function that also used to fit the room-temperature decay data. The parameters calculated by this technique are presented in Table 2. Derived decay constants were used to calculate theoretical variations of the measured quantities. The results for the spectrum area calculated by double integration methods are presented in Fig. 5. It is seen that the calculated decay constant describes the variations of the spectrum area at annealing temperatures fairly well. This was also the case for the measured line intensities. Activation energies of the involved species were also calculated from \( \ln \left( k \right) - \frac{1}{T} \) graphs and the values presented in Table 2 were obtained.
Dose–response curves
Beside qualitative detection, ESR spectroscopy can also be used for dose estimation and dose measurement. In the present work, the dosimetric potential of SC was explored by measuring the assigned resonance line intensities and spectrum area at different gamma radiation doses, about 1 h after the irradiation. Samples irradiated to doses of 1, 2, 3, 5, 7, 10, 15 and 25 kGy were used to produce experimental dose–response curves. The results related to with the Y 1, Y 2, Y 3 and Y 4 intensities and spectrum area are presented in Fig. 6a, b. It is seen that variations of Y 2, Y 4 and spectrum area (Fig. 6a) with applied dose are fairly different from those of the Y 1 and Y 3 intensities (Fig. 6b). While the latter show almost a linear variation with applied dose, the former exhibit rather exponential variations in the investigated dose range (1–25 kGy). The four different functions given in Table 3 (linear, linear-quadratic, exponential and sum of two exponentials) have been tried to describe the experimental dose–response data without forcing them to pass through the origin. In these functions (Table 3), Y’s and D stand for the ESR line intensities and absorbed dose in kGy, respectively, and a, b, c… are the constants to be determined. The parameter values obtained after fitting the experimental dose–response data are also presented in Table 3. It is seen that while a function linear or linear-quadratic in dose describes best the dose–response data for the Y 1 and Y 3 intensities, a different function including two exponentials should be used to describe the Y 2, Y 4 intensities and spectrum area as a function of dose. This result shows once more that the origin of the Y 2 and Y 4 lines is different from that of the Y 1 and Y 3 lines.
Variations of measured intensities and spectrum area with applied radiation dose. Symbols: experimental; solid lines: calculated data using a sum of two exponentials [filled triangle (Y 2); down pointing filled triangle (Y 4) and filled circle (spectrum area)]; and b a linear-quadratic function (filled square (Y 1) and right pointing filled triangle (Y 3)]
A material suitable for dose measurements should show a high radical yield (high G value), radicals that are stable with time, and exhibit a linear dose–response curve. Gamma-irradiated SC was calculated to have a G value of 0.21 ± 0.05 radical/100 eV. Such a G value falls into the range of G values typical for most organic compounds. In other words, the sensitivity of SC toward gamma radiation is not high. Furthermore, the radicals produced after irradiation are not stable even at room temperature (Fig. 4). That is, they experience a very significant decrease during the days after the irradiation. These two negative features of SC do not meet those required for a good dosimetric material. In other words, SC is not a suitable material for measuring normal and/or accidental doses in the range of 1–25 kGy with reasonable accuracy. However, ESR spectroscopy could be used to distinguish between irradiated and unirradiated SC one or even 2 month after the irradiation.
Conclusion
The following conclusions can be drawn from the results obtained in the present work:
-
\( {\text{COOHCH}}_{2} \mathop {\text{C}}\limits^{ \bullet } {\text{OHCH}}_{2} {\text{COOH}}\,\left( {\text{I}} \right) \) and \( {\text{COOHCH}}_{2} {\text{COHCOOH}}\mathop {\text{C}}\limits^{ \bullet } {\text{HCOOH}}\,\left( {\text{III}} \right) \) radicals manifesting as pentate and a doublet ESR signals, respectively, are also produced after irradiated of solid SC with gamma radiation, as is the case of irradiated three carboxylic acid and its derivatives (Finch et al. 1979; Russell 1965; Zeldes and Livingston 1971).
-
Another radical (radical IV) that gives rise to a singlet resonance line was also produced in gamma-irradiated SC. This species of unknown structure is likely the species reported by Finch et al. (1979) after irradiation of single crystals of citric acid with X-rays.
-
The radicals produced upon SC irradiation were found to be unstable even at room temperature. They experience fast decays after irradiation. Radical VI represents the most unstable species with an activation energy of 36.76 kJ/mol.
-
Radical I and III show higher activation energy (Table 2), as expected from variable-temperature findings (see Fig. 3).
-
Radiosensitivity of SC is relatively low, and the corresponding G value (0.21 ± 0.05 radical/100 eV) after gamma irradiation falls into the range of most organic compounds. Thus, gamma irradiation of the foods containing SC as additive is not expected to creating severe a drawback in the functional characteristics of SC.
-
Low radical yields, the unstable characteristics of the produced radicals, and low microwave power saturation values of the signals let us conclude that SC does not present the characteristic features of a useful dosimetric material.
References
Bayés B, Bonal J, Romero R (1999) Sodium cirate for filling haemodialysis catherters. Nephrol Dial Transplant 14:2532–2533
Finch LL, Johnson JE, Moulton GC (1979) An ESR and ENDOR study of citric acid single crystals x irradiated at 4.2 K. J. Chem Phys 70:3662–3668
Fischer A, Palladino G (2003) Trisodium citrate dehydrate. Acta Cryst E 59:m1080–m1082
Oöpik V, Saaremets I, Medijainen L, Karelson K, Janson T, Timpmann S (2003) Effects of sodium citrate ingestion before exercise on endurance performance in well trained college runners. Br J Sport Med 37:485–489
Russell DB (1965) Electron spin resonance of γ-irradiated sodium citrate pentahydrate and pentadeuterate. J Chem Phys 43:1996–2000
Sakhaee K, Nicar M, Hill K, Pak CYC (1983) Contrasting effects of potassium citrate and sodium citrate therapies on urinary chemistries and crystallization of stone-forming salts. Kidney Int 24:348–352
Salant W, Wise LE (1916) The action of sodium citrate and its decomposition in the body. J Biol Chem 28:27–58
Schubert J, Wallace H (1950) The effect of zirconium and sodium citrate on the distribution and excretion of simultaneously injected thorium and radiostrontium. J Biol Chem 183:157–166
Tuner H, Korkmaz M (2007a) Radiostability of solid butylated hydroxytoluene (BHT): an ESR study. Nucl Instrum Meth B 258:388–394
Tuner H, Korkmaz M (2007b) ESR study of ascorbic acid ırradiated with gamma rays. J Radioanal Nucl Chem 273:609–614
Tuner H, Korkmaz M (2009) Kinetic features of the radical species produced in γ-irradiated dl-tartaric acid and the dosimetric potential of this acid. Radiat Res 172:120–128
Ustundag IO, Korkmaz M (2009) Spectroscopic, kinetic and dosimetric features of the radical species produced after radiodegradation of solid triclosan. Radiat Environ Biophys 48:159–167
Zeldes H, Livingston R (1971) Paramagnetic resonance study of liquids during photolysis. XI. Citric acid and sodium citrate in aqueous solution. J Am Chem Soc 93:1082–1085
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tuner, H., Korkmaz, M. Effects of gamma radiation on solid trisodium citrate dihydrate: radical kinetics, radiosensitivity and dosimetry. Radiat Environ Biophys 49, 723–729 (2010). https://doi.org/10.1007/s00411-010-0304-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-010-0304-2