Abstract
The aim of this study was to investigate the in vivo frequency of chromosomal aberrations (primarily dicentric chromosomes and chromatid breaks) potentially induced by 224Ra α-radiation in peripheral lymphocytes. The study was designed to serve as a cytogenetic analysis along with the therapeutic procedure of ankylosing spondylitis patients who were undergoing a treatment with 224Ra-chloride. The total administered activity was 10 MBq, and the treatment followed a schedule of 10 i.v. injections per week, each with a dose of 1 MBq of 224Ra. The calculation of absorbed doses delivered to the blood used the models suggested by the ICRP and yielded a value of 4.7 mGy/MBq. The frequency of chromosomal aberrations observed during the course of therapy was related to the blood dose. The frequency of dicentric chromosomes induced in vivo was found to agree well with the corresponding value of dicentrics induced in vitro. However—given that peripheral lymphocytes are in the cell cycle’s G0 stage—an unexpected increase with dose in the yield of chromatid breaks was observed, with about 95% of them occurring in cells without any other chromosome-type aberrations. Reasons for the production of chromatid breaks are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the 1940s and 1950s, a drug containing Thorium X (224Ra) was introduced in Germany as an effective compound (with additional traces of platinum and eosin) for the treatment of patients suffering from ankylosing spondylitis or bone tuberculosis [1]. At that time, patients received multiple high-dose intravenous administrations that resulted in a cumulative activity of up to 140 MBq. In a subgroup of those patients who were treated as children or juveniles before the age of 21 years, a striking excess of malignant bone tumours occurred [1, 2]. In October 2000, 224Ra was re-approved in Germany—however, limited to the treatment of ankylosing spondylitis patients [2] and the levels of activity to be applied are much lower now than in the earlier treatments.
The short-lived α-emitter 224Ra has a half-life of 3.66 days. In addition to α-particles, β- and γ-rays are emitted. After intravenous injection, there is a quick decrease of the 224Ra activity in the blood, but an increase in other tissues. Three days after injection, merely about 0.1% of the initial activity can be measured in the peripheral blood. The treatment for ankylosing spondylitis patients includes 10 i.v. injections per week, each with 1 MBq of 244Ra. Bone surface, red bone marrow, liver and colon are the primary organs to accumulate the administered 224Ra activity. The absorbed dose due to a total activity of 10 MBq was calculated to be highest in the bone surface (dose equivalent of 88 Sv) with a resulting effective dose of 2.5 Sv. Red bone marrow, liver, kidneys and colon are exposed to doses of 8.5, 2.6, 1.5 and 1.4 Sv, respectively [3].
To our knowledge, no human in vivo data have up to now been available for the frequency of chromosome aberrations potentially induced by α-particles in relation to the blood dose. It was, therefore, the aim of this study to determine the frequency of chromosome aberrations (i.e. dicentric chromosomes and chromatid breaks) in the peripheral lymphocytes of six ankylosing spondylitis patients during their treatment with 224Ra.
Patients, material and methods
Patients and blood sampling
A cytogenetic study was designed with the aim to serve as an additional analysis along with the therapeutic procedure of ankylosing spondylitis patients who underwent a treatment with 224Ra-chloride.
The study group was selected from the Clinic of Nuclear Medicine at the University Hospital Schleswig Holstein, Kiel, Germany, and contained six patients with manifested ankylosing spondylitis, who had been suffering from long-term painful and active mineralization processes in the axis skeleton and who were resistant to any type of pharmacotherapy and physiotherapy. The indication for 224Ra therapy was based on both clinical symptoms and the results of a two-phase bone scintigraphy using 600 MBq Tc-99m-HDP. Following clinical and radiological classification [4], the delayed images 3 h p.i. show in some particular cases areas of enhanced tracer uptake, indicative for progressive ossification in the sacroiliac joints as well as in at least two spine regions that refer to the disease stages II and III of ankylosing spondylitis [5, 6]. The patients suffer from persisting low back pain, severe morning stiffness in the back and the spine, as well as breathing impairment due to progredient inflammation and stiffening of their chest.
The patients (five men, one woman) were between 32 and 58 years of age (mean age 45 years) under therapy. They were well informed about the investigation, and all of them gave their written consent to the 224Ra treatment and the following investigation using repeated blood sampling.
Ankylosing spondylitis is a disease that requires some balancing therapy of pain and other secondary effects. The patients have therefore been receiving various types of drug therapy prior to the 224Ra therapy, such as corticoids (Urbason) in combination with analgetics (Diclofenac) or dermatics (Triamcinolon) in combination with antiphlogistics (Azulfidine), or corticoids (Dicortin) in combination with analgetic/antirheumatic agents (Valoron). Drug therapy of the patients was continued unchanged during radiotherapy, except for the intake of corticoids which was slightly reduced.
From each patient, three to five blood samples were taken during therapy. Prior to the first intravenous injection of 224Ra, one blood sample was taken as control. The patients received 1 MBq of 224Ra per week over a period of 10 weeks which resulted in a total activity of 10 MBq 224Ra during therapy. Further blood samples were regularly taken 1 week after every injection. After 1 week, the injected activity should have disappeared from the peripheral blood, which means that the blood has received the maximum dose from the administered 224Ra of the corresponding fraction.
Cytogenetic protocol
The blood samples for chromosome analyses were collected in heparinized syringes and sent to the laboratory by express courier. Whole blood lymphocyte cultures were set up for 48 h, using RPMI 1640 medium supplemented with 10% foetal calf serum, 2 mM glutamine, 2% PHA, 10 μM BrdU and antibiotics. The cells were treated with 0.1 μg colcemid for the last 3 h of culture time. After hypotonic treatment with 75 mM KCl, the cells were fixed in methanol-acetic acid (3:1). According to our standard protocol, chromosome aberrations were analysed exclusively in complete first division cycle metaphases [7].
Dose calculation
Dose calculation was done in line with the biokinetic and dosimetric model of the ICRP’s “Publication 67” [8] for 224Ra. The number of nuclear transformations has been calculated for all radionuclides of the 224Ra decay chain for all source compartments, assuming direct uptake of 224Ra into the blood.
The dosimetric model used by the ICRP [8], however, does not consider blood as a target organ; therefore, to assess the absorbed dose to blood, special assumptions for penetrating and nonpenetrating radiation have to be included. For nonpenetrating radiation, it was readily assumed that all energies emitted in blood would be absorbed in blood. This is a conservative assumption, especially for small blood vessels. On the other hand, the blood dose may be underestimated due to the second assumption that energies emitted apart from the blood would not reach the blood. In any event, this seems to be an adequate blood dose estimation for nonpenetrating radiation. Refinements of the dosimetric models would require information on local activity distributions which cannot be achieved by the biokinetic ICRP models; they are designed at an organ level, and not at a voxel level. The energies listed in the ICRP’s “Publication 38” [9] have been used for the calculations. Finally, the equivalent dose was determined by application of a radiation weighting factor of 20 for α-radiation [10].
For penetrating radiation, muscle tissue was chosen as target tissue, because due to its spreading over the whole body it is a qualified surrogate for blood. However, in the case of 224Ra, the dose from α-radiation exceeds the doses from β- and γ-radiation by some orders of magnitude. Therefore, the assumptions on penetrating radiations do not considerably affect the results of dose calculation.
This dosimetric approach yielded a dose coefficient of 9.4×10−8 Sv/Bq or 4.7×10−9 Gy/Bq. The value is higher by a factor of about 2 compared with the dose coefficients given for soft tissues other than liver and kidneys [3].
Statistical analysis of data on dicentric and chromatid breaks
The determination of the error bands was performed according to a method developed by Kellerer [11]. By this approach the model parameters are transformed in a way that they become uncorrelated, i.e. orthogonal, which has the advantage that the confidence intervals—or standard errors—of the model parameters can be propagated in terms of their sum of squares in order to obtain the uncertainty of the estimated effect levels.
Results
Table 1 presents the study results from six ankylosing spondylitis patients. In addition to the analysis during therapy, control values have been determined prior to the onset of therapy. In total, 27,111 cells were analysed. From five patients, 5,269 cells were scored as controls in which 8 dicentrics, 2 centric rings, 12 excess acentric fragments, 24 chromatid breaks, and 2 chromatid exchanges were observed.
In 21,842 cells taken during therapy, 279 dicentrics, 23 centric rings and 161 excess acentric fragments were registered. In the 279 dicentrics, 13 tricentric chromosomes are included which were evaluated as 26 dicentrics. As chromatid aberrations, 179 chromatid breaks and 8 chromatid exchanges were observed. In Table 1, chromatid exchanges are included in chromatid breaks as 2 break events. Most chromatid breaks (170 out of 179 chromatid breaks) and the 8 chromatid exchanges occurred in cells without any other aberration, i.e. 4.6% of the chromatid breaks occurred in cells containing additional chromosome-type aberrations, whereas 95.4% occurred in cells without chromosome-type aberrations. Table 1 demonstrates that for all structural aberrations, including chromatid breaks, a dose-dependent increase is observed.
The distribution of dicentrics on cells is given in Table 2. The values of the dispersion ratio (σ2/y) and the u value [12] indicate overdispersion for the number of dicentrics during therapy, since the dispersion ratio is different from 1 and the u value is >1.96. The dicentric data (corrected for overdispersion) fitted a linear model (y=c+αD) where α was determined to be 0.28±0.03 Gy−1. The adaptation to a linear-quadratic model (y=c+αD+βD2) is shown in Fig. 1. The β-coefficient of this equation is (2.3±2.3)10−3 Gy−2 which means that the quadratic term can also be zero.
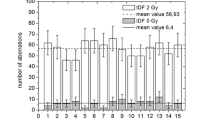
Figure 2 shows the frequency of chromatid breaks (cbr) versus absorbed dose. The parameters of a linear-quadratic fit are α=(−0.11±1.00)10−2 Gy−1 and β=(3.25±2.25)10−2 Gy−2. The α-coefficient in a linear function is 0.14 ± 0.03 Gy−1.
Discussion
In the present study, frequencies of structural chromosome aberrations are reported which were observed in peripheral lymphocytes of ankylosing spondylitis patients during radiation therapy with 224Ra. The mean frequency of dicentrics (1.52 ± 0.54 dicentrics/1,000 cells) determined prior to the onset of therapy is not significantly increased in comparison with our control value of 0.95 ± 0.14 dicentrics/1,000 cells (P=0.25). This is also true for the frequencies of excess acentric fragments (2.28±0.66/1,000 cells in patients and 2.5±0.3/1,000 cells in control) and chromatid breaks (5.31±1.00/1,000 cells in patients and 6.5±0.6/1,000 cells in control) [7], and it means that neither X-ray examinations nor pharmaceutical treatments of the patients undertaken before/under 224Ra therapy have affected the occurrence of chromosomal aberrations.
To our knowledge this is the first report on chromosomal aberrations in human peripheral lymphocytes after in vivo exposure to a well defined amount of α-emitters that had been intravenously administered, and with the blood samples for chromosome analysis that were collected shortly after administration. The patients received 1 MBq of 224Ra per fraction per week over a time interval of 10 weeks. Calculations of dose equivalent have been performed using ICRP models [8] and yielded a blood dose of 4.7 mGy per fraction. The therapy duration (10 weeks) should have no influence on the aberration frequency in peripheral blood lymphocytes, since the half-life of dicentrics is about 3 years [13]. The intravenously administered 224Ra was subsequently distributed in blood where the lymphocytes were consequently exposed to external α-irradiation.
Schmid et al. [14] established an in vitro dose–response curve with 241Am α-particles in the dose range from 0.02 to 1.0 Gy. Summing up all radiation-induced dicentrics, centric rings and excess acentrics, the ratio is 10:1:11. The sum of chromosome-type aberrations observed during the therapy of the patients results in a ratio of 12:1:7. From these ratios it can be concluded that irradiation with α-particles produces chromosome-type aberrations in vivo (dose range 0.009–0.047 Gy) and in vitro (dose range 0.02–1.0 Gy) with similar efficiencies.
The fit of the dicentrics frequency observed during radiation therapy to a linear dose–response curve revealed an α-coefficient of 0.28 ± 0.03 Gy−1, which is in good agreement with those obtained in in vitro experiments of 0.27 ± 0.02 Gy−1 [14], 0.29 ± 0.15 Gy−1 [15] and 0.29 ± 0.02 Gy−1 [16]. Furthermore, there is good agreement in the observed cellular distribution patterns of dicentrics which show significant overdispersion (Table 1). These comparisons indicate that after in vivo or in vitro exposure of lymphocytes with α-particles, similar frequencies of dicentrics per dose unit are induced [14, 15]. Figure 3 illustrates this as well, and the α-coefficients of the dose–response curves (in vitro and in vivo) indicated above, demonstrate that in the dose range from 0.009 Gy up to 4.18 Gy a unique slope can be used to describe the relation between radiation dose and the induced yield of dicentrics. In other words, the dose-dependent yield of dicentrics can be derived over about 3 decades of absorbed dose.
Peripheral lymphocytes use to be in the cell cycle’s Go stage. After exposure of lymphocytes to ionising radiation, a dose-dependent increase is therefore only expected for chromosome-type aberrations, such as dicentrics, centric rings and excess acentrics. In the present study, however, an unexpected dose-dependent increase in the yield of chromatid breaks was observed. About 95% of the chromatid breaks occurred in cells without any chromosome-type aberration. Earlier in vitro experiments with lymphocytes after α-particle irradiation did not show any influence on the frequency of chromatid breaks. One explanation may be the fact that in those experiments, single lymphocytes have separately been attached to a foil without any fluid between them [14].
The blood for our chromosome analyses was collected every 8 days after the administration of the respective 224Ra fraction, but already 3 days after the injection no more than 0.1% of the initial activity was measurable [3]. The collected blood sample did not consequently contain any substantial amount of 224Ra that would have been able to induce the observed chromatid aberrations during culturing.
The majority of the administered 224Ra activity is accumulated in the bone surface and red bone marrow. That means that some lymphocyte precursor cells may have been irradiated and chromatid aberrations may have been induced in those cells that are in the cell cycle’s late S or G2 phase. These cells, however, have to pass the mitosis stages in order to result in mature peripheral lymphocytes and when passing the anaphase the chromatid break will lead to a deletion and thus the chromatid break would no more be observable in the descendent lymphocyte. Consequently chromatid breaks will most likely be induced in mature lymphocytes only. If so, the chromatid-type inducing agent must act during the late S or G2 phase of the first cell cycle after culture initiation. As indicated above, therapeutical drugs alone that have additionally been dispensed to the patients do not induce chromatid breaks, but that does not exclude the possibility of any combination effects.
A number of reports are dealing with the effect that in experiments with α-particles more cells were damaged than were traversed by α-particles [17–19]. For the production of this phenomenon, the so-called bystander effect, the cell nuclei must be traversed by one α-particle [20]; more than one hit to one cell nucleus do not result in an increased bystander response in its neighbours [21]. It was reported that the type of mutations induced as a result of a bystander effect is qualitatively different from that of a direct nuclear hit [22, 23], but on the other hand, micronuclei were observed in irradiated as well as in bystander cells [24]. More recently published results showed that for the transmission of the bystander signal, cell-to-cell contact is an important mediator of the effect [25], but it was also shown that this signal can be transmitted over considerable distances [26].
Since dicentrics and centric rings are characteristic of ionizing radiation, it can be assumed that nuclei containing ≥1 dicentric or ≥1 centric ring, or both, aberrations are traversed by one or more α-particles. In line with the statements by Little et al. [19], Sawant et al. [20], Brenner et al. [21], Nagasawa et al. [22], and Zhou et al. [23], a dose-dependent increase in the frequencies of chromatid breaks should be observed. The data presented in Fig. 2 show this correlation between the absorbed dose and the frequency of chromatid breaks. Consequently, the data are in agreement with features being attributed to a bystander effect. Hence, it may be hypothesized that the nuclei of lymphocytes (from ankylosing spondylits patients) that have possibly been traversed by one or more α-particles from 224Ra may have sent out a signal which led to chromatid aberrations in bystander cells. In order to produce this effect, a soluble extracellular factor must have been released and transmitted from irradiated to unirradiated cells as has been reported earlier [26].
In conclusion, to our knowledge this is the first time that effects have been described for humans which may be explained by the phenomenon ‘bystander effect’. Although the results are in agreement with all criteria postulated for a bystander effect, further experimental investigations are necessary to verify our results as an effect produced in bystander cells.
References
Spiess H (2002) Peteosthor—a medical disaster due to radium-224. A personal recollection. Radiat Environ Biophys 41:163–172
Nekolla EA, Kreisheimer M, Kellerer AM, Kuse-Isingschulte M, Gössner W, Spiess H (2000) Induction of malignant bone tumors in radium-224 patients: risk estimates based on the improved dosimetry. Radiat Res 153:93–103
Lassman M, Nosske D, Reiners Ch (2002) Therapy of ancylosing spondylitis with 224Ra-radium chloride: dosimetry and risk considerations. Radiat Environ Biophys 41:173–178
Linden S van der, Valkenburg A, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum 27:361–368
Ott VR (1972) Klinik und Therapie der ankylosierenden Spondylitis (Morbus Strümpell-Marie-Bechterew). In: Brügel H (ed) Fortschritte auf dem Gebiet der rheumatischen Erkrankungen und der degenerativen Gelenkerkrankungen. Schattauer, Stuttgart, pp 92–104
Schilling F (1964) Spondylitis ankylopoetica: die sogenannte Bechterew’sche Krankheit und ihre Differentialdiagnose (einschliesslich Spondylitis hyperostotica, Spondylitis psoriatica und chronischem Reiter Syndrom). In: Diethelm L (ed) Handbuch der medizinischen Radiologie, Bd. IV, Teil 2. Springer, Berlin Heidelberg New York, pp 452–689
Stephan G, Pressl S (1999) Chromosomal aberrations in peripheral lymphocytes from healthy subjects as detected in first cell division. Mutat Res 446:231–237
ICRP (1994) Publication 67. Age-dependent doses to members of the public from intake of radionuclides, Part 2: ingestion dose coefficients. International Committee on Radiological Protection. Pergamon, Oxford
ICRP (1983) Publication 38. Radionuclide transformations: energy and intensity of emissions. International Committee on Radiological Protection. Pergamon, Oxford
ICRP (1991) Publication 60. The 1990 recommendations of the International Commission of Radiation Protection. International Committee on Radiological Protection. Pergamon, Oxford
Kellerer AM (2003) The use of orthogonal parameters to quantify the uncertainty of dose-effect relations. Technical Report SBI 222/12.2003, Ludwig-Maximilians University of Munich
Rao CM, Chakravarti IM (1956) Some small sample test of significance for a Poisson distribution. Biometrics 12:265–282
IAEA (1986) Biological dosimetry: chromosomal aberration analysis for dose assessment. Technical Report Series No. 260. International Atomic Energy Agency, Vienna
Schmid E, Hieber L, Heinzmann U, Roos H, Kellerer AM (1996) Analysis of chromosome aberrations in human peripheral lymphocytes induced by in vitro α-particle irradiation. Radiat Environ Biophys 35:179–184
Edwards AA, Purrott RJ, Prosser JS, Lloyd DC (1980) The induction of chromosome aberrations in human lymphocytes by alpha-radiation. Int J Radiat Biol 38:83–91
Baquinero JF, Stephan G, Schmid E (2004) Effect of americium-241 α-particles on the dose–response of chromosome aberrations in human lymphocytes analysed by fluorescence in situ hybridization. Int J Radiat Biol 80:155–164
Nagasawa H, Little JB (1992) Induction of sister chromatid exchanges by extremly low doses of α-particles. Cancer Res 52:6394–6396
Nagasawa H, Little JB (1999) Unexpected sensitivity to the induction of mutations by very low doses of alpha particle radiation: evidence for a bystander effect. Radiat Res 152:552–557
Little MP, Wakeford R (2001) The bystander effect in C3H 10T1/2 cells and radon-induced lung cancer. Radiat Res 156:695–699
Sawant SG, Randers-Pehrson G, Geard CR, Brenner J, Hall EJ (2001) The bystander effect in radiation oncogenesis, I: transformation in C3H 10T1/2 cells in vitro can be initiated in the unirradiated neighbours of irradiated cells. Radiat Res 155:397–401
Brenner DJ, Little JB, Sachs RK (2001) The bystander effect in radiation oncogenesis, II: a quantitative model. Radiat Res 155:402–408
Nagasawa H, Little JB (1999) Unexpected sensitivity to the induction of mutations by very low doses of alpha-particle radiation: evidence for a bystander effect. Radiat Res 152:552–557
Zhou H, Randers-Pehrson G, Waldren CA, Vannais D, Hall EJ, Hei TK (2000) Induction of a bystander mutagenic effect of alpha particles in mammalian cells. Proc Natl Acad Sci U S A 97:2099–2104
Ponnaiya B, Jenkins-Baker G, Brenner DJ, Hall EJ, Randers-Pehrson G, Geard CR (2004) Biological responses in known bystander cells relative to known microbeam-irradiated cells. Radiat Res 162:426–432
Mitchell SA, Randers-Pehrson G, Brenner DJ, Hall EJ (2004) The bystander response in C3H 10T1/2 cells: the influence of cell-to-cell contact. Radiat Res 161:397–401
Mothersill C, Seymour C (1997) Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of unirradiated cells. Int J Radiat Biol 71:421–427
Acknowledgements
The authors would like to express their gratitude to Mrs T. Roedler-Vogelsang for her valuable consideration and help in preparing this manuscript. The authors also thank Mrs S. Wenzel and D. Westpfahl for skilful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stephan, G., Kampen, W.U., Noßke, D. et al. Chromosomal aberrations in peripheral lymphocytes of patients treated with radium-224 for ankylosing spondylitis. Radiat Environ Biophys 44, 23–28 (2005). https://doi.org/10.1007/s00411-005-0275-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-005-0275-x