Abstract
After the Chernobyl accident a statistically significant increase in the number of children with thyroid tumours was observed. In this study 166 children with and 75 without thyroid tumours were analysed for micronucleus formation in peripheral blood lymphocytes using the cytochalasin B approach. The following factors did not significantly affect micronucleus formation: gender, age at the time of the first 131I treatment, tumour stage, tumour type, or metastases; a statistically significant increase in the number of micronuclei, however, was observed for the residents of Gomel compared to other locations, such as Brest, Grodno, and Minsk. The children with tumours received 131I treatment after surgical resection of the tumours. This gave us the opportunity to systematically follow the effect of 131I on micronucleus formation. A marked increase was observed 5 days after the 131I treatment followed by a decrease within a 4–7 months interval up to the next application, but the pre-treatment levels were not achieved. Up to 10 therapy cycles were followed each including an analysis of micronucleus formation before and 5 days after 131I application. The response of the children was characterised by clear individual differences and the increase/decrease pattern of micronucleus frequencies induced by iodine-131 was correlated with a decrease/increase pattern in the number of lymphocytes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1996 a study on micronucleus formation in peripheral lymphocytes of children from the vicinity of Chernobyl was published [1]. At that time our primary goal was the evaluation of the possibility to reconstruct individual doses on the basis of micronuclei frequencies several years after the Chernobyl accident. It turned out that this was not possible because no differences in micronucleus formation were seen between the children from the vicinity of Chernobyl and unexposed control children. A major factor being responsible for this outcome was the relatively long interval of several years between exposure and analysis.
Some results on the 131I therapy of children with thyroid cancer were reported as well [1]. The analyses were restricted to the very first radioiodine treatment. A fairly good correlation was observed between the frequency of micronuclei and the calculated dose from the 131I activity administered.
Meanwhile, we have considerably extended the study on the radioiodine treatment of children with thyroid cancer. In particular we followed the response of micronuclei in peripheral lymphocytes over many (up to 10) therapy cycles and the results for the entire population studied (166 children with and 75 without thyroid cancer) are described. We will, in a future paper, show individual kinetics and attempt to correlate micronucleus frequencies and therapeutic outcome.
Materials and methods
Patients
Blood of the first patient was analysed on 9 January 1992 and the study was terminated on 9 November 1998. Table 1 summarises the most important characteristics of the patients (n=166) and of the healthy controls (n=75). Both groups were matched for gender, time of birth, age at the time of the accident and at the time of analysis. All children were from Belarus, but there were some differences with regard to the specific home town with Brest being under-represented in the control group. In both groups, most of the children originated from Gomel.
The higher frequency of girls with thyroid cancer is in accordance with the experience that thyroid cancer is more frequent in females than in males. More than 80% of the tumours were of stage T4 and of the papillary type. About 50% of the tumours showed metastases.
131I Therapy
The diagnostic protocol included ultrasonography and scintigraphy of the neck, chest radiographs, computer tests of pulmonary function, determination of thyroglobulin, TSH, FT4 and FT3 in serum, as well as measurements of calcium, phosphate and differential blood cell counts (using standard Coulter counter automatic procedures). Additionally, in a subset of children, x-ray computer tomography (CT) and whole body counter measurements of incorporated radionuclides were performed.
For elimination of thyroid remnants, 50 MBq of iodine-131 per kg body weight was administered. For ablation of metastases, 100 MBq of iodine-131 per kg body weight was administered. Two days after treatment, replacement therapy with levothyroxin that had been withdrawn 4 weeks before treatment, was restarted. The mean dose amounted to 2.5 mg of levothyroxin per kg body weight. For staging, whole body scans were performed 4 days after the iodine application. The mean interval between 2 consecutive treatment courses was 4.6 months (minimum 4, maximum 7.5 months).
Blood samples for micronucleus analysis were drawn immediately before and 5 days after administration of iodine-131.
Micronucleus analysis
Details of micronucleus analysis have been published previously [1, 2]. We used the cytochalasin B technique in lymphocytes [3] with slight modifications as described in [1]. Briefly, heparinized blood was collected immediately before 131I administration and 5 days thereafter. Lymphocytes of whole blood cultures in RPMI 1640 were stimulated by the addition of phytohaemagglutinin (PHA) and approximately 44 h after stimulation, cytochalasin B (5 µg/ml) was added to induce the formation of binucleated lymphocytes. About 25 h after application of cytochalasin B, lymphocytes were fixed on slides and stained with Giemsa. Usually, 1,000 binucleated cells per donor per time point were checked for the presence of micronuclei at a magnification of 10×50. In total 26,138 micronuclei were scored in 875,668 binucleated cells. In addition, proliferation was monitored by counting the number of binucleated per 200 mononucleated cells (trinucleated and tetranucleated cells were rarely observed under our culture conditions). The analysis was done by one experienced person (more than 1 year of training) and reliability of scoring was checked at regular intervals through counting of a series of standard slides.
Micronuclei have the advantage that in vivo and in vitro responses after radiation exposure are comparable [2], which means that in vivo responses can be estimated through in vitro calibration.
There was one technical problem to be considered when interpreting the micronucleus results: 238 slides from a total of 1,163 could not be scored for 2 reasons: 108 blood samples did not enter the analysis procedure (blood was not withdrawn, lost on the mailing route or lost during preparation of the slides) and the quality of a significant number of slides (127 from the patients and 3 of the controls) was so poor (e.g. no cytoplasm detectable, disintegrated cell nuclei, no binucleated cells present) that scoring was impossible. Most of these slides (n =109) that could not be scored were obtained after iodine therapy, indicating that the integrity of lymphocytes was affected by radiation. One of our original goals was (and still is) to study whether a correlation exists between micronucleus results and therapy response at an individual level. This, quite obviously, is difficult when taking into consideration that complete data sets with at least 3 therapy cycles are only available from 10 out of 166 patients. A possible solution of the problem will be addressed at the end of the Results and discussion section.
Statistics
Micronucleus frequencies were compared using the Mann Whitney U-test (GraphPad Prism version 3.02; GraphPad Software, San Diego, CA) and Bonferroni correction was used for multiple comparisons.
Results and discussion
Blood of 75 healthy controls and 166 children with thyroid carcinomas was analysed (see Table 1). In order to avoid misinterpretations, one important fact has to be kept in mind: quite a number of the children already received therapy elsewhere, before they could be analysed for micronucleus formation in our institute (7 chemotherapy, 20 radiotherapy, 50 iodine-131, some of the children that had received chemotherapy or radiotherapy were also treated with iodine-131, that means, 65 of the 166 children came after pretreatment). As we knew the details of the pretreatment, we could take care of this problem.
Table 2 shows that lymphocytes could be scored from 153 out of a total of 166 patients before they received their first treatment in Essen or Würzburg, and from 92 previously untreated children. The “all patients” group showed significantly higher numbers of micronuclei as compared to those patients without pretreatment. The latter group, on the other hand, had significantly less micronuclei than the control group. The frequency of binucleated cells was the same in all groups.
The reason for the discrepancy in the number of micronuclei for all patients (11.2 per 1,000 cells) and for all patients without pretreatment (7.2 per 1,000 cells) is explained by the data of Table 3. All pretreated children had higher micronucleus frequencies than those without pretreatment. The increase was statistically significant after radiotherapy and radioiodine treatment, but with just 2 patients receiving chemotherapy only, testing for significance is not reasonable. Binucleation, however, was not affected by any of the pretreatment procedures.
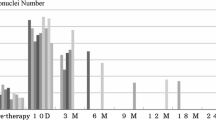
In addition to Table 3, Fig. 1 also gives the information on the effects of other treatment modalities on micronucleus formation in our first analysis. Again, all pretreatment procedures increased micronucleus frequencies, although in many cases the number of children in the group was very small. No attempts were made to look for the impact of time between treatment and analysis, because in most groups only very few donors were available.
Effect of pretreatment in other hospitals on our first analysis (CT chemotherapy, RT radiotherapy, RIT radioiodine therapy, numbers in parentheses number of patients. For two patients we had the information that they were pretreated, but no information on the type of pretreatment. When comparing Table 4 and Fig 1, please note that this box and whisker plot gives the median and not the arithmetic mean)
Figure 2 summarises the effect of radioiodine therapy on micronucleus formation and the percentage of binucleated cells. Intentionally, this graph shows all data available to give a first impression. in all cases of radioiodine treatment the number of micronuclei clearly increased by about 11 micronuclei per Gy and decreased until the next treatment was applied by about 7 per month of those micronuclei induced by radioiodine, but never reached the level prior to therapy. In the course of time, there was a tendency to higher levels of persisting micronuclei. One has to take into consideration, however, that only therapy-resistant children received many (up to 10) therapies and that this subgroup perhaps has different retention times for radioiodine. Proliferation of lymphocytes after stimulation responded in most cases with a drop in the number of binucleated cells after radioiodine administration.
There are two problems when pooling all the data available. First, children with pretreatment were included and pretreatment has some effect (see Table 3 and Fig. 1). This was no problem for the major group of pretreated children, i.e. those with radioiodine pretreatment. As we knew the number of therapies applied previously, we could introduce the children into our analysis taking into consideration the number of radioiodine pretreatments. If, for example, a child had already undergone three radioiodine treatments other than in our hospital, then the first treatment in our hospital was counted as the fourth one (we did of course, not know the micronucleus response of the first three therapies). Second, for some patients we had results either before or after a specific therapy, but not before and after therapy.
In order to minimise both problems, Fig. 3 includes only those children for whom micronucleus numbers before and after a specific radioiodine administration were available and who had not received either radiotherapy or chemotherapy before. The result essentially agrees with the one presented in Fig. 2. Due to the high number of patients that were analysed, pretreatment and missing values did not have much impact on the mean values. But there is still another problem: for some of the children the complete data of the first and third therapies were available, but not of the second one and for those children having received radioiodine therapies other than in our hospital, the micronucleus analyses are missing for these therapies. This is also the major reason for the fact that in Fig. 3 less patients are listed for the fourth therapy than for the fifth: several children previously received four therapies and thus entered our study at the beginning of the fifth therapy cycle.
Effect of radioiodine therapy on micronucleus formation and binucleation in those patients without previous chemotherapy or radiotherapy for whom complete data sets were available before and after radioiodine therapy (in parentheses number of children studied, mnc micronuclei, BNC binucleated cells)
Figure 4 therefore shows the data only for those 10 children for whom all possible micronucleus frequencies could be obtained and who received all non-surgical treatments in Essen or Würzburg (there was, thus, no pretreatment problem). The results of the first 3 therapy cycles are particularly valuable, because all 10 children that met the conditions for being included into this subgroup underwent at least 3 therapies. The data of the subsequent therapies are less reliable, because they are based on less patients. Nevertheless, the basic pattern from Fig. 2 with the raw data is very similar to this “optimum” group shown in Fig. 4.
In addition to micronucleus formation and binucleation we analysed some more endpoints, e.g. the number of lymphocytes. Figure 5 shows that an increase in the number of micronuclei after radioiodine application is reflected by a very similar decrease in lymphocytes (Fig. 5). Lymphocyte numbers appear to be a mirror image of micronucleus numbers. Thus, there is a marked inverse correlation between micronucleus and lymphocyte numbers, a correlation that is statistically significant at P<0.001 (Pearson’s r, based on 819 XY pairs).
Table 4 gives detailed information on the effects of gender, age at the time of the first treatment, place of residence, tumour stage, tumour type, and metastases. The age of 11.8 years was chosen because it is the median of ages in the entire group of healthy individuals and patients. Unfortunately, the median of the healthy individuals (11.1 years) is slightly different from that of the patients (12.4 years), therefore the number of individuals is not the same in the groups below or above the median when considering healthy donors and patients separately. The result, however, is essentially the same when one uses the separate median values for healthy individuals (8.6 micronuclei per 1,000 cells below 11.1 years and 8.9 per 1,000 above) and patients (7.8 per 1,000 below 12.4 years and 6.8 above).
In the case of tumour stage, tumour type, and metastases, none of the micronucleus numbers is significantly different from the corresponding values (i.e. there is no statistically significant difference in the formation of micronuclei in lymphocytes of children with T4 and stages less than T4 tumours). Much more complicated is the situation for gender, age at the time of the first 131I treatment, and place of residence. P-values at <0.001 were found for the following comparisons: healthy females versus female patients; healthy donors of ≥11.8 years and patients of ≥11.8 years; healthy donors from Gomel and healthy donors who did not live in Gomel and, particularly, all donors (healthy + patients) from Gomel compared to all donors from other locations than Gomel. A serious problem, however, arises when interpreting these results: we used multiple statistical comparisons (a total of 11 comparisons), so that with a certain probability we will find significance in some cases although there is none. In order to avoid this “fishing for significance”, we used the Bonferroni correction and we learned that instead of an alpha level of P<0.01 for a single test we had to obtain a P<0.0009 to correct for multiple testing. After applying the Bonferroni correction, there remained only one significant result: children living in Gomel had higher micronucleus frequencies than children living in other locations. Nevertheless, also the significances at P<0.01 are useful, not for a conclusion in the context of this publication, but for hypothesis generation. Such hypotheses have to be examined in a study designed specifically for this purpose.
It should be stressed that this study was not intended to analyse radiation effects induced by the Chernobyl accident. Due to the long time interval between the accident and the analyses, any possible initial increases in micronucleus frequencies would have disappeared [1]. Our major interest was focussed on the formation of micronuclei in lymphocytes possibly induced by 131I therapy for thyroid carcinoma.
Quite a number of studies have dealt with the effects of 131I therapy on micronucleus formation in peripheral lymphocytes [1, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13]. Almost all of these studies reported a significant increase in the number of micronuclei in peripheral lymphocytes after application of therapeutic activities of iodine-131. It is, however, very difficult to compare the specific results, because there are many differences among the individual studies. These differences refer to, for example, the types of disease treated, the levels of radioactivity applied, numbers of treatment cycles, time points of micronucleus analysis, age at the time of exposure, and aim of the study.
One peculiarity of our study when comparing our results to those of other studies is that all our donors were children. Thus, many aspects may have different impacts when lymphocytes of children or adults are analysed for micronuclei after 131I exposure, e.g. lymphocyte sensitivity, spectrum of lymphocyte subpopulations, lymphocyte turnover, amount of iodine storing tissue, and retention times of iodine in the body.
Age-dependence may at least partly explain the very heterogeneous results that have been reported with respect to the persistence of micronuclei in lymphocytes after exposure to 131I. Our results suggest that most of the micronuclei induced (about 85%) disappear during the interval of about 4–7 months between the treatments. The results reported in the literature for thyroid cancer therapy differ markedly from almost no decline between 1 and 6 months [13] or between 11 days up to 8 months [4], over a minor decline between the first week and 6 months and a further decline after 1 year without reaching pretreatment frequencies [9], to a complete disappearance of induced micronuclei 1 year following exposure to 131I [6]. As might be expected, there is also a dependence on the type of thyroid disease that has been treated. Gutiérrez et al. [9] reported a gradual increase of micronuclei up to 3 months after 131I therapy of hyperthyroidism with no reduction in the first 6 months, whereas cancer patients showed the highest values 1 week after exposure (unfortunately no data for 3 months were reported) and somewhat lower frequencies after 6 months and 1 year. The authors concluded from their results that micronuclei induced by iodine-131 may persist up to 3 years. That a small fraction of micronuclei can persist for a relatively long time can be demonstrated by our observation that all children that came with a pretreatment (radiotherapy, radioiodine, and/or chemotherapy) showed slightly elevated numbers of micronuclei.
While some factors, such as tumour stage, tumour type and metastases, did not significantly affect micronucleus formation, we did indeed observe some effects on micronucleus formation induced by gender, age at the time of the first 131I treatment, and place of residence. As outlined one has to be very careful with the conclusions, because we used multiple statistical comparisons within the same data set. When taking care of this problem by using Bonferroni correction, it turned out that there is a strong indication for children from Gomel, irrespective of being healthy or suffering from thyroid carcinoma, to show higher micronucleus frequencies in their lymphocytes (9.1±6.7, n=89) than children from other Belorussian or Ukrainian locations (6.3±6.7, n=69). This result, in a sense, is in line with a previous publication by Mikhalevich et al. [14] who found a higher number of micronuclei in mononucleated lymphocytes of children living in the Gomel region, when compared to children living in Minsk, but not in binucleated lymphocytes. This indicates that micronucleus frequency can be affected by living in a comparatively highly contaminated region (Gomel district), but in their study this was only true for mononucleated lymphocytes. One reason why Mikhalevich et al. [14] did not find an increase in binucleated lymphocytes might be the comparatively low sample size (10 children from Minsk and 20 children from the Gomel district), whereas we analysed the blood of 69 non-Gomel and 89 Gomel residents.
The group of healthy children showed a lower micronucleus frequency of about 9 (Gomel residents) or 6 (non-Gomel residents) micronuclei per 1,000 binucleated cells at an average age of about 11 years when compared to adults showing 16 micronuclei per 1,000 binucleated cells (our own historical background frequency being based on 110 healthy controls at an average age of 40 years). Some increase of micronucleus numbers with age has been observed many times (e.g. [15, 16]).
An interesting result of our analysis was the observation that the numbers of micronuclei showed an increase and the numbers of lymphocytes an almost corresponding decrease after radioiodine application. At first glance this correlation does not seem to be very surprising because it is well known that micronuclei represent a semi-quantitative indicator of cell death [15]. However, lymphocytes were analysed in our study and lymphocytes usually do not divide in vivo, so that they do not “recognize” that they harbour a micronucleus. In addition, the mechanism of cell death induced by a micronucleus is assumed to be through the loss of genetic material during cell division; this loss, however, is not possible for lymphocytes in vivo.
On the other hand, the comparatively close inverse correlation between micronucleus and lymphocyte numbers may be useful when we look for individual predictions of therapeutic success or failure in the future, because this correlation may help to encompass the problem of missing micronucleus values. Those analyses that have to wait for a complete picture of the therapy success rates will also address another important aspect that has been mentioned above: the individual responses of the children to 131I applications are markedly different, even in those cases where the same activities have been given. This is another example showing that the response of the body to radiation exposure does not only depend on activities and doses, but also on individual biological parameters. There are many biological parameters which affect this response, so that it can only be assessed by the determination of biological endpoints and not by physical model calculations.
References
Wuttke K, Streffer C, Müller W-U, Reiners C, Biko J, Demidchik E (1996) Micronuclei in lymphocytes of children from the vicinity of Chernobyl before and after 131I therapy for thyroid cancer. Int J Radiat Biol 69:259–268
Gantenberg H-W, Wuttke K, Streffer C, Müller W-U (1991) Micronuclei in human lymphocytes irradiated in vitro or in vivo. Radiat Res 128:276–281
Fenech M, Morley AA (1985) Measurement of micronuclei in lymphocytes. Mutat Res 147:29–36
Livingston GK, Foster AE, Elson HR (1993) Effect of in vivo exposure to iodine-131 on the frequency and persistence of micronuclei in human lymphocytes. J Toxicol Environ Health 40:367–375
Catena C, Villani P, Nastasi R et al. (1994) Micronuclei and 3AB-index in patients receiving iodine-131 therapy. J Nucl Biol Med 38:586–593
Gutierrez S, Carbonell E, Galofrea P, Xamena N, Creus A, Marcos R (1995) A cytogenetic follow-up study of thyroid cancer patients treated with131I. Cancer Lett 91:199–204
Ramirez MJ, Surralles J, Galofre P, Creus A, Marcos R (1997) Radioactive iodine induces clastogenic and age-dependent aneugenic effects in lymphocytes of thyroid cancer patients as revealed by interphase FISH. Mutagenesis 12:449–455
Watanabe N, Yokoyama K, Kinuya S et al. (1998) Radiotoxicity after iodine-131 therapy for thyroid cancer using the micronucleus assay. J Nucl Med 39:436–440
Gutierrez S, Carbonell E, Galofre P, Creus A, Marcos R (1999) Cytogenetic damage after 131-iodine treatment for hyperthyroidism and thyroid cancer. A study using the micronucleus test. Eur J Nucl Med 26:1589–1596
Monsieurs MA, Thierens HM, Wiele CV van de et al. (1999) Estimation of risk based on biological dosimetry for patients treated with radioiodine. Nucl Med Commun 20:911–907
Catena C, Conti D, Trenta G et al. (2001) Micronucleus yield and colorimetric test as indicators of damage in patients’ lymphocytes after 131I therapy. J Nucl Med 41:1522–1524
Monsieurs MA, Thierens HM, Vral AM, Wiele C Van De, Ridder LI, Dierckx RA De (2001) Adaptive response in patients treated with 131I. J Nucl Med 41:17–22
Monteiro Gil O, Oliveira NG, Rodrigues AS et al. (2001) Cytogenetic alterations and oxidative stress in thyroid cancer patients after iodine-131 therapy. Mutagenesis 15:69–75
Mikhalevich LS, Zwart FA de, Perepetskaya GA, Chebotareva NV, Mikhalevich EA, Tates AD (2000) Radiation effects in lymphocytes of children living in a Chernobyl contaminated region of Belarus. Int J Radiat Biol 76:1377–1385
Müller W-U, Streffer C (1994) Micronucleus assays. Adv Mutag Res 5:1–133
Barale R, Chelotti L, Davini T et al. (1998) Sister chromatid exchange and micronucleus frequency in human lymphocytes of 1,650 subjects in an Italian population: II. Contribution of sex, age, and lifestyle. Environ Mol Mutagen 31:228–242
Acknowledgements
This work was supported by a grant from the “Vereinigung Deutscher Elektrizitätswerke (VDEW) e.V.”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Müller, WU., Dietl, S., Wuttke, K. et al. Micronucleus formation in lymphocytes of children from the vicinity of Chernobyl after 131I therapy. Radiat Environ Biophys 43, 7–13 (2004). https://doi.org/10.1007/s00411-004-0233-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-004-0233-z