Abstract
The Cerro del Almirez massif (Spain) represents a unique fragment of serpentinized oceanic lithosphere that has been first equilibrated in the antigorite stability field (Atg-serpentinites) and then dehydrated into chlorite–olivine–orthopyroxene (Chl-harzburgites) at eclogite facies conditions during subduction. The massif preserves a dehydration front between Atg-serpentinites and Chl-harzburgites. It constitutes a suitable place to study redox changes in serpentinites and the nature of the released fluids during their dehydration. Relative to abyssal serpentinites, Atg-serpentinites display a low Fe3+/FeTotal(BR) (=0.55) and magnetite modal content (=2.8–4.3 wt%). Micro-X-ray absorption near-edge structure (μ-XANES) spectroscopy measurements of serpentines at the Fe–K edge show that antigorite has a lower Fe3+/FeTotal ratio (=0.48) than oceanic lizardite/chrysotile assemblages. The onset of Atg-serpentinites dehydration is marked by the crystallization of a Fe3+-rich antigorite (Fe3+/FeTotal = 0.6–0.75) in equilibrium with secondary olivine and by a decrease in magnetite amount (=1.6–2.2 wt%). This suggests a preferential partitioning of Fe3+ into serpentine rather than into olivine. The Atg-breakdown is marked by a decrease in Fe3+/FeTotal(BR) (=0.34–0.41), the crystallization of Fe2+-rich phases and the quasi-disappearance of magnetite (=0.6–1.4 wt.%). The observation of Fe3+-rich hematite and ilmenite intergrowths suggests that the O2 released by the crystallization of Fe2+-rich phases could promote hematite crystallization and a subsequent increase in fo2 inside the portion of the subducted mantle. Serpentinite dehydration could thus produce highly oxidized fluids in subduction zones and contribute to the oxidization of the sub-arc mantle wedge.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In subduction zones, the dehydration of serpentinites is believed to be one of the key processes to transfer water from the slab to the mantle wedge (Ulmer and Trommsdorff 1995). Furthermore, these rocks are an essential reservoir of trace, fluid mobile, halogen, and volatile elements (Alt and Shanks 2003; Vils et al. 2008; Delacour et al. 2008a, b; Kodolanyi et al. 2012; Marchesi et al. 2013). It follows that serpentine dehydration could lead to the modification of the composition of the mantle wedge and thus that of arc magmas (Savov et al. 2007; Vils et al. 2011; Scambelluri and Tonarini 2012; Debret et al. 2013a, 2014a). In this context, the evolution of serpentinite iron redox state is a key parameter to better understand the speciation of the released C–S–O–H-bearing fluids that influence the mantle wedge solidus and the properties of the resulting melts (Frost 1991; Evans 2012; Debret et al. 2014b).
Numerous studies have shown that arc magmas are oxidized relative to MORB and suboceanic mantle, suggesting that oxidized slab-derived fluids (e.g., CO2, SOX) are transferred to the mantle wedge and modify its redox properties (Arculus 1994; Parkinson and Arculus 1999; Andersen and Neumann 2001; Kelley and Cottrell 2009; Evans and Tomkins 2011; Bouilhol et al. 2012; Evans 2012; Laubier et al. 2014). Nevertheless, the oxidized nature of sub-arc mantle has been the subject of controversy. Indeed, the study of V/Sc and Zn/Fe ratios in arc basalts and the observation of CH4-, C-, and H2-rich fluid inclusions in olivine from orogenic harzburgites suggest that sub-arc mantle is not more oxidized than the MORB mantle source (Lee et al. 2005, 2010; Song et al. 2009), while the redox state of key elements like iron remains poorly constrained in the residual slab (Padrón-Navarta et al. 2011; Debret et al. 2014b). The study of ophiolitic material is thus an alternative approach to the study of arc volcanism and mantle wedge xenoliths for constraining the redox state of iron in subduction zones (Malaspina and Tumiati 2012; Debret et al. 2014b; Tumiati et al. 2015).
In oceanic settings, the serpentinization of mantle peridotite results in olivine and orthopyroxene alteration into chrysotile/lizardite and magnetite assemblages. This episode is associated with an iron oxidation and a discharge of H2-rich fluids (Frost 1985; Berndt et al. 1996; Oufi et al. 2002; Charlou et al. 2002; Bach et al. 2006; Klein and Bach 2009; McCollom and Bach 2009; Klein et al. 2013). In a fluid-dominated system, the progressive serpentinization of peridotite is accompanied with an increase in magnetite modal amount and Fe3+/FeTotal ratio in serpentine (Marcaillou et al. 2011; Andreani et al. 2013; Frost et al. 2013; Klein et al. 2013). Serpentinites could thus constitute an essential reservoir of fluids but also of Fe3+ in subduction zones where they can potentially play a major role in redox processes.
During subduction, from greenschist-to-blueschist facies conditions, the transition from lizardite/chrysotile to antigorite is accompanied with a decrease in Fe3+/FeTotal ratio in serpentine and dissolution of oceanic magnetite (Evans et al. 2012; Debret et al. 2014b). These results attest for an iron reduction in serpentinites during the first stages of prograde metamorphism (Debret et al. 2014b). Such redox reactions could be coupled with oxidation of reduced phases allowing the formation of highly oxidized fluids (e.g., SOX, CO2) that can metasomatize the mantle wedge (Debret et al. 2014a, b). At eclogite facies conditions, the prograde metamorphism of serpentinites results eventually in the loss of H2O and the growth of olivine + orthopyroxene + chlorite assemblages (Trommsdorff et al. 1998). Although antigorite breakdown is the most important source of H2O liberation at depth (Ulmer and Trommsdorff 1995), the redox aspects of this reaction are poorly documented because of the scarcity of fully dehydrated serpentinites in eclogitic terrains (e.g., Evans and Trommsdorff 1978).
The Cerro del Almirez massif, in the Betic Cordillera (Spain), is the best occurrence of meta-chlorite harzburgite (Chl-harzburgite) derived from antigorite serpentinites (Trommsdorff et al. 1998; Scambelluri et al. 2001; Garrido et al. 2005; López Sánchez-Vizcaíno et al. 2005; Padrón-Navarta et al. 2010a, b, 2011). Chl-harzburgites are composed of Fe2+-rich minerals, mainly olivine and orthopyroxene, with chlorite, titano-clinohumite (Ti-clinohumite), and oxides (Trommsdorff et al. 1998; Scambelluri et al. 2001; López Sánchez-Vizcaíno et al. 2005), which control the H2O and Fe3+ budget of ultramafic rocks beyond antigorite stability. Furthermore, the massif preserves a dehydration front between Atg-serpentinites and Chl-harzburgites that allows the assessment of the evolution of iron redox state and constrains the nature of the aqueous fluid released during antigorite breakdown in subduction zones. In this study, we measured bulk rock (BR) chemistry and magnetic hysteresis of the different lithologies forming the massif in order to establish their Fe3+/FeTotal(BR) ratio, FeOTotal(BR) content, and magnetite modal amount. We then estimate the Fe3+/FeTotal ratio of the different silicate phases, mostly antigorite and chlorite, involved during antigorite breakdown by performing synchrotron µ-XANES measurements to decipher the redox nature of the dehydration reaction.
Geological setting
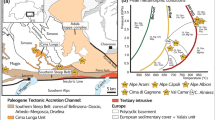
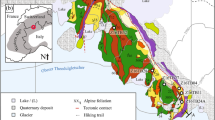
The Cerro del Almirez massif is located in the south of Spain (Fig. 1a), in the Nevado–Filábride Complex, the lowermost unit of the internal zones of the Betic Cordillera. It is an ultramafic body of 2–3 km2 enclosed in Paleozoic metasediments (Fig. 1a; Gómez-Pugnaire et al. 2004). The massif consists of an antigorite serpentinite unit (Atg-serpentinites) of about 200 m thick sitting on the top of a chlorite harzburgite unit (granofels and spinifex-like Chl-harzburgites) (Fig. 1b). The contact between the two lithologies crosscuts the Atg-serpentinite foliation and is interpreted as the antigorite dehydration front that resulted in the growth of olivine (Ol), orthopyroxene (Opx), and chlorite (Chl) at 1.6–1.9 GPa and 660–680 °C (Trommsdorff et al. 1998; López Sánchez-Vizcaíno et al. 2005; Padrón-Navarta et al. 2010b). The dehydration front is marked by a ~10-m-thick transitional zone of partly dehydrated serpentinites (transitional lithologies after Padrón-Navarta et al. 2008, 2011) consisting of Chl–Atg–Ol serpentinites and Atg–Chl–Opx–Ol serpentinites. Beyond this zone, the antigorite is fully dehydrated into Chl-harzburgites displaying granofels (granoblastic) or spinifex-like textures.
The different lithologies from the Cerro del Almirez massif preserve different mineral assemblages representative of the prograde antigorite breakdown in subduction zones (Fig. 2). Therefore, a set of five Atg-serpentinites, three transitional lithologies, four granofels, and four spinifex-like Chl-harzburgites have been selected for this study in order to follow the evolution of Fe redox state during Atg-breakdown (Fig. 1b; Table 1). These samples have been characterized in detail in previous petrological and geochemical studies (Trommsdorff et al. 1998; López Sánchez-Vizcaíno et al. 2005; Garrido et al. 2005; Padrón-Navarta et al. 2008, 2010a, b, 2011; Marchesi et al. 2013).
Methods
Bulk rock ferrous iron was determined by potentiometric analysis using potassium permanganate as oxidation agent. Ferric iron was then calculated from the difference between the total iron measured by XRF and ferrous iron measured by potentiometric analysis. Bulk rock FeOTotal(BR) and Fe2O3(BR) content (in wt%) of the studied sample suite is from Padrón-Navarta et al. (2011, their Table 2) and Marchesi et al. (2013, their S1 in the supplementary material), and they are reported as Fe3+/FeTotal(BR) molar ratio (Table 1).
Magnetic hysteresis loops were measured in a J-meter coercivity spectrometer designed by the University of Kazan up to a maximum applied field of 0.5 T (Jasonov et al. 1998). Three powdered separates and one piece of rock have been measured for each sample (Table 1), giving rise to similar results. Saturation magnetization (Ms) has been computed after ensuring the reversibility of the loop and subtracting the Ms attributed to the paramagnetic fraction (Tauxe 2009). Saturation is reached in all samples at fields below 0.25 T. Additionally, thermomagnetic curves were obtained in all samples in a Vibrating Field Translation Balance (VFTB) up to 700 °C, showing a unique curie temperature of 580 °C, confirming magnetite is the main carrier of Ms in the analyzed samples (e.g., Dunlop and Özdemir 1997). Thus, saturation Ms has been normalized by the pure magnetite value (Ms = 480 kA/m) in order to estimate the bulk percentage of magnetite (Dunlop and Özdemir 1997). Powdered samples and blocks gave identical results within the instrumental error. Average values of magnetite amounts are reported in Table 1.
Silicates and oxides were characterized by Raman spectroscopy and microprobe analyses coupled with thin-section observations. Raman spectroscopy was performed at the ENS of Lyon (France). The Raman signal was acquired over 30–90 s in three accumulating cycles. The laser power was set at 800 µW in order to avoid degradation of serpentine or oxide minerals (De Faria et al. 1997; Schwartz et al. 2013), and the surface was checked after each analysis. In situ major element analyses of minerals were performed with a microprobe CAMECA SX100 at the Laboratoire Magmas et Volcans in Clermont-Ferrand (France). Microprobe analyses mean values of repeated analysis of serpentine, chlorite, olivine, talc, orthopyroxene, and Ti-clinohumite are shown in Table 2.
Iron speciation in silicate minerals was measured by X-ray absorption spectroscopy at the iron K-edge at the LUCIA beamline of SOLEIL synchrotron (Source Optimisée de Lumière d’Energie Intermédiaire du LURE, France). Measurements were taken with the following configuration for the storage ring: current of 400 mA and energy of 2.75 GeV. The X-ray absorption near-edge structure (XANES) spectra were collected using a Si(311) double-crystal monochromator. The energy calibration was performed using an iron foil. XANES spectra were measured in fluorescence mode using a four-element silicon drift diode (SDD) detector with a total active area of 40 mm2. The beam spot size was set to 4 × 4 μm2 by using two dynamically bendable mirrors in Kirkpatrick–Baez configuration.
XANES spectra were acquired from 7050 to 7300 eV. We used a sampling step of 2 eV between 7050 and 7106 eV, 0.1 eV from 7106 to 7120 eV, 0.2 eV between 7120 and 7150 eV, 0.5 eV from 7150 to 7220 eV, and 1 eV between 7220 and 7300 eV. The Fe3+/FeTotal ratios of serpentines, chlorites, and Ti-clinohumite have been derived after fitting the pre-edge region (Fig. 3) with the PeakFit© software. The background of the pre-edge region was modeled using the tail of a Gaussian and Lorenz mixed function, while the pre-edge region was deconvoluted into two pseudo-Voigt functions. This treatment results in absolute uncertainties for the determination of the pre-edge centroid of ±0.05 eV in energy and ±0.025 in integrated area (Wilke et al. 2001; Marcaillou et al. 2011; Andreani et al. 2013; Debret et al. 2014b).
In order to minimize photooxidation effects, serpentine XANES spectra were acquired following the procedure presented in Debret et al. (2014b). Chlorite XANES spectra are not significantly affected by photooxidation effects based on tests performed in this work (Appendix A). Muñoz et al. (2013) have reported strong changes in the pre-edge centroid position correlated with serpentine and chlorite crystal orientation relative to the polarized X-ray beam. These authors report a systematic overestimation of pre-edge centroid energy position of 0.07 eV for measurements taken with the wave vector perpendicular to (001) planes of serpentine and chlorite crystals. With the wave vector perpendicular to (100) or (010) planes, the maximal overestimation is about 0.07 and 0.1 eV, while the underestimate is about 0.1 and 0.12 eV for serpentine and chlorite crystals, respectively. In order to reduce crystal orientation effects during serpentine and chlorite XANES analyses, several spectra (from 4 to 9) were taken on randomly orientated crystals.
Before each XANES measurement, the positioning of the incident beam on the thin section was checked based on a chemical mapping of the region using X-ray fluorescence (XRF) spectroscopy. This chemical mapping was compared to SEM chemical mapping in order to identify accessory microphases (such as magnetite or sulfides) associated with serpentine minerals and to avoid multiphase analyses.
Bulk rock results
In the Cerro del Almirez massif, there is no obvious change in FeOTotal(BR) passing from Atg-serpentinites to Chl-harzburgites (Table 1; Garrido et al. 2005; Padrón-Navarta et al. 2011; Marchesi et al. 2013). Among the studied sample suite, FeOTotal(BR) contents of Atg-serpentinites and transitional lithologies vary from 6.7 to 7.7 wt% and from 6.7 to 8.7 wt%, respectively, while Chl-harzburgite FeOTotal(BR) content ranges from 7.1 to 8.3 wt.% (Table 1). In each lithology, the observed FeOTotal(BR) variations probably reflect slight variations in primary modes. Indeed, it has been proposed that FeOTotal(BR) contents vary little during serpentinization or dehydration processes (Garrido et al. 2005; Godard et al. 2008; Andreani et al. 2013; Marchesi et al. 2013; Debret et al. 2014b). We can thus assume that BR iron content remains roughly constant during antigorite breakdown.
The Fe3+/FeTotal(BR) ratio decreases passing from Atg-serpentinite and transitional lithologies to granofels and spinifex-like Chl-harzburgites (Fig. 4a). Atg-serpentinites and transitional lithologies Fe3+/FeTotal(BR) ratios range from 0.53 to 0.57. These values are lower than the values reported for fully serpentinized peridotites from oceanic settings (~0.7, Andreani et al. 2013) but are similar to values reported for Atg-serpentinites from alpine ophiolites (Fig. 4a). Chl-harzburgites display a substantially lower Fe3+/FeTotal(BR) ratio, ranging from 0.34 to 0.41.
BR measurements of a Fe3+/FeTotal(BR) and b magnetite modal amount in the studied samples suite compared to highly serpentinized abyssal peridotites (serpentinization degree >80 %; Oufi et al. 2002; Andreani et al. 2013) and alpine serpentinites (Debret et al. 2014b). Fe3+/FeTotal(BR) in Cerro del Almirez samples are from Padron-Navarta et al. (2011)
Petrography
Padrón-Navarta et al. (2011) provided a detailed description of the textural variations in Cerro del Almirez serpentinites and metaperidotites. Here, we summarize the main features with emphasis on the samples selected for this study.
Atg-serpentinites are foliated and mostly composed of oriented aggregates of highly ordered antigorite crystals of about 20-µm-wide (Padrón-Navarta et al. 2008, 2011; Atg1; Fig. 5a) olivine porphyroblasts and minor clinopyroxene. Magnetite is the most abundant opaque constituent of Atg-serpentinites. It crystallizes as elongated grains of about 50 µm long. The magnetite modal amount of Atg-serpentinites ranges from 2.8 to 4.3 wt% (Table 1; Fig. 4b). Locally, oxide aggregates of about 300 µm are zoned. They are composed of chromite surrounded by a magnetite corona of about 50–100 µm. Rare accessory phases (Ti-clinohumite, chlorite, tremolite, ilmenite, and hemo-ilmenite) have been observed in the studied samples.
a Atg-serpentinites showing fine-grained elongated Atg1. b Transitional lithology showing Atg2 flakes. The Atg2 could present subehedral plate of chlorite in the center. c Chl-harzburgite with granofels texture showing a Ti-clinohumite porphyroblast surrounded by a thin corona of ilmenite/magnetite and olivine. The dehydrated paragenesis (orthopyroxene, olivine, and Ti-clinohumite) is crossed by late chrysotile veins. d Ilmenite aggregate of a Chl-harzburgite with granofels texture showing Fe-rich exsolutions. e Characteristic Raman spectra of Fe-rich intergrowths corresponding to hematite. f Spinifex-like Chl-harzburgite composed of secondary brown olivine, orthopyroxene, and chlorite aggregates. Chl-harzburgite assemblages are crossed by late chrysotile veins. g Magnetite, hematite, and ilmenite aggregates in a spinifex-like Chl-harzburgite
The transitional lithologies are composed of antigorite, chlorite, magnetite, secondary olivine, +/− secondary orthopyroxene, and talc. Relative to Atg-serpentinite, these rocks are characterized by a modal increase in chlorite, olivine, and orthopyroxene and a decrease in antigorite and magnetite modal amounts, the latter now ranging from 1.6 to 2.2 wt% (Table 1; Fig. 4b). The transitional lithologies are characterized by the crystallization of a new antigorite generation (Atg2) in equilibrium with secondary olivine and chlorite (Padrón-Navarta et al. 2011, 2013; Fig. 5b). Atg2 corresponds to interpenetrating blades of 50–500 µm wide that can contain subhedral plates of chlorite (Fig. 5b). Orthopyroxene is poorly preserved in thin section; it is commonly replaced by retrograde talc (Padrón-Navarta et al. 2011). Magnetite is the most abundant opaque mineral of transitional lithologies. It crystallizes as interstitial grains of 20–50 µm. The occurrence of ilmenite is also noted.
Chl-harzburgites mainly consist of chlorite, coarse-grained olivine, prismatic orthopyroxene, rare Ti-clinohumite, and late chrysotile veins (Fig. 5c). These minerals are associated with a wide range of oxides (magnetite, ilmenite, and hematite). Relative to the transitional lithologies, Chl-harzburgites are characterized by a modal increase in olivine and orthopyroxene, the complete lack of antigorite, and by a decrease in magnetite modal amount ranging from 0.6 to 1.4 wt% (Table 1; Fig. 4b).
In Chl-harzburgites with granofels texture, orthopyroxenes form elongate crystals of 500 µm long, locally replaced by talc (Padrón-Navarta et al. 2011). Olivines form porphyroblasts of 100 µm up to several millimeters in width (Fig. 5c). Orthopyroxene and olivine contain small ilmenite and magnetite inclusions of about 1–10 µm. Rare Ti-clinohumite porphyroblasts of 100 µm up to 1 mm are surrounded by secondary olivine coronas associated with ilmenite grains of 5–20 µm formed by Ti-clinohumite breakdown (Fig. 5c; López Sánchez-Vizcaíno et al. 2005). Orthopyroxenes, olivines, and Ti-clinohumites porphyroblasts are crossed by ~20-µm-wide green or orange chrysotile veins (Fig. 5c). Few oxides are associated with these veins. Chlorite occurs as coarse flakes of about 200 µm grouped into aggregates and associated with interstitial magnetites of variable width (10–100 µm; Fig. 5c). Ilmenite crystallizes as irregular interstitial grains between olivine, orthopyroxene, or chlorite crystals (Fig. 5d). The maximum measured dimension of ilmenite grains is about 200 µm. They contain fine exsolution lamellae of Fe-rich oxide which lay on up to 20 µm in one direction (Fig. 5d). Raman spectra of such Fe-rich oxides are characterized by four peaks at 224, 295, 411, and 1320 cm−1 corresponding to hematite and a weak band ranging from 620 to 660 cm−1 corresponding to a mix between hematite and ilmenite (Fig. 5e; De Faria et al. 1997). In these aggregates, no pure end-members of ilmenite or hematite have been found by in situ analyses (e.g., Raman spot size of ~1 µm).
Spinifex-like olivine and orthopyroxene in Chl-harzburgites range from 1 mm to several centimeters. Commonly, olivine cores are clear and contain small inclusions of magnetite and ilmenite (Fig. 5f). Rims are composed of brown-colored olivine with abundant inclusions of magnetite, ilmenite, chromite, orthopyroxene, fluid, and rare Ti-clinohumite microscopic lamellae intergrowth (Fig. 5f; Trommsdorff et al. 1998; Ruiz Cruz et al. 1999; Scambelluri et al. 2001; Garrido et al. 2005; López Sánchez-Vizcaíno et al. 2005). Orthopyroxene occurs as coarse-grained aggregates (up to 8 cm in length) of radial acicular crystals of 50–100 µm wide and are associated with fine-grained chlorite of 100–300 µm (Fig. 5f). These assemblages are associated with hematite and magnetite aggregates of 20–500 µm (Fig. 5g). Hematite grains display fine exsolutions of ilmenite and are surrounded by an ilmenite corona. The contact between magnetite and ilmenite or hematite boundaries is sharp (Fig. 5g).
Iron content and redox state in serpentine and chlorite
In situ analyses have been performed on four of the studied samples (Tables 2, 3). Electron microprobe has been performed on serpentine, chlorite, olivine, orthopyroxene, and Ti-clinohumite. µ-XANES was performed on serpentine minerals (antigorite and late chrysotile) and chlorites since these phases are the main carriers of Fe3+ among silicates. In addition, we have also analyzed Ti-clinohumite to better constrain its Fe3+ content.
Antigorite occurs in Atg-serpentinites (Atg1) and in the transitional lithologies (Atg2). Some changes in major element contents occurred during the transformation of Atg1 to Atg2 (Table 2; Fig. 6a; Padrón-Navarta et al. 2008, 2011, 2013). The Al2O3 and total FeO contents of Atg1 range from 2.4 to 2.9 wt% and from 3.5 to 3.7 wt%, respectively. Atg2 has more heterogeneous Al2O3 contents and higher FeO than Atg1 (Fig. 6a). The Al2O3 and FeO contents of Atg2 range from 2.3 to 3.7 wt% and from 3.9 to 4.5 wt%, respectively. Chlorite displays similar FeO contents relative to antigorite. In the transitional lithologies, the Al2O3 and FeO contents in chlorite vary from 12.2 to 13.2 wt% and from 4 to 4.4 wt%, respectively. Talc displays low FeO contents, ranging from 1.1 to 1.4 wt%, relative to antigorite or chlorite (Table 2). In granofels and spinifex-like Chl-harzburgites, chlorite displays higher Al2O3 (=12.6–14.1 wt%) and lower FeO (=2.7–3.7 wt%) contents than chlorite from transitional lithologies (Fig. 6b; Table 2). In these rocks, the Cr2O3 content of chlorite increases from core to the rim, while Al2O3 and FeO contents do not vary. Olivine and orthopyroxene show an increase in X Mg [= Mg/(Fe + Mg)] passing from Atg-serpentinites to Chl-harzburgites (Trommsdorff et al. 1998; Padrón-Navarta et al. 2011). FeO contents of Ti-clinohumite are similar to those of olivine and range from 9.3 to 9.6 wt%. The Al2O3 content in orthopyroxene increases passing from transitional lithologies to Chl-harzburgites (Padrón-Navarta et al. 2011). Late chrysotile veins display lower Al2O3 contents (<0.1 wt%) and higher FeO contents (1.5–8.8 wt%) than antigorite in Atg-serpentinite and in the transitional lithologies.
Plot of FeO versus Al2O3 of a Atg1 and Atg2 forming Atg-serpentinites and transitional lithologies, respectively; b chlorite forming transitional lithologies and Chl-harzburgites. Small symbols are values from Padrón-Navarta et al. (2011)
XANES pre-edge features (centroid energy and integrated area) are displayed in Fig. 7 and reported in Table 3. The quantification of Fe3+/FeTotal ratio was obtained by using the calibration variogram of Wilke et al. (2001). This variogram has been reproduced by measuring three powdered standard compounds, namely siderite (VIFe2+), andradite (VIFe3+), and sanidine (IVFe3+), which were already characterized in previous studies (Marcaillou et al. 2011; Muñoz et al. 2013; Andreani et al. 2013; Debret et al. 2014b). The entire dataset plots in the VIFe2+–VIFe3+–IVFe3+ region (Fig. 7a). This suggests the absence of tetrahedral Fe2+ in serpentine and chlorite. The occurrence of mixed tetrahedral and octahedral Fe3+ in serpentine and chlorite is expected as proposed in previous crystallographic and chemical studies (O’Hanley and Dyar 1993; Fuchs et al. 1998; Marcaillou et al. 2011; Evans et al. 2012; Muñoz et al. 2013; Andreani et al. 2013; Debret et al. 2014b).
a Calibration grid modified after Wilke et al. (2001) and b quantification of Fe3+/FeTotal ratio of antigorite and chlorite composing the different lithologies of Cerro del Almirez massif. * Serpentine from Mid-Atlantic ridge (Andreani et al. 2013) and from the Chenaillet (Debret et al. 2014b). ** Mixed (antigorite, lizardite, chrysotile assemblages) and antigorite from the Chenaillet, Monviso, and Lanzo massif (Debret et al. 2014b)
A distinct modification of antigorite and chlorite pre-edge XANES spectra is observed with increasing grade. Antigorite pre-edge peak centroid energy is shifted to higher values, while its integrated area remains unchanged (Fig. 7a; Table 3). This observation corresponds to an increase in Fe3+/FeTotal ratio from 0.48 (+/− 0.05) in Atg1 to 0.60 (+/− 0.05)–0.75 (+/− 0.05) in Atg2 (Fig. 7b). The Fe3+/FeTotal ratio in Atg1 is typical of antigorite in alpine serpentinites. Passing from the transitional lithologies to Chl-harzburgites, chlorite pre-edge peak centroid energy is progressively shifted to lower values, while its integrated area is unchanged (Fig. 7a; Table 3). This corresponds to a decrease in Fe3+/FeTotal ratio from 0.69 (+/− 0.05) to 0.47–0.55 (+/− 0.06). Chlorite Fe3+/FeTotal ratio from the transitional lithologies is heterogeneous, with Fe3+/FeTotal ratio decreasing from 0.69 (+/− 0.05) at grain rims to 0.50 (+/− 0.06) at grain cores (Fig. 7b). In contrast, chlorite Fe3+/FeTotal ratio from Chl-harzburgites is constant and ranges from 0.47 to 0.55 (+/− 0.06). Pre-edge peaks of Ti-clinohumite XANES spectra are characterized by a low energy and area relative to the ones of antigorite or chlorite (Fig. 3; Table 3) and are close to the one reported for siderite (Table 3). This shows that Ti-clinohumite mostly incorporates Fe2+ in its structure and exclusively in octahedral site. Pre-edge peaks of late chrysotile XANES spectra are characterized by a high energy relative to antigorite or chlorite pre-edge peaks. These observations indicate that chrysotile displays a high Fe3+/FeTotal ratio of 0.8 (Fig. 7a; Table 3).
Discussion
There are no obvious changes in Fe3+/FeTotal(BR) ratio in ultramafic rocks passing from Atg-serpentinites to the transitional lithologies, while this ratio sharply decreases in Chl-harzburgites (Fig. 4a). This suggests a two-stage evolution of iron redox during Atg-breakdown. (1) In the transitional lithologies, the initiation of antigorite breakdown is marked by the crystallization of newly formed olivine, chlorite, and Atg2 at the expense of Atg1 (Padrón-Navarta et al. 2011). During this stage, the Fe3+/FeTotal(BR) ratio remains unchanged, while the magnetite amount decreases (Fig. 4) and the Fe3+/FeTotal ratio of antigorite increases (Fig. 7b). This suggests a new partitioning of Fe3+ within the new assemblage (Debret et al. 2014b). (2) Chl-harzburgites result from the complete antigorite dehydration into chlorite, olivine, and orthopyroxene (Trommsdorff et al. 1998). These rocks display a lower Fe3+/FeTotal(BR) relative to Atg-serpentinites or to the transitional lithologies, suggesting a reduction of iron.
Among the different phases involved during antigorite breakdown, olivine is considered to contain negligible amounts of Fe3+, while orthopyroxene can only incorporate a low amount of Fe3+ (a maximum Fe3+/FeTotal of about 0.1, e.g., Malaspina and Tumiati 2012); therefore, these phases do not contribute significantly to the Fe3+ budget of ultramafic rocks during subduction (e.g., Canil and O’Neill 1996). Thus, it is the distribution of Fe3+ between antigorite, chlorite, and oxides that is of interest for the Fe3+/FeTotal(BR) evolution in hydrated ultramafic rocks during subduction. Moreover, this distribution will have an influence on the speciation of C–S–O–H-bearing fluids released during Atg-breakdown. A combination of the measured FeO and Fe3+/FeTotal ratio of the different assemblages from Atg-serpentinite, transitional lithologies, and Chl-harzburgites with their respective phase proportions allows us to determine the Fe3+/FeTotal(BR) by mass balance calculation (Fe3+/Fe CTotal(BR) ). The details and results of this calculation are displayed in Appendix B and in Fig. 8. The modal amount of antigorite, chlorite, olivine, and orthopyroxene is taken from Padrón-Navarta et al. (2011). Magnetite modal amount was retrieved from hysteresis measurements, whereas magnetite and hematite Fe3+/FeTotal ratios are assumed constant and equal to their stoichiometric values of 0.7 and 1, respectively. This calculation allows assessing the relative contribution of each mineral to the bulk Fe3+ budget. In addition, we compare the FeO CTotal(BR) and Fe3+/Fe CTotal(BR) with the FeOTotal(BR) and Fe3+/FeTotal(BR) obtained by Padrón-Navarta et al. (2011) in order to assess the validity of the estimations (Fig. 8). Phase relations are reported as chemographies in Fig. 9 for the simplified system FeO–(MgO)–Fe2O3–SiO2–H2O. These projections are useful to depict changes in the partitioning of Fe3+ between silicates and oxides in addition to net changes in the oxygen budget (i.e., resulting in bulk rock changes along the vector pointing to the oxygen axis, see Fig. 9a, e.g., Tumiati et al. 2015).
FeO CTotal(BR) and Fe3+/Fe CTotal(BR) and relative mineral contribution of the different lithologies composing Cerro del Almirez massif. Two different modal abundances of Chl-harzburgites are displayed: the first one is based on Padrón-Navarta et al. (2011) and the second one takes into account late chrysotile alteration and hematite crystallization. Chrysotile modal abundance is estimated using the LOI, while hematite crystallization has been fixed (1 %) in order to match Fe3+/Fe CTotal(BR) and Fe3+/FeTotal(BR)
a Fe–Si–O plane projected from Fe–Mg-1 exchange and H2O (modified from Thompson 1982). The shaded region corresponds to the physically accessible part of the Fe–Si–O triangle where the subsystem FeO–SiO2–Fe2O3 exists (see Thompson 1982 for details). The triangle resulting from the assemblage ol-en-mgt is depicted and enlarged in b, c, and d after coordinate mapping (computed with CSpace, Torres-Roldán et al. 2000) for a representative sample of Atg-serpentinite, transitional lithology, and Chl-harzburgite, respectively. BR: bulk rock composition BR*: bulk rock corrected for late talc (Padrón-Navarta et al. 2011). Antigorite composition is based on XANES analyses (Tables 2, 3)
Lizardite-to-antigorite phase transition in Atg-serpentinites
In the Cerro del Almirez massif, Atg-serpentinites result from oceanic lizardite-/chrysotile-bearing serpentinites recrystallization into antigorite during alpine subduction (Fig. 2; Alt et al. 2012; Marchesi et al. 2013). The Fe3+/FeTotal(BR) ratio of Atg-serpentinites is lower than that of oceanic serpentinites and similar to that of other alpine Atg-serpentinites (Fig. 4a) supporting the notion that a reduction of iron during the transition lizardite to antigorite. Previous studies have shown that this reduction is concomitant with a decrease in magnetite modal amount and a decrease in Fe3+/FeTotal ratio in serpentine (Evans et al. 2012; Debret et al. 2014b).
At the oceanic stage, in fully serpentinized peridotites, Fe3+ is mostly carried by magnetite, where modal amount can reach up to 10 % (corresponding to ~80 % of the Fe3+ budget in BR); the serpentine Fe3+/FeTotal ratio is close to 0.8–1, and its contribution to the Fe3+ budget in BR is about 20 % (Andreani et al. 2013). In Atg-serpentinites, Fe3+ is equally partitioned into magnetite (~60 % of Fe3+ budget in BR) and antigorite (~40 % of Fe3+ budget in BR, Fig. 8). Although serpentine Fe3+/FeTotal ratio decreases during the transition lizardite to antigorite (Fig. 7b), antigorite is still a significant carrier of Fe3+ in these rocks because its FeO content increases during subduction due to consumption of magnetite (Fig. 9b, Debret et al. 2014b).
Iron redistribution in the transitional lithologies
The transitional lithologies display a Fe3+/FeTotal(BR) ratio close to that of Atg-serpentinites (Figs. 4a, 9c). Nevertheless, in these rocks, Fe3+ is mostly hosted by antigorite (~50 % of Fe3+ budget in BR), and the magnetite contribution to the bulk Fe3+ budget decreases (~40 % of Fe3+ budget in BR). Chlorite carries little Fe3+ (~10 % of Fe3+ budget in BR; Fig. 8). Olivine and orthopyroxene contain negligible ferric iron.
The magnetite modal amount in the transitional lithologies is lower than the one in Atg-serpentinites, suggesting that magnetite is consumed during the onset of Atg-serpentinites dehydration. Indeed, in these rocks, secondary olivine displays a lower X Mg relative to Atg1 (Table 2), suggesting the involvement of Fe-oxide (e.g., magnetite) in the antigorite breakdown reaction (see also Debret et al. 2013b). Moreover, Atg2 displays a higher FeO content and Fe3+/FeTotal ratio relative to Atg1. This suggests that magnetite dissolution is accompanied with a redistribution of Fe3+ and Fe2+ between the newly formed antigorite, olivine, and chlorite. The Fe2+ is mainly integrated into secondary olivine, while Fe3+ is incorporated into the new generation of antigorite (Atg2). The incorporation of Fe3+ into the antigorite structure via Tschermak substitutions (Evans et al. 2012; Padrón-Navarta et al. 2013) could stabilize it to higher temperature and pressure (Bromiley and Pawley 2003; Padrón-Navarta et al. 2008, 2013; Debret et al. 2014b), allowing its crystallization during secondary olivine growth. Chlorite displays a heterogeneous Fe3+/FeTotal ratio: the Fe in the center of the mineral is reduced relative to iron at rims in contact with Atg2 (Fig. 7b). This observation is in agreement with a partitioning of iron and aluminum between chlorite and antigorite. Fe3+ is preferentially incorporated into antigorite, while Al is incorporated into chlorite during the first stages of antigorite breakdown. FeOTotal(BR), Al2O3Total(BR), and MgOTotal(BR) contents vary little passing from Atg-serpentinites to transitional lithologies (Fig. 9b, Padrón-Navarta et al. 2011), and the Fe redistribution between antigorite, chlorite, and olivine can be expressed as:
Atg1, Atg2, and Chl structural formulae are based on Padrón-Navarta et al. (2008, 2011). This mass balance reaction is non-unique and depends on the precise stoichiometry of the considered phases. It is important to note however that the reaction does not involve a net change in the bulk oxygen budget, but rather a distribution of ferric iron between antigorite and magnetite that results in a change in their modal proportions (Fig. 9b, c).
Complete Atg-breakdown and Chl-harzburgites
Chl-harzburgites result from the high-pressure dehydration of Atg-serpentinites described by the following general reaction (Trommsdorff et al. 1998, Fig. 9d):
Granofels and spinifex-like Chl-harzburgites display similar Fe3+/FeTotal(BR) (Table 1). Their Fe3+/FeTotal(BR) is lower than the one reported for Atg-serpentinites and the transitional lithologies (Figs. 4a, 9b–d). Assuming that FeO(BR) is roughly constant passing from Atg-serpentinites to Chl-harzburgites (Garrido et al. 2005; Marchesi et al. 2013), this shows that equation [2] is accompanied by a reduction of iron. This reduction is due to the disappearance of Fe3+-rich phases, mostly magnetite (Fig. 4b) and antigorite, and is associated with an increase in XMg in Fe2+-rich phases (Trommsdorff et al. 1998; Padrón-Navarta et al. 2011) and of Al2O3 content in chlorites (Fig. 6b). This reaction results in a net decrease in the oxygen budget as it emerges from inspection of Fig. 9c. Bulk rock composition is displaced along the O−1 vector passing from Atg-serpentinite and transitional lithologies to Chl-harzburgite with a possible contribution of the Si−1Fe vector (Fig. 9d). The Si-1Fe contribution might be explained by a different solubility of ferric complexes relative to aqueous silica in the fluid.
In detail, the Fe3+/Fe CTotal(BR) in Chl-harzburgite considering exclusively chlorite, olivine, orthopyroxene, and magnetite does not match the measured Fe3+/FeTotal(BR) (Fig. 8). Careful petrographic inspection reveals that late Fe-rich chrysotile veins (Fig. 5f) and hematite exsolutions are common in Chl-harzburgites (Fig. 5d, g). If these phases are incorporated in the calculations, the agreement between calculated and measured Fe3+/FeTotal(BR) is improved significantly (Fig. 8). The amount of chrysotile and hematite in the Chl-harzburgite cannot be easily deduced from bulk rock measurements. Chrysotile crystallizes in veins crossing high-pressure minerals (Fig. 5f) showing that its crystallization corresponds to a retrograde event during exhumation. Its crystallization results in the addition of water to the system that affects the loss on ignition (LOI) bulk rock measurements. In the mass balance calculation, we considered the LOI as being proportional to the modal amount of chlorite and chrysotile in the rock, since these phases are the potential main carriers of volatile in Chl-harzburgite. The chlorite modal amount is from Padrón-Navarta et al. (2011), while chrysotile is assumed to crystallize at the expense of olivine and orthopyroxene. The estimated chrysotile modal amount varies from 5 and 20 % in Chl-harzburgites. Chrysotile could thus contribute to 20 and up to 45 % of the Fe3+/Fe CTotal(BR) (Fig. 8). Such estimates should be considered as maximal since the LOI could be influenced by other phases (e.g., S- or C-bearing phases, tremolite or talc) and iron oxidation effects due to heating during sample preparation. In Chl-harzburgite, hematite with fine ilmenite exsolutions is associated with magnetite and shows a relatively thick ilmenite exsolution delineating an originally sharp magnetite–hematite boundary (Fig. 5g), suggesting that hematite is not a late replacement product of magnetite during exhumation. The crystallization of hematite and ilmenite exsolutions indicates that both phases existed in a solid solution as ferrianilmenite or titanhematite at high temperature (Burton and Davidson 1988) in equilibrium with the Chl–Opx–Ol–Mgt assemblages. Hematite crystallization during antigorite breakdown has also been observed experimentally (Maurice and Bolfan-Casanova 2014). The amount of hematite has been fixed to 1 % in the calculations in order to match calculated and measured Fe3+/FeTotal(BR). Its contribution, based on mass balance calculations, reaches 30–35 % Fe3+/Fe CTotal(BR) (Fig. 8). Considering the Chl–Opx–Ol–Mgt–Hem assemblage and the modal amount of each phase, the Fe3+/Fe CTotal(BR) decreases from 0.5 in the transitional lithologies to ~0.2 in Chl-harzburgites (Fig. 8). This iron reduction can be expressed as:
The crystallization of hematite–ilmenite solid solution could provide constrains on the oxygen fugacity (fo2) of the subducting de-serpentinized mantle. The composition of hematite–ilmenite solid solution is a function of both fo2 and T (Spencer and Lindsley 1981). At one specific temperature, as fo2 increases, the ilmenite content of the solid solution decreases. In the Cerro del Almirez massif, granofels and spinifex-like Chl-harzburgites are formed at similar P–T conditions (Padrón-Navarta et al. 2011). In granofels Chl-harzburgites, hematite occurs as exsolutions in ilmenite grains (Fig. 5d) in which hematite component represents about 10 % of the grain (estimated by image analyses based on optical microphotographs). At 680–710 °C, hematite–ilmenite solid solution could have been equilibrated at a log (fo2) ranging from −16 to −14, equivalent to FMQ ranging from +1 to +3 (Spencer and Lindsley 1981). In spinifex-like Chl-harzburgites, the hematite component is predominant, while ilmenite represents only a minor fraction of the assemblage, forming exsolutions and coronas surrounding hematite grains (Fig. 5g). These assemblages could be equilibrated at relatively higher fo2 ranging from −15 to −13, equivalent to FMQ +3 or +4 (Spencer and Lindsley 1981).
Such high fo2 could play a major role in element mobility in the released fluid during Atg-breakdown. For example, arc magmas commonly display high U/Th ratios relative to MORB, suggesting that the fluids metasomatizing the mantle wedge peridotites are preferentially enriched in U than in Th (e.g., Stolper and Newman 1994). U mobility in aqueous fluids strongly depends on fo2 and fluid salinity, whereas Th is poorly affected by these variables (Bali et al. 2011). The Cerro del Almirez massif spinifex-like olivine and orthopyroxene contain disseminated fluid inclusions which are remnants of the fluid released at these P–T- fo2 conditions (Scambelluri et al. 2001, 2004). These fluid inclusions display a low-salinity range and high U/Th ratio, which could be compatible with high fo2 conditions (Bali et al. 2011).
Change in fo2 in serpentinites during subduction
In oceanic settings, serpentine crystallization occurs within the magnetite stability field; thus, fo2 of the subducted serpentinites is below the hematite–magnetite buffer. Also, mantle peridotites are transformed into serpentinites as the result of seawater circulation through the mantle at low temperature (T < 400 °C). The oceanic serpentinization is an oxidizing reaction. During fluid/rock interaction, Fe2+ contained in mantle olivine and orthopyroxene is transformed into Fe3+-rich phases, mostly magnetite and serpentine (Fig. 10). The onset of the oxidizing reaction (T = 400–200 °C) is mostly controlled by magnetite crystallization, which is the consequence of the Mg-rich nature of serpentine and the sluggish Mg–Fe diffusion in olivine (Evans 2010; Marcaillou et al. 2011). The last stages of serpentinization (<200 °C) are associated with the crystallization of Fe- and Fe3+-rich serpentine with very little magnetite associated (Andreani et al. 2013). During the whole iron oxidation process, fluids discharged from serpentinites can be H2 and CH4 rich (Charlou et al. 2002) and produce extremely reducing conditions, below the FMQ buffer (Frost 1985; Klein and Bach 2009), allowing the precipitation of sulfides and organic carbon (Alt and Shanks 2003; Delacour et al. 2008a, b).
Schematic diagram illustrating Fe redox behavior in serpentinites during subduction (modified after Alt et al. 2012). Boxes show the Fe redox states of serpentinites during subduction and the redox and the amount of Fe3+-bearing minerals (values from Andreani et al. 2013; Debret et al. 2013a, b and this study). Fe3+/FeTotal ratios of arc magmas and MORB are from Kelley and Cottrell (2009). Blue dashed line indicates instability limit of lizardite (after Schwartz et al. 2013) and the red dashed line the one of antigorite (after Ulmer and Trommsdorff 1995). Fluids derived from serpentinites during prograde metamorphism move upward (arrows) and might produce metasomatism of the mantle wedge
During subduction, prograde metamorphism in the slab induces two major mineral reactions within serpentinites: firstly, from greenschist-to-blueschist facies, the transformation of lizardite into antigorite, and then, at eclogite facies, the breakdown of antigorite into olivine ± orthopyroxene ± chlorite (Fig. 10; Trommsdorff et al. 1998; Schwartz et al. 2013). These phase transitions are accompanied by a decoupled evolution between Fe3+/FeTotal(BR) ratio, which decrease during prograde metamorphism, and fo2, which increase up to magnetite–hematite buffer in serpentinites (Fig. 10). Fe3+/FeTotal(BR) evolution in serpentinites is controlled by magnetite modal amount and Fe3+/FeTotal ratio in serpentine minerals, which both decrease during prograde metamorphism (Fig. 10). This result suggests a reduction of iron in serpentinites and the release of an oxygen-rich fluid phase (e.g., CO2, SOX…). Indeed, the different phase transitions of serpentine liberate O2 (Eqs. [1], [3]) that can potentially consume residual reduced species and mobilize oxidized C, H, or S into the aqueous fluid (Debret et al. 2014b). The quantity of oxygen released in the fluid phase is governed by the mobility of redox-sensitive elements in fluids; the abundance of volatile elements such as C, H, and S; and the evolution of the Fe3+/FeTotal(BR) ratio. The sole study of Fe redox state in serpentinites cannot explain the full redox equilibrium between serpentinites and the fluids during subduction, and the fate of other volatile elements should also be considered. The evolution of fo2 in the serpentinites during subduction is monitored by mineral equilibrium rather than by the simple oxidation state of Fe (Frost 1991; Tumiati et al. 2015). Prograde metamorphism in serpentinites ultimately results in H2O release and growth of Fe2+-bearing silicates. The crystallization of hematite in Chl-harzburgites suggests that the fo2 increases in the slab during prograde metamorphism until reaching FMQ + 1 to FMQ + 4. This process is accompanied by an increase in XMg in Fe2+-bearing silicates (Trommsdorff et al. 1998; Padrón-Navarta et al. 2011), which stabilizes them to higher fo2 in the presence of hematite (Frost 1991).
Conclusions
In the Cerro del Almirez, antigorite breakdown occurs through a series of continuous reactions forming chlorite, olivine, and orthopyroxene that progressively consume magnetite and antigorite. The study of Fe redox state indicates a two-stage process. The beginning of antigorite dehydration leads to a partitioning of Fe between olivine that mostly incorporates Fe2+ and antigorite/chlorite that display higher Fe3+/FeTotal ratio. Although the Fe3+/Fetotal(BR) remains constant during this stage, the redistribution of Fe3+ liberates oxygen that can potentially mobilize C and S into aqueous fluids (e.g., CO2 or SO4). At higher temperatures, antigorite breakdown is accompanied by a Fe3+/FeTotal(BR) decrease of 60 % of the initial budget, XMg increases in silicate minerals, and the crystallization of hemo-ilmenite. These observations are consistent with an increase in fo2 in subducted ultramafic rocks during prograde metamorphism. The final assemblage could be equilibrated between FMQ + 1 and FMQ + 4, suggesting that the released fluids during antigorite breakdown have an important oxidizing potential.
Dehydrating subducted serpentinites are considered to be a major source of fluid for arc magmas (Ulmer and Trommsdorff 1995), and trace element or isotope studies highlight a link between the composition of the released fluid during Atg-breakdown and the one of arc magmas (Scambelluri et al. 2004; Savov et al. 2007; Vils et al. 2011; Scambelluri and Tonarini 2012; Debret et al. 2013a, 2014a). This suggests that serpentinite dehydration could significantly contribute to the modification of the composition and properties of the overlying mantle wedge. Our results show that prograde metamorphism is accompanied by a reduction of iron in serpentinites and a subsequent increase in fo2 in serpentinites. Serpentinite dehydration could thus produce highly oxidized fluids that can contribute to oxidized sub-arc mantle as suggested by the study of arc magmas melt inclusions (e.g., Parkinson and Arculus 1999; Kelley and Cottrell 2009).
References
Alt JC, Shanks WC III (2003) Serpentinization of abyssal peridotites from the MARK area, Mid-Atlantic Ridge: sulfur geochemistry and reaction modeling. Geochim Cosmochim Acta 67:641–653
Alt JC, Garrido CJ, Shanks WC III, Turchyn A, Padrón-Navarta JA, López-Sánchez-Vizcaíno V, Gómez Pugnaire MT, Marchesi C (2012) Recycling of water, carbon, and sulfur during subduction of serpentinites: a stable isotope study of Cerro del Almirez, Spain. Earth Planet Sci Lett 327–328:50–60
Andersen T, Neumann ER (2001) Fluid inclusions in mantle xenoliths. Lithos 55:301–320
Andreani M, Muñoz M, Marcaillou C, Delacour A (2013) μXANES study of iron redox state in serpentine during oceanic serpentinization. Lithos 178:70–83
Arculus RJ (1994) Aspects of magma genesis in arcs. Lithos 33:189–208
Bach W, Paulick H, Garrido CJ, Ildefonse B, Meurer WP, Humphris S (2006) Unravelling the sequence of serpentinization reactions: petrography, mineral chemistry, and petrophysics of serpentinites from MAR 15°N (ODP leg 209, site 1274). Geophys Res Lett 33:L13306
Bali E, Audetat A, Keppler H (2011) The mobility of U and Th in subduction zone fluids: an indicator of oxygen fugacity and fluid salinity. Contrib Mineral Petrol 161:597–613
Berndt ME, Allen DE, Seyfried WE (1996) Reduction of CO2 during serpentinization of olivine at 300 °C and 500 bar. Geology 24:351–354
Bouilhol P, Burg JP, Bodinier JL, Schmidt MW, Bernasconi S, Dawood D (2012) Gem olivine and calcite mineralization precipitated from subduction-derived fluids in the Kohistan arc-mantle (Pakistan). Can Mineral 50:1291–1304
Bromiley GD, Pawley AR (2003) The stability of antigorite in the systems MgO–SiO2–H2O (MSH) and MgO–Al2O3–SiO2–H2O (MASH): the effects of Al3+ substitution on high-pressure stability. Am Mineral 88:99–108
Burton BP, Davidson PM (1988) Multicritical phase relations in minerals. In: ESS Ghose, JMD Coey (eds), Advances in physical geochemistry, volume 7, 60. Springer, New York
Canil D, O’Neill HStC (1996) Distribution of ferric iron in some upper-mantle assemblages. J Petrol 37:609–635
Charlou J, Donval JP, Fouquet Y, Jean Baptiste P, Holm N (2002) Geochemistry of high H2 and CH4 vent fluids issuing from ultramafic rocks at the Rainbow hydrothermal field (36°14′N, MAR). Chem Geol 191:345–359
De Faria DLA, Venancio Silva S, de Oliveira MT (1997) Raman microspectroscopy of some iron oxides and oxyhydroxides. J Raman Spectrosc 28:873–878
Debret B, Andreani M, Godard M, Nicollet C, Schwartz S, Lafay R (2013a) Trace element behaviour during serpentinization/deserpentinization of an eclogitized oceanic lithosphere: a LA-ICPMS study of the Lanzo ultramafic massif (Western Alps). Chem Geol 357:117–133
Debret B, Nicollet C, Andreani M, Schwartz S, Godard M (2013b) Three steps of serpentinization in an eclogitized oceanic serpentinization front (Lanzo Massif—Western Alps). J Metamorph Geol 31:165–186
Debret B, Koga K, Nicollet C, Andreani M, Schwartz S (2014a) F, Cl and S input via serpentinite in subduction zones: implications on the nature of the fluid released at depth. Terra Nova 26:96–101
Debret B, Andreani M, Munoz M, Bolfan-Casanova N, Carlut J, Nicollet C, Schwartz S, Trcera N (2014b) Evolution of Fe redox state in serpentine during subduction. Earth Planet Sci Lett 400:206–218
Delacour A, Früh-Green GL, Bernasconi SM (2008a) Sulfur mineralogy and geochemistry of serpentinites and gabbros of the Atlantis Massif (IODP Site U1309). Geochim Cosmochim Acta 72:5111–5127
Delacour A, Früh-Green GL, Bernasconi SM, Schaeffer P, Kelley DS (2008b) Carbon geochemistry of serpentinites in the Lost City hydrothermal system. Geochim Cosmochim Acta 72:3681–3702
Dunlop DJ, Özdemir Ö (1997) Rock magnetism. Cambridge University Press, Cambridge, p 573
Evans BW (2004) The serpentinite multisystem revisited: chrysotile is metastable. Int Geol Rev 46:479–506
Evans BW (2010) Lizardite versus antigorite serpentinite: magnetite, hydrogen, and life(?). Geology 38:879–882
Evans KA (2012) The redox budget of subduction zones. Earth Sci Rev 113:11–32
Evans KA, Tomkins A (2011) The relationship between subduction zone redox budget and arc magma fertility. Earth Planet Sci Lett 308:401–409
Evans BW, Trommsdorff V (1978) Petrogenesis of garnet lherzolite, Cima di Gagnone, Lepontine Alps. Earth Planet Sci Lett 40:333–348
Evans BW, Dyar MD, Kuehner SM (2012) Implications of ferrous and ferric iron in antigorite. Am Mineral 97:184–196
Frost BR (1985) On the stability of sulfides, oxides, and native metals in serpentinite. J Petrol 26:31–63
Frost BR (1991) Introduction to oxygen fugacity and its petrologic importance. In: DH Lindsley (ed), Oxide minerals: petrologic and magnetic significance. Rev mineral 25: 1–8
Frost BR, Evans KA, Swapp SM, Beard JS, Mothersole FE (2013) The process of serpentinization in dunite from New Caledonia. Lithos 178:24–39
Fuchs Y, Linares J, Mellini M (1998) Mössbauer and infrared spectrometry of lizardite-1T from Monte Fico, Elba. Phys Chem Mineral 26:111–115
Garrido CJ, López-Sánchez-Vizcaíno V, Gómez-Pugnaire MT, Trommsdorff V, Alard O, Bodinier JL, Godard M (2005) Enrichment of HFSE in chlorite-harzburgite produced by high-pressure dehydration of antigorite-serpentinite: implications for subduction magmatism. Geochem Geophys Geosyst 6:Q01J15
Godard M, Lagabrielle Y, Alard O, Harvey J (2008) Geochemistry of the highly depleted peridotites drilled at ODP Sites 1272 and 1274 (fifteen-twenty fracture zone, Mid-Atlantic Ridge): implications for mantle dynamics beneath a slow spreading ridge. Earth Planet Sci Lett 267:410–425
Gómez-Pugnaire MT, Galindo-Zaldivar J, Rubatto D, González-Lodeiro F, López-Sánchez-Vizcaíno V, Jabaloy A (2004) A reinterpretation of the Nevado-Filabride and Alpujarride complexes (Betic Cordillera): field, petrography and U-Pb ages from orthogneisses (western Sierra Nevada, S Spain). Schweiz Mineral Petrogr Mitt 84:303–322
Jasonov PG, Nougaliev DK, Burov BV, Heller F (1998) A modernized coercivity spectrometer. Geol Carpath 49:224–225
Kelley K, Cottrell E (2009) Water and the oxidation state of subduction zone magmas. Science 325:605–607
Klein F, Bach W (2009) Fe–Ni–Co–O–S phase relations in peridotite seawater interactions. J Petrol 50:37–59
Klein F, Bach W, Humphris SE, Kahl W-A, Jöns N, Moskowitz B, Berquó TS (2013) Magnetite in seafloor serpentinite—Some like it hot. Geology. doi:10.1130/g35068.1
Kodolanyi J, Pettke T, Spandler C, Kamber BS, Gméling K (2012) Geochemistry of ocean floor and fore-arc serpentinites: constraints on the ultramafic input to subduction zones. J Petrol 53:235–270
Laubier M, Grove TL, Langmuir CH (2014) Trace element mineral/melt partitioning for basaltic and basaltic andesitic melts: an experimental and laser ICP-MS study with application to the oxidation state of mantle source regions. Earth Planet Sci Lett 392:265–278
Lee CTA, Leeman WP, Canil D, Li ZXA (2005) Similar V/Sc systematics in MORB and arc basalts: implications for the oxygen fugacities of their mantle source regions. J Petrol 46:2313–2336
Lee CTA, Luffi P, Le Roux V, Dasgupta R, Albarede F, Leeman W (2010) The redox of arc mantle using Zn/Fe systematics. Nature 468:681–685
López-Sánchez-Vizcaíno V, Trommsdorff V, Gómez-Pugnaire MT, Garrido CJ, Müntener O, Connolly JAD (2005) Petrology of titanian clinohumite and olivine at the high-pressure breakdown of antigorite serpentinite to chlorite harzburgite (Almirez Massif, S. Spain). Contrib Mineral Petrol 149:627–646
Malaspina N, Tumiati S (2012) The role of C–O–H and oxygen fugacity in subduction-zone garnet peridotites. Eur J Mineral 24:607–618
Marcaillou C, Muñoz M, Vidal O, Parra T, Harfouche M (2011) Mineralogical evidence for H2 degassing during serpentinization at 300°C/300 bar. Earth Planet Sci Lett 303:281–290
Marchesi C, Garrido CJ, Padrón-Navarta JA, López-Sánchez-Vizcaíno V, Gómez-Pugnaire MT (2013) Element mobility from seafloor serpentinization to high-pressure dehydration of antigorite in subducted serpentinite: insights from the Cerro del Almirez ultramafic massif (southern Spain). Lithos 178:128–142
Maurice J, Bolfan-Casanova N (2014) Experimental study of serpentine dehydration. Lherzolite conference, Marrakech, Maroc, abstr
McCollom TM, Bach W (2009) Thermodynamic constraints on hydrogen generation during serpentinization of ultramafic rocks. Geochim Cosmochim Acta 73:856–875
Muñoz M, Vidal O, Marcaillou C, Sakura P, Mathon O, Farges F (2013) Iron oxidation state in phyllosilicate single crystals using Fe–K edge and XANES spectroscopy: effects of the linear polarization of the synchrotron X-ray beam. Am Mineral 98:1187–1197
O’Hanley DS, Dyar MD (1993) The composition of lizardite 1 T and the formation of magnetite in serpentinites. Am Mineral 78:391–404
Oufi O, Cannat M, Horen H (2002) Magnetic properties of variably serpentinized abyssal peridotites. J Geophys Res 107-1978-2012
Padrón-Navarta JA, López Sánchez-Vizcaíno V, Garrido CJ, Gómez-Pugnaire MT, Jabaloy A, Capitani G, Mellini M (2008) Highly ordered antigorite from Cerro del Almirez HP–HT serpentinites, SE Spain. Contrib Mineral Petrol 156:679–688
Padrón-Navarta JA, Tommasi A, Garrido CJ, López Sánchez-Vizcaíno V, Gómez-Pugnaire MT, Jabaloy A, Vauchez A (2010a) Fluid transfer into the wedge controlled by high-pressure hydrofracturing in the cold top-slab mantle. Earth Planet Sci Lett 297:271–286
Padrón-Navarta JA, Hermann J, Garrido CJ, López Sánchez-Vizcaíno V, Gómez-Pugnaire MT (2010b) An experimental investigation of antigorite dehydration in natural silica-enriched serpentinite. Contrib Mineral Petrol 159:25–42
Padrón-Navarta JA, López Sánchez-Vizcaíno V, Garrido CJ, Gomez-Pugnaire MT (2011) metamorphic record of high-pressure dehydration of antigorite serpentinite to chlorite harzburgite in a subduction setting (Cerro del Almirez, Nevado-Filabride complex, Southern Spain). J Petrol 52:2047–2078
Padrón-Navarta JA, López Sánchez-Vizcaíno V, Hermann J, Connolly JAD, Garrido CJ, Gómez-Pugnaire MT, Marchesi C (2013) Tschermak’s substitution in antigorite and consequences for phase relations and water liberation in high-grade serpentinites. Lithos 178:186–196
Parkinson IJ, Arculus RJ (1999) The redox state of subduction zones: insights from arc-peridotites. Chem Geol 160:409–423
Ruiz Cruz MD, Puga E, Nieto JM (1999) Silicate and oxide exsolution in pseudospinifex olivine from metaultramafic rocks of the Betic Ophiolitic association: a TEM study. Am Mineral 84:1915–1924
Savov IP, Ryan JG, D’Antonio M, Fryer P (2007) Shallow slab fluid release across and along the Mariana arc-basin system: insights from geochemistry of serpentinized peridotites from the Mariana fore arc. J Geophys Res. doi:10.1029/2006JB004749
Scambelluri M, Tonarini S (2012) Boron isotope evidence for shallow fluid transfer across subduction zones by serpentinized mantle. Geology 40:907–910
Scambelluri M, Bottazzi P, Trommsdorff V, Vannucci R, Hermann J, Gómez- Pugnaire MT, López-Sánchez-Vizcaíno V (2001) Incompatible element-rich fluids released by antigorite breakdown in deeply subducted mantle. Earth Planet Sci Lett 192:457–470
Scambelluri M, Fiebig J, Malaspina N, Müntener O, Pettke T (2004) Serpentinite subduction: implications for fluid processes and trace-element recycling. Int Geol Rev 46:595–613
Schwartz S, Guillot S, Reynard B, Lafay R, Debret B, Nicollet C, Lanari P, Auzende AL (2013) Pressure–temperature estimates of the lizardite/antigorite transition in high pressure serpentinites. Lithos 178:197–210
Song S, Su L, Niu Y, Lai Y, Zhang L (2009) CH4 inclusions in orogenic harzburgite: evidence for reduced slab fluids and implication for redox melting in mantle wedge. Geochim Cosmochim Acta 73:1737–1754
Spencer KJ, Lindsley DH (1981) A solution model for coexisting iron-titanium oxides. Am Mineral 66:1189–1201
Stolper E, Newman S (1994) The role of water in petrogenesis of Mariana trough magmas. Earth Planet Sci Lett 121:293–325
Tauxe L (2009) Essentials of paleomagnetism. University of California Press, San Diego, p 512
Thompson JB (1982) Composition space: an algebraic and geometric approach. Rev Mineral Geochem 10:1–31
Torres-Roldán RL, García-Casco A, García-Sanchez PA (2000) CSpace: an integrated workplace for the graphical and algebraic analysis of phase assemblages on 32-bit wintel platforms. Comput Geosci 26:779–793
Trommsdorff V, López Sánchez-Vizcaíno V, Gomez-Pugnaire MT, Müntener O (1998) High pressure breakdown of antigorite to spinifex-textured olivine and orthopyroxene, SE Spain. Contrib Mineral Petrol 132:139–148
Tumiati S, Godard G, Martin S, Malaspina N, Poli S (2015) Ultra-oxidized rocks in subduction mélanges? Decoupling between oxygen fugacity and oxygen availability in a Mn-rich metasomatic environment. Lithos. doi:10.1016/j.lithos.2014.12.008
Ulmer P, Trommsdorff V (1995) Serpentine stability to mantle depths and subduction-related magmatism. Science 268:858–861
Vils F, Pelletier L, Kalt A, Müntener O, Ludwig T (2008) The Lithium, Boron and Beryllium content of serpentinized peridotites from ODP Leg 209 (Sites 1272A and 1274A): implications for lithium and boron budgets of oceanic lithosphere. Geochim Cosmochim Acta 72:5475–5504
Vils F, Müntener O, Kalt A, Ludwig T (2011) Implications of the serpentine phase transition on the behaviour of beryllium and lithium-boron of subducted ultramafic rocks. Geochim Cosmochim Acta 75:1249–1271
Wilke M, Farges F, Petit PE, Gordon EB, Martin F (2001) Oxidation state and coordination of Fe in minerals: an Fe K-XANES spectroscopic study. Am Mineral 86:714–730
Acknowledgments
We acknowledge SOLEIL for provision of synchrotron radiation facilities on LUCIA beamline (Project No. 20121036). We thank J.-L. Devidal (LMV, Clermont-Ferrand) for his assistance during microprobe analyses, P. Boulhiol (Durham University) for instructive discussions, and MR Reyes-González for sample preparation. We thank N. Malaspina and K. Evans for critical comments on an earlier version of this article, and O. Müntener for his careful editorial handling. The Raman spectroscopy facility at the ENS Lyon is supported by CNRS INSU. This work was supported by ANR11JS5601501 HYDEEP, grant to Nathalie Bolfan-Casanova. The first author is supported by the ERC HabitablePlanet (306655), grant attributed to Helen Williams (Durham University, UK). JAPN has been supported by a EU-FP7-funded Marie Curie postdoctoral grant under contract agreement PIOF-GA-2010-273017. JAPN, VLSV, MTGP, and CJG are supported by “Ministerio de Economía y Competitividad” Grants CGL2012-32067 and CGL2013-42349-Pand Junta de Andalucía Grants RNM-145, RNM-131, and P09-RNM-4495, funded by the European Regional Development Fund. The authors further acknowledge support by the Marie Curie ITN-ZIP funded under Grant agreement PITN-GA-2013-604713.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Othmar Müntener.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Debret, B., Bolfan-Casanova, N., Padrón-Navarta, J.A. et al. Redox state of iron during high-pressure serpentinite dehydration. Contrib Mineral Petrol 169, 36 (2015). https://doi.org/10.1007/s00410-015-1130-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00410-015-1130-y