Abstract
Background
Inflammation plays a central role in chronic obstructive pulmonary disease and lung cancer carcinogenesis. Inhaled corticosteroids (ICS) reduce inflammation. This study has investigated whether ICS use is associated with a lower risk of lung cancer.
Materials and Methods
Data from the Nord-Trøndelag Health Study (HUNT2 Survey, 1995–1997) were merged with The Cancer Registry of Norway and Norwegian Cause of Death Registry. From a total of 65,215 participants, those with chronic airway inflammation, defined by FEV1% < 70 and/or chronic cough and expectorate phlegm, were included (N = 4136). Of these, 3041 individuals reported regarding ICS use and were observed for a period of 12 years. Cox regression models were used to calculate the risk of lung cancer with a 95% confidence interval (CI) with sex, age, smoking pack years and FEV1% < 70 as known confounders.
Results
Among ICS users (N = 1095). we found a higher, but not significant, incidence of lung cancer N = 39 (3.6%), compared to non-users (N = 1946) with N = 65 (3.3%) cases. Age and smoking were associated with a higher risk, while sex and lung function were not. After adjusting for confounders, ICS use did not change the risk of lung cancer, hazard ratio (HR) 0.968, (95% CI, 0.608–1.540), and p value 0.890.
Conclusion
ICS use is not associated with a reduced risk of lung cancer in our study population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, the burden of lung cancer is still a problem considering both public health care and economics. Lung cancer is the most common cause of cancer-related death in the United States [1]. The 5-year overall survival rate is still poor (10–15%) [2]. At the time of diagnosis, the disease is often in an advanced stage and curation is not possible. Considering the fatal outcome, prevention is a better approach to avoid new cases [3].
Chronic obstructive pulmonary disease (COPD) and chronic airway inflammation is an important public health challenge and the third leading cause of death worldwide. More than 3 million people died of COPD in 2012 [4].
Cancer-related inflammation comprises both inflammatory mediators and cells, as seen in chronic inflammatory responses and tissue repair [5]. Chronic inflammation can contribute to unrestricted cell proliferation and invasion, inducing angiogenesis and increasing mutagenesis [3].
Several pathophysiological mechanisms may link COPD and lung cancer. Factors as inflammation, smoking, presence of specific proteinases, genetic and epigenetic changes are associated with COPD and lung cancer [6].
The pathological structural changes and the chronic inflammation remain despite smoking cessation and increase with COPD severity [7]. Young et al. concluded that COPD is both a common and important independent risk factor associated with the development of lung cancer [8]. Smokers with COPD have five times higher risk of developing lung cancer compared to smokers with normal lung function [9]. In addition to smoking and COPD, other known risk factors for developing lung cancer are sex and age [10,11,12].
By suppressing the inflammatory process in patients with COPD with corticosteroids, there exists a potential for reducing the tumor-promoting effect [13, 14].
Previous studies examining the association between inhaled corticosteroids (ICS) and the risk of lung cancer present conflicting results. Several studies have shown a decreased risk, but others did not [15,16,17,18,19,20,21]. These studies have some limitations, such as their study population and short follow-up time [15, 16, 18,19,20].
We wanted to study the hypothesis that ICS reduce the risk of lung cancer in a large general population. A population-based cohort study, the Nord-Trøndelag Health Study (HUNT), with a long follow-up period can contribute to answer this question.
Materials and Methods
Source of Data
Data applied in this study were obtained from the HUNT Study, a large population-based cohort study [22]. Three surveys have been performed: the HUNT1 (1984–1986), the HUNT2 (1995–1997), and the HUNT3 (2006–2008). All inhabitants of the Nord-Trøndelag County, older than 20 years, have been invited to participate in the study. In total 77,212 (89.4%), 65,215 (69.5%), and 50,807 (54.1%) individuals have participated in HUNT1, 2, and 3, respectively.
Study Population
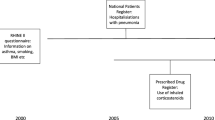
Since the HUNT1 survey did not provide information about the use of ICS, we included only participants from the HUNT2 Survey with chronic inflammation (N = 4136). Chronic airway inflammation was defined by reduced forced expiratory volume in 1 s/forced vital capacity (FEV1%) (lower than 70%), and/or participants that answered “yes” to the question whether they have had “persistent cough and expectorate phlegm in the morning at least 3 months the last 2 years” or not. A total of 3041 individuals answered the question regarding ICS treatment. 1095 did not answer and were excluded to avoid a non-responder bias (Fig. 1). The HUNT2 Survey provides no information about the length of the treatment with ICS before entering the HUNT2 Survey. To avoid an under- or overestimation of the effect of ICS on the risk of lung cancer, we wanted to ensure a sufficient exposure time of ICS. Therefore, patients diagnosed with lung cancer prior to 2002 were excluded. The observational period went from the day of inclusion in the HUNT2 Survey until the diagnosis of lung cancer, death or at the end of the study in December 2008, whichever occurred first.
The data from the HUNT Study were matched with data from the Cancer Registry of Norway (CRN) and the Norwegian Cause of Death Registry at Statistics Norway [23].
Outcome and Exposure Variable
Lung cancer diagnosis is based on the classification released by the World Health Organization (WHO) [24]. All histological types of lung cancer were included in our study.
ICS use was defined as the exposure variable. The participants were classified as ICS users by answering “yes” to the following question: “Have you ever regularly used medicines like Becotide (beclomethasone), Flutide (fluticasone), Pulmicort (budesonide) or Viarox (beclomethasone)?”
Age at the time of inclusion, sex, smoking pack years, and FEV1% < 70 were included as confounders.
Statistical Analysis
The statistical analysis was performed using PASW version 22 (Predictive Analytics Soft Ware, IBM Corporation, New York 10589, USA). First, by using the Chi-square test, we investigated the differences in known prognostic factors between the two groups ICS users (N = 1095) versus non-users (N = 1946). Second, applying the cox regression model, we estimated the hazard ratio (HR) with a 95% confidence interval for developing lung cancer, stratified by known potential confounders in both the univariate and multivariate analyses. All participants in the period from 1996 to 2008 were included. Both pack years and age were tested for linearity. Third, we performed a sensitivity analysis including only participants using ICS over a 12-year period.
To test a possible effect modification, we did separate analyses stratified for sex and smoking. Only two-sided tests were used, and the statistical significance was defined as p < 0.05.
Ethics
The study was approved by the Regional Committee for Medical and Health Research Ethics (2015/1801/REK midt).
Results
Participant Characteristics
We found a prevalence of lung cancer and chronic airway inflammation of 3.0 and 6.4%, respectively. Data from 3041 participants, N = 1946 non-ICS users and N = 1095 ICS users, were analyzed. Non-ICS users were in mean 3 years younger, and had a better lung function compared to ICS users. There was no difference in sex and burden of smoking (Table 1). 40% of the ICS users had used ICS more than 4 years.
In the ICS user group, we found N = 39 (3.6%) cases of lung cancer, versus N = 65 (3.3%) (p = 0.747). The mean age at diagnosis was 70 years in the ICS user group versus 72 years (p = 0.742).
We found a significant higher death rate in the group using ICS versus the group not using ICS (p < 0.001).
The unadjusted analysis identified sex, age, pack years, and lung function as factors increasing the risk of lung cancer. In the multivariate analysis, only age and smoking increased the risk. ICS use did not decrease the risk (Table 2).
Analyses stratified by sex and smoking did not change the results (results not shown).
Sensitivity Analysis
First, we estimated the risk of lung cancer among participants using ICS over 12 years. Still ICS use did not decrease the risk of lung cancer, HR 0.750 (95% CI, 0.120–4.67) (p value 0.758). Secondly, we excluded all patients diagnosed with lung cancer prior to 2002, to avoid selection bias. The results did not indicate that patients using ICS had a decreased risk of lung cancer with HR 0.909 (95% CI, 0.543–1.521) (p value 0.716) in patients using ICS.
Discussion
In contrast to our hypothesis, we did not find a protective effect of ICS among patients with COPD. However, the 95% CI is wide; there may be a small effect of ICS that is not detected in this study considering the low number of cases. Only smoking pack years and higher age were associated with a higher risk of lung cancer in our study. Alberg et al. showed that smokers have a 20-fold increased risk, compared with lifetime non-smokers [25]. The carcinogenesis induced by smoking is a cumulative process that takes place over several decades. Consequently, lung cancer peaks in the elderly population, and is seldom found with individuals younger than the age of 30 [1]. As shown in our results, both male sex and reduced lung function increased the risk of lung cancer. We found a higher incidence of lung cancer in men, consistent with data worldwide. Compared to those with preserved lung function, patients with the lowest pulmonary function have the highest risk. This correlation is alinear, meaning that a small disparity in FEV1 increases the risk of lung cancer even though it is considered within normal range [26].
A decision of ICS use or non-use is not random, and deeply depends on the severity of patient conditions including the severity of air-flow limitation. This fact, in theory, remains a significant bias even with an adjustment with FEV1% value. It is most likely that individuals with use of ICS are more at risk for lung cancer than those without it. We found in our total study population a significant higher death rate in the group using ICS. Early death before emerging lung cancer may underestimate lung cancer incidence and death. However, a sub-analysis which includes only participants participating in HUNT2 and 3, and were still alive at the end of the study, did not change our results. We assume that the difference in the death rate is not the reason for our findings.
Several studies have shown that chronic inflammation promotes susceptibility to occurrence of a variety of cancers. Chronic inflammatory environment with inflammatory cells, chemokines, and cytokines can trigger transcription of proto-oncogenes, tumor suppressor genes, and epigenetic mechanisms that promote carcinogenesis. This process may be activated by several mechanisms, such as autoimmune diseases and infections. Colon cancer is associated with inflammatory bowel disease; gastric cancer is related to helicobacter pylori. The risk of cancer decreases with the use of non-steroidal anti-inflammatory drugs, in both colon cancer and breast cancer [5]. This further supports the association of inflammation and cancer.
A systematic review investigated ICS therapy among COPD patients and the correlation with lung cancer risk [21]. It is based on four RCTs and two observational studies. These studies included COPD patients at age 40 and older, treatment with ICS alone, and ICS in combination with β-agonists. The primary or secondary outcome was either lung cancer diagnosis or mortality. In one of the observational studies included, Parimon et al. followed 10,474 patients in a median of 3.8 years, and proved that ICS use was effective. They found a risk reduction when using higher doses of ICS, suggesting a dose-dependent relationship. As opposed to our study, their study population mainly consisted of males (97%) [18]. This fact makes it difficult to generalize the results.
The latency period in lung cancer is prolonged. This was considered in this study by excluding participants that reported to have lung cancer until 2002 in a sensitivity analysis. Parimon et al. had a much shorter observational time and did not include this latency period. They excluded patients with lung cancer the first year after inclusion. In comparison, we excluded the same group the first 6 years [18].
Kiri et al. used a case-control study, and the cohort included 7079 patients. Their study showed that regular use of ICS in monotherapy and ICS in combination with LABA reduced the risk of lung cancer with 36 and 50%, respectively. The risk was further reduced with the use of higher doses, as presented by Parimon et al. In Kiris’ population, only former smokers were included, thereby their study may lack some transmissibility [17, 18]. Despite suffering from either mild or serious COPD, many do not accomplish tobacco smoking cessation, and need help in order to do so [27]. Tobacco-smoking cessation is essential in lung cancer control. If smokers of 15 cigarettes or more per day reduce their intake by 50%, they will reduce the risk of lung cancer [28]. Kiri et al. included only 30% women, in comparison to 45% female participants in our study [17]. Despite the fact that previous studies have reported men to have higher prevalence and mortality of COPD than women, new evidence suggests a rather balanced gender distribution [7].
Unlike the observational studies, the four RCTs from the systematic review did not indicate a statistically significant effect of ICS use. This result corresponds with the results in our study. The study populations contain few lung cancer diagnosis and deaths, making these studies more prone to type II error (“false negative”). The prolonged latency period with lung cancer requires a long follow-up period, to identify a significant effect of ICS use [21]. Due to the population size and the follow-up, those studies are underpowered to detect an effect.
For an optimal ICS treatment, patients need to adhere to a proper treatment regimen and use the inhaler correctly. Sriram and Percival performed an observational cross-sectional study which demonstrated that 58% of COPD patients had suboptimal adherence [29]. In a systematic review based on data derived from 144 articles, covering 54,354 subjects completing 59,584 observed tests of technique, Sanchis et al. found that inadequate inhaler technique is frequent. The most regular errors in inhaler use have not improved over the last 40 years [30]. In a study, 37 COPD patients were observed as they demonstrated their inhalation technique. The results showed that N = 22 (59%) did critical errors during the observed demonstration. The patients who used metered-dose inhalers made more critical errors than patients using other inhalers [31]. We do not have information concerning inhalation technique and devices used in our study. It is crucial in future studies that the correct technique and adherence are achieved by patient groups. Simple measures will increase the value of studies.
Considering the clinical complexity of COPD, it is now clear that these patients derive from a heterogeneous group with different associated subgroups. It is possible that the effect of ICS use depends on the phenotype and is influenced by genetic involvement [6]. To improve clinical outcomes, it will be valuable to provide individualized treatment [32].
All histological types of lung cancer were included in our study. This study is therefore not suitable to unveil whether ICS use had a chemoprotective effect in any of the histological types individually. In our study, participants with chronic airway inflammation are defined by FEV1% < 70 and/or chronic cough and expectorate phlegm in the morning. It would be preferable with spirometry results of all participants. Compared to Parimon et al. which had not spirometry, we received results from half of the population [18]. To ensure that most COPD patients were incorporated in this study, we also included the question concerning chronic cough.
Most of our data are based on a questionnaire, this may have given both an underestimate or overestimate of our results. For example, 1095 participants did not answer the question regarding ICS use. We have no information why these participants did not answer the question. Multiple imputation (MI) is one method that may increase the strength of our study. However, we have only four covariates included in our study and we concluded that MI will probably not increase the strength.
The data in this study are based on the HUNT Study which has several strengths. It is a large database with high participation, with different known risk factors for developing lung cancer. The region in the population-based prospective cohort study consists of both coastal, and inland municipalities with a population aged 20 years and older, thereby providing a group with relatively diverse exposures [22]. The prevalence of lung cancer and the median age of 71 years is in line with other studies, indicating high validity of our study. Raherison and Girodet found a COPD prevalence of 7.6% (95% CI, 6–9.2%) in the general population [33]. In our study population, the COPD prevalence was 6.4%. The fact that the prevalence in our population corresponds with global data, strengthening our study. Since we have a population-based study, our results have great transmissibility. The main features of the population in HUNT are typical of the Norwegian population [34]. Additionally, the population has remained stable throughout the study. All data are individually connected to The Cancer Registry of Norway and Norwegian Cause of Death Registry, increasing the validity and reliability of the study [22]. Compared with other similar studies, our study had the longest follow-up period.
This study contains several potential limitations. Firstly, diseases and risk factors concerning health can be related to socioeconomic status. Since participants in population-based studies compared to nonparticipants have a higher socioeconomic status, this can contribute to selection bias [35].
Furthermore, the inhabitants of Nord-Trøndelag are found to be smoking less than the average population in Norway. This may ultimately influence the results of our study [36,37,38].
In our study, we have used ICS use as a binary variable, in lack of more information from the HUNT Survey. Unfortunately, the current data set contains no information concerning the daily dose of ICS. Parimon et al. found in their study a dose-dependent decreased risk with a cut-off value of ≥ 1200 µg/day adjusted HR 0.39 (CI 0.67–1.90) [18]. There is possibly a protective effect of ICS in our study, but we could not show this effect. The lack of dosage-based segmentation in our study may have influenced our results.
Conclusion
We found no protective effect of ICS use on the incidence of lung cancer. A large prospective population-based study including the dose of ICS use and the severity of air-flow limitation is needed to further answer the question definitively.
References
Torre LA, Siegel RL, Jemal A (2016) Lung cancer statistics. Adv Exp Med Biol 893:1–19
WHO (2014) World cancer report 2014. International Agency for Research on Cancer, Lyon
Gomes M, Teixeira AL, Coelho A, Araújo A, Medeiros R (2014) The role of inflammation in lung cancer. In: Aggarwal BB, Sung B, Gupta SC (eds) Inflammation and cancer. advances in experimental medicine and biology, vol 816. Springer, Basel
Terzikhan N, Verhamme KM, Hofman A, Stricker BH, Brusselle GG, Lahousse L (2016) Prevalence and incidence of COPD in smokers and non-smokers: the Rotterdam Study. Eur J Epidemiol 31(8):785–792
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A (2009) Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 30(7):1073–1081
Houghton AM (2013) Mechanistic links between COPD and lung cancer. Nat Rev Cancer 13(4):233–245
Disease GIfCOP (2017) GOLD 2017 global strategy for the diagnosis, mangement, and prevention of chronic obstructive pulmonary disease http://goldcopd.org/2017
Young RP, Hopkins RJ, Christmas T, Black PN, Metcalf P, Gamble GD (2009) COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J 34(2):380–386
Durham AL, Adcock IM (2015) The relationship between COPD and lung cancer. Lung Cancer 90(2):121–127
El-Zein M, Parent ME, Nicolau B, Koushik A, Siemiatycki J, Rousseau MC (2013) Body mass index, lifetime smoking intensity and lung cancer risk. Int J Cancer 133(7):1721–1731
Powell HA, Iyen-Omofoman B, Baldwin DR, Hubbard RB, Tata LJ (2013) Chronic obstructive pulmonary disease and risk of lung cancer: the importance of smoking and timing of diagnosis. J Thorac Oncol 8(1):6–11
Yang Y, Dong J, Sun K, Zhao L, Zhao F, Wang L et al (2013) Obesity and incidence of lung cancer: a meta-analysis. Int J Cancer 132(5):1162–1169
Barnes PJ, Adcock I, Spedding M, Vanhoutte PM (1993) Anti-inflammatory actions of steroids: molecular mechanisms. Trends Pharmacol Sci 14(12):436–441
Lee CH, Hyun MK, Jang EJ, Lee NR, Kim K, Yim JJ (2013) Inhaled corticosteroid use and risks of lung cancer and laryngeal cancer. Respir Med 107(8):1222–1233
Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW et al (2007) Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 356(8):775–789
Group LHSR (2000) Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med 343(26):1902–1909
Kiri VA, Fabbri LM, Davis KJ, Soriano JB (2009) Inhaled corticosteroids and risk of lung cancer among COPD patients who quit smoking. Respir Med 103(1):85–90
Parimon T, Chien JW, Bryson CL, McDonell MB, Udris EM, Au DH (2007) Inhaled corticosteroids and risk of lung cancer among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 175(7):712–719
Pauwels RA, Lofdahl CG, Laitinen LA, Schouten JP, Postma DS, Pride NB et al (1999) Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N Engl J Med 340(25):1948–1953
Tashkin DP, Rennard SI, Martin P, Ramachandran S, Martin UJ, Silkoff PE et al (2008) Efficacy and safety of budesonide and formoterol in one pressurized metered-dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease: results of a 6-month randomized clinical trial. Drugs 68(14):1975–2000
Raymakers AJ, McCormick N, Marra CA, Fitzgerald JM, Sin D, Lynd LD (2017) Do inhaled corticosteroids protect against lung cancer in patients with COPD? a systematic review. Respirology 22(1):61–70
Krokstad S, Langhammer A, Hveem K, Holmen TL, Midthjell K, Stene TR, Bratberg G, Heggland J, Holmen J (2013) Cohort profile: The HUNT Study, Norway. Int J Epidemiol. https://doi.org/10.1093/ije/dys095
Norway CRo (2016) Cancer in Norway 2015—cancer incidence, mortality, survival and prevalence in Norway Oslo: Cancer Registry of Norway https://www.kreftregisteret.no/globalassets/cancer-in-norway/2015/cin_2015.pdf. Accessed 27 March 2017
Sobin LH (1982) The World Health Organization’s histological classification of lung tumors: a comparison of the first and second editions. Cancer Detect Prev 5(4):391–406
Alberg A, Nonemaker J (2012) Who is at high risk for lung cancer? population-level and individual-level perspectives. Semin Respir Crit Care Med.
Wasswa-Kintu S, Gan WQ, Man SFP, Pare PD, Sin DD (2005) Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: a systematic review and meta-analysis. Thorax 60(7):570–575
Tønnesen P (2013) Smoking cessation and COPD. Eur Respir Rev 22(127):37–43
Godtfredsen N, Prescott E, Osler M (2005) Effect of smoking reduction on lung cancer risk. JAMA ;294
Sriram KB, Percival M (2016) Suboptimal inhaler medication adherence and incorrect technique are common among chronic obstructive pulmonary disease patients. Chronic Respir Dis 13(1):13–22
Sanchis J, Gich I, Pedersen S (2016) Systematic review of errors in inhaler use: has patient technique improved over time? Chest 150(2):394–406
Batterink J, Dahri K, Aulakh A, Rempel C (2012) Evaluation of the use of inhaled medications by hospital inpatients with chronic obstructive pulmonary disease. Can J Hosp Pharm 65(2):111–118
Vermylen JH, Kalhan R (2013) Revealing the complexity of chronic obstructive pulmonary disease. Transl Res 162(4):203–207
Raherison C, Girodet P-O (2009) Epidemiology of COPD. Eur Respir Rev 18(114):213–221
Lie TM, Bomme M, Hveem K, Hansen JM, Ness-Jensen E (2017) Snus and risk of gastroesophageal reflux. A population-based case-control study: the HUNT study. Scand J Gastroenterol 52(2):193–198
Langhammer A, Krokstad S, Romundstad P, Heggland J, Holmen J (2012) The HUNT study: participation is associated with survival and depends on socioeconomic status, diseases and symptoms. BMC Med Res Methodol 12(1):143
Earnings of all employees, 2016. Statistics Norway. 2016. https://www.ssb.no/en/arbeid-og-lonn/statistikker/lonnansatt. Accessed 28 March 2017
Level of education for men and woman 16 years and older. County of residence. Numbers and per cent. Statistics Norway. 2016. https://www.ssb.no/en/utdanning/statistikker/utniv/aar/2016-06-20?fane=tabell&sort=nummer&tabell=270245. Accessed 28 March 2017
Smoking habits, 2015. Statistics Norway. 2016 https://www.ssb.no/en/helse/statistikker/royk/aar/2016-01-14. Accessed 28 March 2017
Acknowledgements
We thank the staff at HUNT Research Center, Norwegian Cancer Registry and Statistics Norway for cooperation and help in data management, and, last but not least, all the participants in the HUNT Study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Sørli, K., Thorvaldsen, S.M. & Hatlen, P. Use of Inhaled Corticosteroids and the Risk of Lung Cancer, the HUNT Study. Lung 196, 179–184 (2018). https://doi.org/10.1007/s00408-018-0092-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-018-0092-z