Abstract
Purpose
Cystic fibrosis (CF) patients suffer from chronic lung inflammation. This inflammation may activate platelets. There are limited data on the role of platelet-secreted cytokines in CF. Platelet cytokines with inflammatory effects include vascular endothelial growth factor (VEGF) and transforming growth factor-β1 (TGF-β1). As levels of these cytokines are tenfold greater in serum than plasma due to platelet release, serum levels may be one index of platelet content, but a more specific index is release during the aggregation of isolated platelets. We postulated that altered release of these platelet cytokines occurs in CF.
Methods
We obtained sera and plasma from CF outpatients (n = 21) and from healthy controls (n = 20), measured VEGF and TGF-β1, assessed for correlations with platelet number, analyzed cytokine release during platelet aggregation to collagen, and analyzed differences in maximal platelet aggregation.
Results
Platelet number and maximal aggregation levels were higher in CF. Plasma and serum levels of TGF-β1 and VEGF were higher in CF, but these levels were similar after adjusting for platelet number (serum cytokines correlated with platelet count). The release of VEGF and TGF-β1 during aggregation was decreased in CF platelets (by 52 and 29 %, respectively).
Conclusion
Platelet release is not a source of the elevated blood proinflammatory cytokines TGF-β1 and VEGF in CF, as platelets from CF patients actually release less of these cytokines. These data provide further evidence for platelet defects in CF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cystic fibrosis (CF) is a lethal disease where chronic inflammation causes progressive lung destruction. Other sites such as the pancreas, sinuses, and liver are also frequently involved. [1, 2] Recurrent acute bacterial infections amplify the effects of chronic infection and thereby contribute substantially to the progressive loss of lung function and untimely death that characterize this disease. Leukocyte-triggered damage is clearly a primary destructive factor in CF, but platelets activated by systemic inflammation may also be important as they can release the contents of their granules in the lung (including proinflammatory cytokines) and thus worsen inflammation [3–6]. Thus, platelets and their secreted substances have been of interest in CF as both potential biomarkers of disease activity and as targets for therapeutic interventions. Mutations in CFTR may also affect platelet function independent of inflammation, and CF platelets have been reported to be hyperaggregable and secrete more thromboxane A2 and soluble CD40 ligand. [6–10] The beneficial effect of ibuprofen in CF could be related to its inhibition of platelet aggregation. Interestingly, inhibition of platelets also decreases lung inflammation in LPS-challenged cystic fibrosis transmembrane conductance regulator (CFTR) mutant mice, further supporting a potential role for platelets in the inflammatory cascade of CF [11]. Pseudomonas components can activate CF platelets directly [12]. Platelet counts and ex vivo platelet aggregation are also known to be higher in CF patients, as is typical of many diseases of chronic inflammation. However, it remains undetermined if platelet cytokine levels increase in response to the many stimuli of chronic inflammation in CF [10, 13, 14].
There are limited data available on platelet-derived cytokines in CF. Platelet-secreted cytokines with inflammatory effects that have been examined in CF include vascular endothelial growth factor (VEGF) and transforming growth factor-beta 1 (TGF-β1) [15–17]. Serum and/or plasma levels of these two cytokines have been shown to be elevated in CF patients, to increase with acute exacerbations, and to be associated with the severity of lung disease and/or pseudomonas colonization [18–21]. Notably, VEGF and TGF-β1 are highly expressed in platelets, so their serum levels (measured in coagulated blood) are up to tenfold greater than plasma levels (measured in anticoagulated blood) [18, 21]. Serum cytokine levels thus may an index of the platelet stores of these cytokines, related to both platelet number and platelet content [22, 23]. As higher platelet counts in CF may confound interpretation of the serum levels of these platelet cytokines, serum values should be adjusted for platelet count [23], but this has not been done in the CF studies to date. A more accurate assessment of platelet cytokine content/release can be determined after in vitro aggregation of isolated platelets, which has also not been described in CF. Plasma cytokine concentrations, on the other hand, are an index of circulating cytokine levels, reflecting release from many tissues. Plasma TGF-β1 is also increased in CF patients, both with or without acute infections, where it appears to be associated with the severity of lung disease and with pseudomonas colonization [21, 24].
We postulated that platelet release of the profibrotic and proinflammatory cytokines TGF-β1 and VEGF would be altered in CF as further evidence of defective platelet function in CF. To test this hypothesis, we studied blood and platelets from CF outpatients who had not recently been hospitalized or treated for an acute infection.
Materials and Methods
Human Subjects
The study was approved by the Medical College of Wisconsin Institutional Review Board in Milwaukee, Wisconsin. All subjects gave informed written consent, and all procedures were performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. All CF patients had lung disease (based on abnormal imaging studies and/or abnormal lung function tests in the past year), abnormal sweat chloride tests, or carriage of two well-characterized CFTR mutations on DNA sequencing. Subjects were a convenience sample enrolled prospectively over 3 months as they presented for routine follow-up visits at a multidisciplinary CF clinic at the Medical College of Wisconsin clinics. Lung function tests were not abstracted as part of study data. Healthy controls were recruited from hospital staff. Inclusion criteria were age 18–40 years. Exclusion criteria were hospitalization in the past 30 days, lung transplant, acute infection requiring antibiotics (or use of chronic azithromycin), smoking, and use of aspirin or nonsteroidal anti-inflammatory agents in the past 2 weeks.
General
All plastics, solutions, chemicals, and buffers were from Sigma (St. Louis, MO) unless otherwise stated.
Serum and Plasma Samples
Preparation of plasma and serum followed the published procedures using heparin-containing tubes for plasma and plain glass tubes for serum [25]. Ten milliliter of blood was drawn by venipuncture from arm veins with a 21 gauge or larger needle. Blood was allowed to stand for 30 min at 22 °C to ensure full clotting of serum. The samples were then centrifuged at 750 xg for 10 min at 4 °C; then supernatants were aliquoted and frozen at −70 °C. Cell counts were not assessed in whole blood.
Platelet Isolation
Platelet-rich plasma (PRP) was isolated from whole blood as described using ACD (acid citrate dextrose)-filled tubes [25]. Whole blood was centrifuged at 90×g for 15 min at 22 °C to yield PRP, and then a 1 ml aliquot of PRP was removed and centrifuged at 2000×g for 10 min to yield platelet-poor plasma (PPP). Platelet counts were determined from on PRP on a commercial cell sorter in the hospital clinical lab. WBC counts were similarly measured, and in all cases, isolated PRP had <0.1 × 106 WBC/ml.
Platelet Aggregation
Platelet aggregation was performed in a standard manner as published [25]. Bovine skin collagen, stir bars, and siliconized cuvettes were obtained from Chronolog (Havertown, PA). PRP was adjusted with filtered Tyrode’s buffer to platelet counts of 250,000/ml and to 2 mmol/l CaCl2. After standing at 22 °C for 30 min, 0.5 ml of adjusted PRP diluted was then added to paired cuvettes in a dual chamber lumiaggregometer (Chronolog) and preequilibrated at 37 °C with constant stirring at 900 rpm for 7 min, and then the experiment was begun by the addition of the aggregating agent (collagen) or vehicle (PBS). The final concentration of collagen was selected to achieve maximal aggregation (125 μg/ml). Agonist-induced aggregation was verified by characteristic tracings. As a control, the lack of aggregation after PBS vehicle addition was likewise verified. Maximal aggregation was defined as peak aggregation (in percent) with 100 % aggregation defined by the light transmission of PPP. After 7 min of aggregation, samples were centrifuged at 10,000×g at 4 °C for 10 min, and the supernatants were frozen at −70 °C for later analysis. In quality control experiments using ten healthy subjects, the concentrations of TGF-β and VEGF in the centrifuged supernatants of unaggregated PRP preparations were found to be ≤5 % of the concentrations seen after collagen aggregation, so a significant plasma (nonplatelet) contribution of these cytokines to aggregation release data is unlikely. These techniques are consistent with international society guidelines for length of aggregation using collagen [26] and are modeled after other reports that assessed platelet cytokine secretion [27–29]. In this assay, cytokine release has been shown to be proportional to the degree of platelet aggregation in normal subjects [29, 30].
ELISA
The concentrations of all cytokines were determined in duplicate with commercial ELISA assays (R&D Systems, Inc. Minneapolis, MN, kit DVE00 for VEGF; and Promega, Madison, WI, Emax Immuno Assay System G1230 for TGF-β1). General techniques were as published for VEGF [25]. These assays are sensitive to 9 pg/ml (0.2 pM) VEGF and 32 pg/ml (0.04 pM) TGF-β1. Samples used for VEGF assays were undiluted. Samples for TGF-β1 were diluted 1:50–1:150 to place them in the range of the standard curve and were acidified per manufacturer’s directions to measure total TGF-β1 (active and latent; as opposed to only active TGF-β1). Optical densities were measured on a plate reader and corrected for background. Linear regression from a standard curve was used to determine concentrations. Dilutions were corrected before data analysis. We did not test other substances released from platelets such as thromboglobulin that may be more platelet specific as their role in inflammation and CF is unclear [31].
Statistical Analysis
A nonparametric unpaired student’s t test was used to compare groups. Group data are presented as mean ± SEM or SD, based on normality. Correlations were performed between variables using Pearson or Spearman analyses, as appropriate (based on normality), and significance was determined in relation to degrees of freedom. Released cytokine concentrations from isolated platelets were corrected for platelet count. A multivariate analysis was performed to assess associations of gender, age, and steroid use with outcome variables. All analyses were two-tailed and were performed with GraphPad software (San Diego, CA). Significance was defined as p < 0.05.
Results
Demographics
Features of the study populations are detailed in Table 1. CF subjects were younger and some were using oral corticosteroids (all doses were ≤20 mg/day), compared with control subjects (p < 0.01). All CF subjects were White except for one African-American subject, whereas healthy controls were representative of the more diverse demographics of the hospital staff recruited for this population.
Platelet Count Correlations
Platelet counts were higher in CF patients than in healthy controls (Fig. 1; CF 338 ± 23 cells/ul, Controls 249 ± 16 cells/ul, mean ± SEM; p = 0.002). Mean platelet volume was similar between groups (6.1 ± 0.77 vs. 6.3 ± 0.46 femtoliters, CF vs. control, mean ± SD, p = 0.40). Platelet count was significantly correlated with both serum TGF-β1 and serum VEGF, with the strongest correlation being for TGF-β1 (Fig. 2, TGF-β1: r = 0.59, p = 0.001; VEGF: r = 0.41, p = 0.02). These data for correlations between serum cytokines and platelet number are presented with both CF and control values combined, but those were similar and remained significant when CF and control groups were analyzed separately. Platelet count was not significantly correlated with plasma TGF-β1 or plasma VEGF (r/p values of 0.42/0.13 and 0.13/0.30, respectively). These data indicated that serum levels of TGF-β1 and VEGF are best compared after adjustment for platelet count. Platelet count was not correlated with use of steroids in a multivariate analysis (p = 0.27).
Correlation of platelet number in blood with serum VEGF and TGF-β1 concentrations in cystic fibrosis patients (CF) and healthy controls, shown with 95 % confidence intervals (dotted lines). Each dot represents one individual. p and r coefficient values are in the Results section; both correlations were statistically significant, whereas plasma values were not correlated with platelet counts
Plasma Levels of TGF-β1 and VEGF are Increased in CF
Plasma TGF-β1 and VEGF were higher in CF patients compared with healthy controls (Fig. 3). Plasma TGF-β1 and VEGF levels were not adjusted for platelet counts as plasma levels were not significantly correlated with platelet counts (p values were 0.21 for VEGF and 0.19 for TGF-β1). Plasma VEGF in CF was not correlated with use of steroids (p = 0.42).
Plasma and serum concentrations of VEGF and TGF-β1 as quantified by ELISA (serum samples are shown both unadjusted and after adjustment for platelet counts) for cystic fibrosis patients (CF) and healthy controls (Control). Results are depicted as group mean ± SEM. NS not significant; * p < 0.05; ** p < 0.01
Plasma TGF-β 1 : CF: 28,472 ± 2446 pg/ml, range 16,350–52,987 pg/ml; Controls: 19,396 ± 1440 pg/ml, range 9556–28,280 pg/ml; mean ± SEM, p = 0.002.
Plasma VEGF: CF: 89.5 ± 11.6 pg/ml, range 8–209 pg/ml; Controls: 59.9 ± 7.8 pg/ml, range 16–139 pg/ml; mean ± SEM, p = 0.04.
Serum Levels of TGF-β1 and VEGF are not Increased in CF (When Adjusted for Platelet Counts)
Serum TGF-β1 and serum VEGF (unadjusted for platelet counts) were higher in CF patients compared with healthy controls (Fig. 3). Serum cytokine concentrations in CF were not correlated with use of steroids (p = 0.56).
Unadjusted Serum TGF-β 1 : CF: 79,646 ± 9303 pg/ml, range 30,550–134,535 pg/ml; controls: 53,433 ± 9001 pg/ml, range 18,250–103,747 pg/ml, mean ± SEM, p = 0.026).
Unadjusted Serum VEGF: CF: 481± 46 pg/ml, range 152–955 pg/ml; controls: 344 ± 47 pg/ml, range 67–742 pg/ml, mean ± SEM, p = 0.04).
Since platelet counts were higher in CF patients, to rule out confounding we compared serum TGF-β1 and VEGF after adjusting for platelet number (expressed as the ratio of pg cytokine/106 platelets). Platelet-adjusted serum levels of TGF-β1 and VEGF were not significantly different between CF and healthy controls (Fig. 3, bottom graphs).
Platelet Count-adjusted Serum TGF-β 1 : CF: 243 ± 29 pg/106 platelets (plt), range 107–504 pg/106 plt; controls 255 ± 41 pg/106 plt, range 77–513 pg/106 plt; mean ± SEM, p = 0.86).
Platelet Count-adjusted Serum VEGF: CF: 1.4± 0.1 pg/106 plt, range 0.2–2.4 pg/106 plt; controls 1.5 ± 0.2 pg/106 plt, range 0.1–3.3 pg/106 plt; mean ± SEM, p = 0.53).
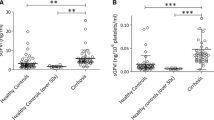
Isolated CF Platelets Release Less TGF-β1 and VEGF During Aggregation
Platelets from CF patients released less TGF-β1 and VEGF after collagen-stimulated maximal aggregation compared with healthy controls (Fig. 4). This occurred despite maximal aggregation being slightly increased in the CF group (76.6 ± 13.5 vs. 69.1 ± 9.3 % of maximal, CF vs. controls, mean ± SD, p = 0.04). Platelet VEGF and TGF-β1 were not correlated with use of steroids (p = 0.29 and 0.19, respectively).
a Release of VEGF and TGF-β1 after collagen-stimulated in vitro platelet aggregation using platelet-rich plasma from cystic fibrosis patients (CF) and healthy controls (Control). Concentrations were measured by ELISA and adjusted to platelet count. Results are depicted as group mean ± SEM; * p < 0.05. b Representative platelet aggregation curves showing aggregation of platelet-rich plasma (PRP) in response to addition (arrows) of collagen or PBS vehicle. In this experiment using samples from a healthy control (channels 1, 3) and from a CF patient (channels 2, 4), there is no aggregation with PBS (channels 1 and 2, stable lines at bottom of figure), but maximal aggregation (gain in light transmission to 70–80 % of the value seen with platelet-poor plasma) is seen 5 min after addition of collagen (channels 3 and 4; upwardly deflecting curves)
TGF-β 1 Release: CF: 121± 15 pg/106 plt, range 62–260 pg/106 plt; controls: 170 ± 23 pg/106 plt, range 102–409 pg/106 plt; mean ± SEM, p = 0.029.
VEGF Release: CF: 0.21 ± 0.05 pg/106 plt, range 0.01–0.55 pg/106 plt; controls: 0.44± 0.07 pg/106 plt, range 0.11–1.03 pg/106 plt; mean ± SEM, p = 0.018.
Discussion
In the current study, we found that isolated platelets from stable CF outpatients secreted less of the proinflammatory/profibrotic cytokines VEGF and TGF-β1 during in vitro aggregation compared with healthy controls. This occurred despite more pronounced aggregation in CF platelets. These data provide further evidence for platelet defects in CF which to date have included increased aggregability, alterations in cell lipids, decreased exocytosis, and decreased integrin binding [4, 5]. Our techniques appear sound as blood cytokine levels, VEGF and TGF-β1 release measured during platelet aggregation, and correlation coefficients of serum VEGF and TGF-β1 with platelet number in healthy controls are typical of those reported in other investigations [22, 24, 32–35].
Our findings are important as a number of investigations have implicated platelets as contributors to the lung inflammation of CF at a level worthy of therapeutic targeting [4, 11, 36]. As the profibrotic cytokine TGF-β1 and the proinflammatory and proangiogenic cytokine VEGF are both highly expressed in platelets, it is logical to suspect that the platelet levels of these cytokines might increase due to systemic inflammation during their formation in bone marrow (since mature platelets do not have a nucleus) and that downstream release of these two cytokines during aggregation may be important in the pathophysiology of CF inflammation. As others have reported, we found platelet counts, maximal platelet aggregation, plasma TGF-β1 and VEGF, and unadjusted serum levels of TGF-β1 and VEGF to be higher in CF patients [5, 24]. However, when we adjusted serum levels for platelet counts, we found no differences between CF and healthy subjects [21]. This differs from the situation seen in cancer patients, where the platelet content of VEGF has also been shown to be increased in subjects with high serum VEGF levels even after adjustment for platelet counts [35]. While our serum and plasma data do not directly inform on platelet behavior, it is of interest that circulating (plasma) levels of these cytokines are elevated despite decreased platelet release in vitro. The elevations in plasma cytokines of stable CF patients that we and others have observed are unlikely to be from platelet release—since plasma levels are obtained from anticoagulated blood, since we observed less cytokine release from isolated platelets, and as plasma cytokine levels were not correlated with platelet counts. Notably in our work plasma concentrations were 20–33 % of serum concentrations of these cytokines, so baseline plasma levels (in unaggregated blood) likely make a “contribution” to serum levels (in ex vivo aggregated blood). Our data suggest that unadjusted serum concentrations of platelet-secreted cytokines are unlikely to be helpful in CF as biomarkers, whereas plasma levels may give some useful information.
These data do not rule out a role for platelets during acute infections in CF, but rather suggest that platelet-secreted TGF-β1 and VEGF are unlikely to have a role in enhancing chronic tissue inflammation in CF. Importantly, the decreased release of VEGF and TGF-β1 we observed during aggregation of CF platelets suggests that decreased synthesis of these cytokines occurs within platelet precursors (megakaryocytes) in the bone marrow, despite the systemic inflammation often seen in CF. These expression of these two cytokines typically increase in most cell lines after inflammatory stimuli.
Our study has limitations. We tested stable CF outpatients, so we cannot be sure that platelet cytokine release is the same during exacerbations or hospitalizations. We did not assess sputum samples for chronic colonization with pseudomonas or other bacteria that could increase the level of inflammation in CF patients. We did not test blood WBC counts to assess whether they were correlated with blood cytokine levels, but we did verify that WBC contamination of platelet suspensions was minimal, and other investigations have shown that blood WBC counts do not typically correlate with these cytokine levels in health or disease [35, 37–39], whereas serum levels of TGF-β1 and VEGF do typically correlate with platelet counts [32].
In conclusion, elevated serum levels of VEGF and TGF-β1 in CF appear to be artifacts of the increased platelet numbers typical of chronic inflammatory illnesses, whereas increased plasma levels of these cytokines may be a useful biomarker of inflammation in CF. Decreased release of these cytokines from CF platelets provides further evidence for platelet abnormalities in CF and underscores that more research is needed to better understand the platelet secretome in CF. Whether such platelet defects are due to direct effects of CFTR dysfunction or indirect effects of chronic inflammation and infection all warrant further study.
Abbreviations
- CF:
-
Cystic fibrosis
- CFTR:
-
Cystic fibrosis transmembrane conductance regulator
- NSAID:
-
Nonsteroidal anti-inflammatory drug
- PRP:
-
Platelet-rich plasma
- PPP:
-
Platelet-poor plasma
- TGF-β1 :
-
Transforming growth factor-beta 1
- VEGF:
-
Vascular endothelial growth factor
References
Rowe SM, Miller S, Sorscher EJ (2005) Cystic fibrosis. New Engl J Med 352(19):1992–2001
Stoltz DA, Meyerholz DK, Welsh MJ (2015) Origins of cystic fibrosis lung disease. New Engl J Med 372(16):1574–1575
O’Sullivan BP, Linden MD, Frelinger AL 3rd et al (2005) Platelet activation in cystic fibrosis. Blood 105(12):4635–4641
O’Sullivan BP, Michelson AD (2006) The inflammatory role of platelets in cystic fibrosis. Am J Respir Crit Care Med 173(5):483–490
Sturm A, Hebestreit H, Koenig C, Walter U, Grossmann R (2010) Platelet proinflammatory activity in clinically stable patients with CF starts in early childhood. J Cyst Fibros 9(3):179–186
Stead RJ, Barradas MA, Mikhailidis DP, Jeremy JY, Hodson ME, Batten JC, Dandona P (1987) Platelet hyperaggregability in cystic fibrosis. Prostaglandins Leukot Med 26(2):91–103
Agam G, Aviram M, Zilberman-Kaufman M, Rothstein A, Livne AA (1995) Cyclic AMP-related and cation-affected human platelet chloride transport regulation. Eur J Clin Chem Clin Biochem 33(6):329–335
Mattoscio D, Evangelista V, De Cristofaro R et al (2010) Cystic fibrosis transmembrane conductance regulator (CFTR) expression in human platelets: impact on mediators and mechanisms of the inflammatory response. FASEB J 24(10):3970–3980
Falco A, Romano M, Iapichino L, Collura M, Davi G (2004) Increased soluble CD40 ligand levels in cystic fibrosis. J Thromb Haemost 2(4):557–560
Mikhailidis DP, Stead RJ, Barradas MA, Hodson ME, Batten JC, Dandona P (1990) Platelet abnormalities in patients with cystic fibrosis and obligate heterozygotes. Haematologica 75(2):137–140
Zhao C, Su EM, Yang X, Gao Z, Li L, Wu H, Jiang Y, Su X (2013) Important role of platelets in modulating endotoxin-induced lung inflammation in CFTR-deficient mice. PLoS One 8(12):e82683
Konig B, Jaeger KE, Konig W (1994) Induction of inflammatory mediator release (12-hydroxyeicosatetraenoic acid) from human platelets by Pseudomonas aeruginosa. Int Arch Allergy Immunol 104(1):33–41
Uysal P, Tuncel T, Olmez D, Babayigit A, Karaman O, Uzuner N (2011) The role of mean platelet volume predicting acute exacerbations of cystic fibrosis in children. Ann Thorac Med 6(4):227–230
Hasselbalch HC (2012) Perspectives on chronic inflammation in essential thrombocythemia, polycythemia vera, and myelofibrosis: is chronic inflammation a trigger and driver of clonal evolution and development of accelerated atherosclerosis and second cancer? Blood 119(14):3219–3225
Schweighofer B, Testori J, Sturtzel C, Sattler S, Mayer H, Wagner O, Bilban M, Hofer E (2009) The VEGF-induced transcriptional response comprises gene clusters at the crossroad of angiogenesis and inflammation. Thromb Haemost 102(3):544–554
Taylor AW (2009) Review of the activation of TGF-beta in immunity. J Leukoc Biol 85(1):29–33
Watts KD, McColley SA (2011) Elevated vascular endothelial growth factor is correlated with elevated erythropoietin in stable, young cystic fibrosis patients. Pediatr Pulmonol 46(7):683–687
McColley SA, Stellmach V, Boas SR, Jain M, Crawford SE (2000) Serum vascular endothelial growth factor is elevated in cystic fibrosis and decreases with treatment of acute pulmonary exacerbation. Am J Respir Crit Care Med 161(6):1877–1880
Tirelli AS, Colombo C, Torresani E et al (2013) Effects of treatment in the levels of circulating cytokines and growth factors in cystic fibrosis and dialyzed patients by multi-analytical determination with a biochip array platform. Cytokine 62(3):413–420
Eickmeier O, Boom L, Schreiner F et al (2013) Transforming growth factor beta1 genotypes in relation to TGFbeta1, interleukin-8, and tumor necrosis factor alpha in induced sputum and blood in cystic fibrosis. Mediat Inflamm 2013:913135
Harris WT, Muhlebach MS, Oster RA, Knowles MR, Clancy JP, Noah TL (2011) Plasma TGF-beta(1) in pediatric cystic fibrosis: potential biomarker of lung disease and response to therapy. Pediatr Pulmonol 46(7):688–695
Zimmermann R, Koenig J, Zingsem J, Weisbach V, Strasser E, Ringwald J, Eckstein R (2005) Effect of specimen anticoagulation on the measurement of circulating platelet-derived growth factors. Clin Chem 51(12):2365–2368
Jelkmann W (2001) Pitfalls in the measurement of circulating vascular endothelial growth factor. Clin Chem 47(4):617–623
Schwarz KB, Rosensweig J, Sharma S, Jones L, Durant M, Potter C, Narkewicz MR (2003) Plasma markers of platelet activation in cystic fibrosis liver and lung disease. J Pediatr Gastroenterol Nutr 37(2):187–191
Maloney JP, Silliman CC, Ambruso DR, Wang J, Tuder RM, Voelkel NF (1998) In vitro release of vascular endothelial growth factor during platelet aggregation. Am J Physiol 275(3 Pt 2):H1054–H1061
Cattaneo M, Cerletti C, Harrison P et al (2013) Recommendations for the standardization of light transmission aggregometry: a consensus of the working party from the platelet physiology subcommittee of SSC/ISTH. J Thromb Haemost 11:1183–1189
Jonnalagadda D, Izu LT, Whiteheart SW (2012) Platelet secretion is kinetically heterogeneous in an agonist-responsive manner. Blood 120(26):5209–5216
Ollivier V, Syvannarath V, Gros A, Butt A, Loyau S, Jandrot-Perrus M, Ho-Tin-Noe B (2014) Collagen can selectively trigger a platelet secretory phenotype via glycoprotein VI. PLoS One 9(8):e104712
Kato H, Adachi S, Doi T et al (2010) Mechanism of collagen-induced release of 5-HT, PDGF-AB and sCD40L from human platelets: role of HSP27 phosphorylation via p44/p42 MAPK. Thromb Res 126(1):39–43
Coppinger JA, O’Connor R, Wynne K et al (2007) Moderation of the platelet releasate response by aspirin. Blood 109(11):4786–4792
Zhang C, Thornton MA, Kowalska MA et al (2001) Localization of distal regulatory domains in the megakaryocyte-specific platelet basic protein/platelet factor 4 gene locus. Blood 98(3):610–617
Kropf J, Schurek JO, Wollner A, Gressner AM (1997) Immunological measurement of transforming growth factor-beta 1 (TGF-beta1) in blood; assay development and comparison. Clin Chem 43(10):1965–1974
Okuda K, Kawase T, Momose M, Murata M, Saito Y, Suzuki H, Wolff LF, Yoshie H (2003) Platelet-rich plasma contains high levels of platelet-derived growth factor and transforming growth factor-beta and modulates the proliferation of periodontally related cells in vitro. J Periodontol 74(6):849–857
Salgado R, Benoy I, Bogers J, Weytjens R, Vermeulen P, Dirix L, Van Marck E (2001) Platelets and vascular endothelial growth factor (VEGF): a morphological and functional study. Angiogenesis 4(1):37–43
Salven P, Orpana A, Joensuu H (1999) Leukocytes and platelets of patients with cancer contain high levels of vascular endothelial growth factor. Clin Cancer Res 5(3):487–491
Pieroni L, Finamore F, Ronci M et al (2011) Proteomics investigation of human platelets in healthy donors and cystic fibrosis patients by shotgun nUPLC-MSE and 2DE: a comparative study. Mol BioSyst 7(3):630–639
Liem LM, Fibbe WE, van Houwelingen HC, Goulmy E (1999) Serum transforming growth factor-beta1 levels in bone marrow transplant recipients correlate with blood cell counts and chronic graft-versus-host disease. Transplantation 67(1):59–65
Di Raimondo F, Azzaro MP, Palumbo GA et al (2001) Elevated vascular endothelial growth factor (VEGF) serum levels in idiopathic myelofibrosis. Leukemia 15(6):976–980
Enjoji M, Nakamuta M, Yamaguchi K et al (2005) Clinical significance of serum levels of vascular endothelial growth factor and its receptor in biliary disease and carcinoma. World J Gastroenterol 11(8):1167–1171
Acknowledgments
We thank the cystic fibrosis patients and healthy subjects who volunteered for this study. This work was supported by Grant K08 HL035454 from the United States National Institutes of Health, National Heart Lung, and Blood Institute (JM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None.
Rights and permissions
About this article
Cite this article
Maloney, J.P., Narasimhan, J. & Biller, J. Decreased TGF-β1 and VEGF Release in Cystic Fibrosis Platelets: Further Evidence for Platelet Defects in Cystic Fibrosis. Lung 194, 791–798 (2016). https://doi.org/10.1007/s00408-016-9925-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-016-9925-9