Abstract
Purpose
The objective of this study was to measure plasma cytokine levels and blood neutrophil functions as well as clinical outcomes in hospitalized patients with community-acquired pneumonia (CAP) treated with or without macrolide use—a known modulator of inflammatory response.
Methods
Subjects with CAP had peripheral blood analyzed for some neutrophil functions (degranulation of secretory vesicles and specific granules, respiratory burst response and phagocytosis) and ten cytokine levels measured in serum and sputum supernatants. Neutrophil function in healthy volunteers was also measured for reference. Values were measured on the day of enrollment, days 2–4 and 5–7, depending on a patient’s length of stay. Early and late clinical outcomes were also evaluated. All values were compared between those treated with or without a macrolide.
Results
A total of 40 subjects were in this study; 14 received macrolide treatment, and 26 did not. Neutrophil function in the macrolide group was not significantly different compared to the non-macrolide group. None of the median cytokine levels or IQRs were statistically significant between the groups. However, a trend toward decreased IL-6, IL-8, and IFN-γ levels, and favorable clinical outcomes were present in the macrolide group.
Conclusions
This pilot study showed no statistical difference between cytokine levels or neutrophil activity for CAP patients prescribed a macrolide containing regimen. Considering the trend of lower cytokine levels in the macrolide group when comparing the 5- to 7-day time period with the non-macrolide group, a full study with an appropriate sample size may be warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Community-acquired pneumonia (CAP) has been one of the top ten causes of death in the US over the last 60 years despite the widespread use of antimicrobials [1]. Recommended treatment by the Infectious Diseases Society of America/American Thoracic Society is either a β-lactam plus a macrolide or a respiratory quinolone [2]. Whether one regimen should be recommended over the other is unknown as both cover Streptococcus pneumoniae and atypical pathogens, such as Legionella pneumophila, but only macrolides have been reported to have modulatory effects on host inflammation and immunity, including the ability to (1) suppress production of pro-inflammatory cytokines [3], (2) to interfere with the attachment of pneumococci from respiratory epithelial cells [4], and (3) to favor apoptosis over necrosis in the presence of neutrophils [5]. However, it is unclear whether macrolides affect neutrophil function and cytokine levels in patients with CAP.
To establish a clinical link with immunomodulation, immune function needs measured in patients whose clinical outcomes are also being recorded. This has never been coordinated previously. With this in mind, we performed this pilot study with the objective to characterize the immune function (neutrophil function and cytokine levels) as well as the clinical outcomes for hospitalized patients with CAP treated with or without a macrolide.
Methods
Study Design, Participant Recruitment, and Sample Collection
This was a prospective study by members of the Community-Acquired Pneumonia Inflammatory Study Group (CAPISG) at the University of Louisville Hospital and the Robley Rex Veterans Affairs Medical Center in Louisville, KY from February 14, 2011 until April 14, 2012. Informed consent was obtained from all individual participants included in this study. Institutional review board approval was obtained from each institution (IRB #07.0182, IRB #0009, respectively). Patients with CAP were consented to have their blood drawn for neutrophil function and cytokine levels, and then were observed on antimicrobial treatment prescribed by their admitting physician. Patients were identified by screening the respective emergency department admission logs for patients with CAP. Once a patient was consented (within 48 h of admission), blood was obtained on day one. Their blood was collected zero to two more times during the first week while they were in hospital depending on their length of stay. The blood was transported to the lab within an hour for analysis of neutrophil function. Another portion was centrifuged and frozen for subsequent batched cytokine measurement.
Clinical data were collected by chart review including demographic information, pneumonia severity index, comorbidities, nursing home status, and influenza and pneumococcal vaccination history. Basic laboratory values were collected, including C-reactive protein and procalcitonin, as well as treatment information.
Outcomes
Two types of mortality were recorded: in-hospital mortality and 30-day mortality. Rehospitalization was defined within 30 days. Length of stay (LOS) was defined as the number of days spent in the hospital including the day of admission. Time to clinical stability was defined as the number of days to fulfill all four of the following criteria: afebrile for at least 8 h, having a normal leukocyte count (4.1–10.8 cells × 1000/mL3) or a decreased leukocyte count compared to the previous day by at least 10 %, maintaining oral toleration, and being clinically improved per the managing physician. Early time to clinical stability was considered to be less than 3 days.
Neutrophil Assays
Neutrophil function/activation was determined in whole blood by measuring basal secretory vesicle (CD35) and specific granule (CD66b) exocytosis by flow cytometry as described [6]. Briefly, whole blood samples from subjects with CAP or from healthy donors were incubated with phycoerythrin-conjugated monoclonal anti-CD35 (Pharmingen, SanDiego, CA) or fluorescein isothiocyanate-conjugated monoclonal anti-CD66b (Accurate Chemical, Westubury, NY). Following antibody treatment, the red blood cells were lysed (BD red cell lysis buffer; Becton, Dickinson and Company (BD), Franklin Lakes, NJ), washed with phosphate-buffered saline solution containing 0.05 % sodium azide, and fixed with 1 % paraformaldehyde and analyzed by flow cytometry (FACSCalibur instrument; BD, Franklin Lakes, NJ) [6, 7]. In addition, neutrophil phagocytosis and phagocytosis-stimulated respiratory burst activity were measured as previously described [8]. For analysis purposes, blood samples taken at days two through four were grouped as 2–4, and for patients with a longer hospitalization of 5–7 days, blood samples were collected and grouped as 5–7. In order to compare results of the neutrophil functional assays from CAP patients with those of healthy individuals, blood samples were also obtained from a control group (n = 12) of healthy adult donors (approved by the University of Louisville IRB #191.06).
Cytokine and Chemokine Analysis
A total of ten cytokines were measured in plasma samples obtained from each patient on the day of enrollment and for some on subsequent days. The pro-inflammatory cytokines evaluated were IL-1β, IL-6, IL-8, IL-12p40, IL-17, inducible protein (IP)-10, TNF-α, and IFN-γ. The anti-inflammatory cytokines evaluated were IL-10 and IL-1ra. Cytokine levels were measured in plasma and sputum using a Milliplex kit (Millipore, Billerica, MA) as described previously [9].
Sputum samples were collected from a total of 15 patients (seven treated with macrolide, eight with non-macrolides) on the day of hospital admission. Patients were instructed to rinse their mouth with water and collect any spontaneously produced sputum into a 50-mL sterile disposable polypropylene centrifuge tube. The sample was kept at 4 °C and transported to the laboratory within 2 h of collection. Upon receipt, sputum samples were weighed and incubated with four times volume of 0.1 % dithiothreitol solution in phosphate-buffered saline, aspirated, and dispensed several times using a disposable pipette and agitated in a vortex followed by being incubated in a rocking platform for 15 min at 37 °C with aspiration every 5 min for homogenization of the mucus. The samples were then filtered through a 48-µm of mesh or cheese cloth into a pre-weighed conical tube, followed by centrifugation at 790 gravity for 10 min at room temperature, and the cell-free supernatants were then aliquoted and stored frozen at −80 °C until used for the measurement of cytokine levels.
Statistics
Comparison of clinical outcomes between subjects in the macrolide group and the non-macrolide group was performed using Fisher’s Exact test for categorical data, and Mann–Whitney U test for continuous data. A P value of <0.05 was considered significant. Analyses were performed for the following time periods: enrollment day, days 2–4, and days 5–7. Median values with interquartile ranges (IQR) were also performed for the two groups for the latter time period: days 5–7.
Results
Among a total of 40 patients, 14 were treated with a macrolide (azithromycin in all cases), while 26 were not. Mean ages were similar between the two groups (52 vs. 60 years; P = 0.178). Demographic data are compared in Table 1, which show no statistically significant differences. Other relevant values are in Table 2. None of the comparisons were statistically significant except that a higher proportion of patients who received a non-macrolide treatment had a pleural effusion (zero vs. 12 (46 %); P = 0.003). Nine patients had S. pneumoniae identified. Three of those patients were treated with a macrolide. Among those three cases, one isolate was sensitive to azithromycin, and two had been diagnosed based on a positive S. pneumoniae urinary antigen, and therefore, no antimicrobial sensitivity analysis data were performed. The remaining six patients with S. pneumoniae were in the non-macrolide group. Two isolates were intermediately sensitive to azithromycin, one was resistant, and three were diagnosed based on a positive S. pneumoniae urinary antigen.
At admission day, exocytosis of secretory vesicles (CD35 expression) and specific granules (CD66b expression) were significantly higher than the control group for all subjects with CAP, irrespective of the antibiotic treatment. Both phagocytosis and respiratory burst activity were also significantly higher in the CAP group compared to control group. Overall, in vitro assays performed with circulating neutrophils from patients diagnosed with CAP showed enhanced neutrophil degranulation, phagocytosis, and respiratory burst activity (Fig. 1). However, no statistical differences were observed for any neutrophil function between the macrolide and non-macrolide groups in the pilot study.
Basal neutrophil activation measured among two groups of subjects with community-acquired pneumonia: those treated with a macrolide (M) or a non-macrolide (NM) containing regimen. Values were taken over three time periods: admission, days 2–4, and days 5–7. Measurements were made of plasma membrane expression of a CD35, b CD66b, c phagocytosis-stimulated respiratory burst, and d phagocytosis. The dashed lines represent median values for healthy volunteers. Data are expressed as box plots, where the horizontal lines represent the median of mean fluorescence intensity (MFI), and the lower and upper ends of the box represent the 25 and 75 % percentiles, respectively. Dots represent patients outside of 1.5 interquartile ranges of the lower and upper quartiles
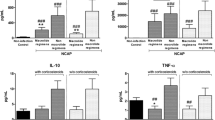
The plasma levels of pro- and anti-inflammatory cytokines of each group over three time periods are shown in Fig. 2. Although, there were again no statistical differences between groups for cytokine levels or between the median values and the IQR, four cytokines trended lower in the macrolide group for IFN-γ, IL-6, IL-8, and IL-12p40 when comparing the later time period, 5–7 days, to the non-macrolide group. Figure 3 shows cytokine values from sputum. All values were similar between macrolide and non-macrolide groups, but no control values were available.
Clinical outcomes were compared for each group (Table 3). Three patients in the non-macrolide group died during their hospitalization (P = 0.539), one of whom a month after being admitted. The difference of three deaths to zero was not significant in the present study.
Discussion
This is the first CAP study known to the authors designed to assess the effects of macrolide treatment on two innate immunity components and to evaluate the clinical outcomes in the same patients. In our study, the time to clinical stability, length of stay, and mortality approached significant improvement in the macrolide group, as was expected since large observational studies evaluating clinical outcomes alone (without innate immunity analysis) found improved clinical outcomes in patients who received macrolide therapy [10–12]. In the macrolide arm of the present study, there were no deaths, LOS was shorter by 2 days, and time to clinical stability was shorter by a half day. In terms of potential responsible mechanisms, the levels of several cytokines (IL-6, IL-8, and IFN-γ) in the macrolide group approached significantly lower levels. Although the present study was not powered for significant differences, our results are consistent with evidence that favor the macrolide group over the non-macrolide group and warrant a larger study focusing on cytokines and clinical outcomes.
The modulatory effects of macrolides on innate immunity cytokines have been investigated by a number of studies, both experimental animals and humans with varying results [3, 4, 13–16]. Several studies have shown a benefit in clinical outcomes in patients with CAP who received a macrolide [11, 17, 18]. Furthermore, there is evidence to support the notion that macrolide immunomodulation alone treats CAP in patients [17]. In patients with severe CAP, 90-day mortality was significantly better in patients who received initial treatment with a macrolide [12 vs. 34 %; hazard ratio (HR) of 0.3; 95 % confidence interval (CI) 0.2–0.6; P = 0.001], even in those patients who were infected with a pathogen resistant to a macrolide [12, 19].
The major implication of knowing what the mechanism of macrolide immunomodulation is, is to determine if it enhances antimicrobial activity or not. If a benefit is attributable to immunomodulatory effects, then it would justify using a macrolide preferentially over a fluoroquinolone, despite the resistance pattern. Such a finding would justify changing the IDSA/ATS guidelines for CAP.
The present study was strengthened by the diagnosis of CAP being supported by an increased neutrophil function in the blood compared to controls and by the cytokine levels in the sputum. The innate immunity components of patients were also linked to their clinical outcomes, which has not been done in human studies until now. As this is a pilot study, generalizability was limited. Although cytokines were evaluated in both serum and sputum specimens, neutrophil function was only evaluated from circulating neutrophils.
In conclusion, patients treated with a macrolide had four cytokines trend lower, and had more favorable outcomes compared to patients treated with a non-macrolide. The present study indicates that future studies may reveal a statistically significant difference.
References
Hoyert DL, Xu J (2012) Deaths: preliminary data for 2011. Natl Vital Stat Rep 61:38
Mandell LA, Wunderink RG, Anzueto A et al (2007) Infectious diseases Society Of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44(suppl2):S27–S72
Demartini G, Esposti D, Marthyn P et al (2004) Effect of multiple doses of clarithromycin and amoxicillin on IL-6, IFN-γ and IL-10 plasma levels in patients with community-acquired pneumonia. J Chemther 16:82–85
Lagrou K, Peetermans WE, Jorissen M et al (2000) Subinhibitory concentrations of erythromycin reduce pneumococcal adherence to respiratory epithelial cells in vitro. J Antimicrob Chem 46:717–723
Koch CC, Esteban DJ, Chin AC et al (2000) Apoptosis, oxidative metabolism and interleukin-8 production in human neutrophils exposed to azithromycin: effects of streptococcus pneumoniae. J Antimicrob Chem 46:19–26
Uriarte SM, Rane MJ, Luerman GC et al (2011) Granule exocytosis contributes to priming and activation of the human neutrophil respiratory burst. J Immunol 187:391–400
Haslett C, Guthrie LA, Kopaniak MM et al (1985) Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol 119:101–110
Ward RA, Nakamura M, McLeish KR (2000) Priming of the neutrophil respiratory burst involves p38 mitogen-activated protein kinase-dependent exocytosis of flavocytochrome b558-containing granules. J Biol Chem 275:36713–36719
Fernandez-Botran R, Uriarte SM, Arnold FW et al (2014) Contrasting inflammatory responses in severe and non-severe community-acquired pneumonia. Inflammation 37:1158–1166
Khair OA, Devalia JL, Abdelaziz MM et al (1995) Effect of erythromycin on haemophilus influenzae endotoxin-induced release of il-6, il-8 and sicam-1 by cultured human bronchial epithelial cells. Eur Resp J 8:1451–1457
Rios AM, Mejias A, Chavez-Bueno S et al (2004) Impact of cethromycin (abt-773) therapy on microbiological, histologic, immunologic, and respiratory indices in a murine model of mycoplasma pneumoniae lower respiratory infection. Antimicrob Agents Chemother 48:2897–2904
Rios AM, Fonseca-Aten M, Mejias A et al (2005) Microbiologic and immunologic evaluation of a single high dose of azithromycin for treatment of experimental mycoplasma pneumoniae pneumonia. Antimicrob Agents Chemother 49:3970–3973
Rodrigo C, Mckeever TM, Woodhead M et al (2013) Single versus combination antibiotic therapy in adults hospitalised with community acquired pneumonia. Thorax 68:493–495
Arnold FW, Summersgill JT, Lajoie AS et al (2007) A worldwide perspective of atypical pathogens in community-acquired pneumonia. Am J Resp Crit Care Med 175:1086–1093
Kovaleva A, Remmelts HHF, Rijkers GT et al (2012) Immunomodulatory effects of macrolides during community-acquired pneumonia. J Antimicrob Chemother 67:530–540
Restrepo MI, Mortensen EM, Waterer GW et al (2009) Impact of macrolide therapy on mortality for patients with severe sepsis due to pneumonia. Eur Resp J 33:153–159
Martin-Loeches I, Lisboa T, Rodriguez A et al (2010) Combination antibiotic therapy with macrolides improves survival in intubated patients with community-acquired pneumonia. Intensive Care Med 36:612–620
Asadi L, Sligl WI, Eurich DT et al (2012) Macrolide-based regimens and mortality in hospitalized patients with community-acquired pneumonia: a systematic review and meta-analysis. Clin Infect Dis 55:371–380
Yanagihara K, Izumikawa K, Higa F et al (2009) Efficacy of azithromycin in the treatment of community-acquired pneumonia, including patients with macrolide-resistant streptococcus pneumoniae infection. Internal Med 48:527–535
Acknowledgments
This material is based upon work approved by the University of Louisville Hospital and the Department of Veteran Affairs Offices of Research and Development, as well as the Veterans Health Administration. This work was unfunded.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no disclosures.
Research Involving Animal and Human Rights
This research involved humans.
Informed Consent
Informed consent was obtained.
Additional information
For the Community-Acquired Pneumonia Inflammatory Study Group.
Rights and permissions
About this article
Cite this article
Arnold, F.W., Bordon, J., Fernandez-Botran, R. et al. Macrolide Use and Neutrophil Function/Cytokine Levels in Hospitalized Patients with Community-Acquired Pneumonia: A Pilot Study. Lung 194, 155–162 (2016). https://doi.org/10.1007/s00408-015-9822-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-015-9822-7