Abstract
Background
Extrapulmonary tuberculosis has been an AIDS-defining condition. Individual studies that highlight the association between HIV and extrapulmonary TB are available. Our objectives were to synthesis evidence on the association between extrapulmonary tuberculosis and HIV and to explore the effective preventive measures of these two diseases.
Methods
This is a meta-analysis of observational studies reporting effect estimates on how HIV is associated with extrapulmonary tuberculosis. We searched for the eligible studies in the electronic databases using search terms related to HIV and extrapulmonary tuberculosis. Where possible, we estimated the summary odds ratios using random effects meta-analysis. We stratified analysis by the type of study design. We assessed heterogeneity of effect estimates within each group of studies was assessed using I 2 test.
Results
Nineteen studies (7 case control studies and 12 cohort studies) were identified for the present study. The pooled analysis shows a significant association between HIV and extrapulmonary tuberculosis (summary odds ratio: 1.3; 95 % confidence interval (CI) 1.05–1.6; I 2: 0 %). In a subgroup analysis with two studies, a significant association was found between CD4+ count less than 100 and the incidence of extrapulmonary tuberculosis (summary OR: 1.31; 95 % CI 1.02–1.68; I 2: 0 %).
Conclusions
Findings show evidence on the association between extrapulmonary tuberculosis and HIV, based on case control studies. Further studies to understand the mechanisms of interaction of the two pathogens are recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coexistent tuberculosis (TB) and HIV are known to make TB control difficult especially in high HIV settings. Of the 8.8 million incident cases of TB in 2010, a best estimate of 13 % (1.1 million) are people living with HIV infection (PLH) [1] in resource-limited countries. Both TB and HIV have profound effects on the immune system, because they are capable of disarming the host’s immune responses through mechanisms that are not fully understood [2].
Generally, a relatively small proportion of people infected with Mycobacterium tuberculosis will develop TB disease [1]. As such, clinically apparent TB develops in approximately 10 % of infected patients, soon after primary infection or years later via reactivation [3]. TB presents with an array of clinical manifestations. It typically affects the lungs (pulmonary TB, PTB) but can affect other sites as well (extrapulmonary TB, EPTB) [1]. A patient with EPTB is defined as a patient with TB of organs other than the lungs (e.g., pleura, lymph nodes, abdomen, genitourinary tract, skin, joints and bones, meninges) [4].
Of note is that EPTB has been an AIDS-defining condition, indicating clinical stage 4 in adults [4, 5]. In countries, such as the United States, where PTB and EPTB case counts have both decreased, EPTB has increased as percentage of total TB cases [5]. Such disproportionate increase highlights the needs to understand the factors contributing to EPTB. However, the increasing rate of EPTB also was reported from countries, such as Saudi Arabia, where prevalence of HIV is relatively very low [6].
The Millennium Development Goals target, which is endorsed by the “Stop TB Partnership” is to reduce the prevalence of TB and to reduce deaths due to TB by 50 % by the year 2015 compared with a baseline level of 1990 [1, 7]. To achieve this target, one component is to scale-up collaborative TB/HIV activities [1, 3]. Although many studies focus on PTB, it appears that studies that address EPTB are relatively limited. Our objectives were to synthesize evidence on the association between EPTB and HIV and to explore the effective preventive measures of these two diseases.
Methods
Search Strategy and Study Selection
To identify eligible studies that address the association between HIV and EPTB, we searched MEDLINE (from 1980 to December 2011) and EMBASE (from 1980 to December 2011) using the following combination of search terms:
#1 extrapulmonary tuberculosis
#2 extrapulmonary TB
#3 gastrointestinal tuberculosis
#4 genitourinary tuberculosis
#5 pleural tuberculosis
#6 #1 OR #2 OR #3 OR #4 OR #5
#7 HIV infections
#8 human immunodeficiency virus
#9 risk factors
#10 cohort study
#11 case control study
#12 epidemiologic study
#13 observational study
#14 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13
#15 #6 AND #14
We also searched the abstracts of the conferences of the International TB Society (inception to March 2011) and the references of retrieved papers for additional references. Searches were limited to articles about human studies published in English-language journals. For this review, EPTB refers to TB of organs other than the lungs as indicated following WHO criteria [4]. We updated our searches on July 27, 2012.
Inclusion criteria of the studies were met if they (1) were of case–control, cohort, or cross-sectional design; (2) included a comparator group having isolated PTB; (3) include exposure of interest that was serologically confirmed HIV; (4) provided or computed data on relative risk (RR), odds ratio (OR), or hazard ratio (HR) with corresponding 95 % confidence interval (CI); and (5) had performed stratification by age groups. If data were duplicated in more than one study, the most relevant study was included in the present analysis.
Data Extraction
All searches were conducted independently by two investigators. The same two authors independently extracted data on the name of the first author, year of publication, country, study design, number of exposed/unexposed people or cases/controls, adjusted ORs or RRs with 95 % CI, adjustment factors, and methods of confirmation of HIV and EPTB. Any discrepancy was resolved by consensus, and if needed, by consultation with the third author.
Statistical Analyses
We assume that the RR from cohort studies approximates the ORs from case–control studies [8] and HR from cohort studies. We stratified analysis by the type of study design. We assessed heterogeneity of effect estimates within each group of studies using I 2 test, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance [9]. For those studies that reported age, gender, race/ethnicity, or birthplace stratified-specific effects, we entered the ratio measures of the (adjusted) effect as a log OR and the standard error of the log OR using generic inverse-variance weighting method [9], and then included this summary estimate in the meta-analyses.
For robustness of results, we performed sensitivity analyses by the studies with isolated EPTB. To investigate the potential publication bias, we visually examined the funnel plots. Data entry and analyses were carried out with RevMan 5.1.1 (The Nordic Cochrane Centre, 2011).
Results
Description of Studies
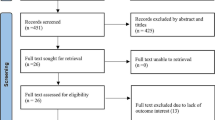
Figure 1 provides the flow chart indicating the literature review process. Based on the inclusion criteria, 45 full articles were retrieved and 19 of these were included in final analysis. Some studies were excluded for the following reasons: (1) no provision of specific data on EPTB [10–17]; (2) information on EPTB was insufficient [18–21]; and (3) there was no comparison group for EPTB [22–25]; (4) there were no HIV-positive patients [6, 26–34]. All of these 19 studies were published in English. Table 1 presents the characteristics of included studies. These included 7 case control studies (36.8 %) and 12 cohort studies (63.2 %). Seven studies (36.8 %) were performed in the United States [5, 35–40], whereas two (10.5 %) were in Spain [41, 42] and one each (5.3 %) in Africa [43], India [44], Taiwan [45], France [46], Hong Kong [47], Nepal [48], Netherland [49], Switzerland [50], South African [51], and Thailand [52]. Of total TB cases, the highest proportion of EPTB (48.5 %) was reported in Nepal [48], whereas the lowest 12.1 % was in the United States [40]. Regarding the localization of EPTB as reported in the studies that were included for analysis, lymph node involvement was the most frequent site of EPTB [5, 35, 36, 38, 42, 48, 49, 52], followed by pleura [38, 47], bones and joints [40, 45], and urogenital regions [43]. Two studies identified for the current analysis did not mention the specific sites of EPTB infection [41, 46].
Effect Estimations
Figure 2 summarizes the adjusted effect estimates of the 19 studies categorized by the study designs. We did not report the summary effect estimates of the cohort studies due to substantial heterogeneity among studies (I 2: 100 %). The forest plot shows a significant association between HIV and EPTB in the case–control studies regardless of age (summary OR: 1.2; 95 % CI 1.13–1.28; I 2: 0 %).
To identify the relationship between CD4+ counts and the development of EPTB, a subgroup analysis was done with two studies [49, 50], based on available data. A significant association was found between CD4+ count less than 100 and the incidence of EPTB (summary OR: 1.31; 95 % CI 1.02–1.68; I 2: 0 %).
Sensitivity Analysis
After removing a study with children [51], the significant association between HIV and EPTB in the case–control studies was summary OR: 1.3; 95 % CI 1.05–1.6 I 2: 0 %. Regarding the studies having isolated EPTB [5, 37, 46, 47], we could not make the summary estimation because of a substantial heterogeneity among studies (I 2: 100 %).
Regarding the summary measures of the association between EPTB and HIV by site of EPTB, based on the data from four studies [38, 39, 43, 49], the involvement of pleura was only significantly lower risk in HIV-infected EPTB than that of non-HIV-infected patients in a study [48] (OR: 0.76; 95 % CI 0.63–0.82). Based on data from two studies [38, 49], there is no significant increase risk of foreign-born males having HIV-infected EPTB (summary OR: 1.02; 95 % CI: 0.06–1.75; I 2: 0 %; Fig. 2). Funnel plot analysis detected publication bias as the shape of the plots seemed asymmetrical (Fig. 3).
Discussion
EPTB refers to TB outside of the lungs [1, 49]. Studies have reported that although EPTB and PTB case counts have both decreased [5, 49], the reduction in case of EPTB has been smaller [42], resulting in a proportional increase in EPTB compared with PTB [40, 49]. Factors perpetuating EPTB may be poorly recognized compared with those perpetuating PTB [5]. The present study showed evidence on the association between EPTB and HIV based on observational studies. This relationship has biological plausibility and could be explained in the current understanding of pathogenesis of TB. Acquired specific responses to TB is mediated by the orchestrated expansion of cell mediated immunity, which seeks to control (influx of cell mediating portion) and contain (florid influx of macrophages to wall off the infection) [53]. In this context, CD4+ T cell subsets, such as Th 17 cell and FoxP3+ regulatory CD4+ cells, seem to play an important role in containing overall inflammatory response [2, 53] to M. tuberculosis antigens. During the emergence of acquired specific responses to mycobacterial infection, the cellular response is mediated by the production of large number of cytokines and chemokines, leading to granulomatous inflammatory responses [53] where M. tuberculosis is contained and thus potentially prevented from causing active diseases [2, 53]. CD4+ T cells and TNF are important to maintain granuloma organization [2]. The hallmark of HIV infection is the depletion of CD4+ T cells. A dysfunctional T cell population displays loss of functional potential. Hence, granuloma formation may fail in an individual with a compromised immune system. As a result, whereas the majority of adult TB is preferentially confined to the lungs due to the aerobic nature of M. tuberculosis, which grows most successfully in tissues with high oxygen content [54], in HIV-infected patients, TB can be a systemic disease involving multiple organs that lack well-defined granulomas [2]. Thus, all forms of EPTB have been described in patients with HIV [2], and as immunosuppression progresses, EPTB becomes increasingly common [55]. Patients with CD4+ count less than 100 who had a higher incidence of EPTB in the present analysis did support this evidence. Also, a study in the United States assessing 320 EPTB cases during a 12-year study period, among HIV-infected patients demonstrated that severe forms of EPTB, such as CNS/meningeal, were independently associated with low CD4+ T cell counts [25].
The current study showed that the involvement of lymph node was the most frequent site of HIV-infected EPTB, followed by pleura. EPTB is a protean disease that can involve virtually all organs, including relatively inaccessible sites [37]. It also has been reported that gender differences exist in relation to the common sites of occurrence of EPTB. Lymph node EPTB involvement tends to be more common in women, whereas pleural EPTB is seen more in men [46]. Common wisdom holds, for instance, that pleural TB is a marker of recent acquisition of infection [26, 39]. However, given the importance of the integrity of the delayed-type hypersensitivity response, TB pleuritis would be expected to be more common in HIV seronegative persons and persons with high CD4+ cell counts in the HIV seropositive [39]. Previously published individual studies demonstrated a possible relationship between HIV and EPTB in which cases with concurrent PTB and EPTB were recorded as EPTB in these studies [35, 45]. This may be attributed to the effect of HIV infection on an individual’s immune system. Of note is that the progressive decline in immunity may have resulted in the progression of PTB to EPTB [45].
An association exists between EPTB and HIV, which we identified, but it is weak due to the variation in diagnosis and/or defining criteria for EPTB and/or confirmation of HIV status among studies identified for the present review. For instance, some studies that identified patients as having EPTB also included patients with concomitant PTB and EPTB [37, 52], whereas some studies restricted inclusion to patients with isolated EPTB [5, 39, 48, 49]. The impact of this inconsistency in definition of EPTB was documented in a study [5] in which the effect estimate becomes stronger from an RR of 1.1 to 1.7 when liberal diagnosis of EPTB was applied. It was found that some studies did not describe confirmation procedures of EPTB [44] or HIV status [38], or both [46]. As stated earlier, because diagnosis of EPTB in itself often is difficult, EPTB may have been underdiagnosed [47]. Taken together, should there be underreporting, this will lead to insufficient data to provide a weak, but significant, association rather than a stronger association.
Contradictory to the present study, some individual studies reported a high frequency of EPTB in HIV-negative patients [47, 56] even in countries, such as Malawi where HIV prevalence is reportedly high [46, 57]. This suggests that the development of EPTB may be truly unrelated to HIV serostatus [57]. However, patients with an unknown HIV serostatus among the study population may have been interpreted as HIV seronegative, leading to selection bias. We acknowledge the limitation of our estimations; the studies identified for the present analysis were of observational designs and therefore reported crude and adjusted ORs could be biased due to unmeasured or unknown confounders [25]. Regarding the confirmation of EPTB, in the context of diagnosis, EPTB compounds the difficulty imposed by their having a lower frequency of sputum smear positivity [58]. As with earlier reviews [58, 59], a recent systematic review addressing 25 studies showed that the commercial serological tests provided inaccurate and imprecise estimates of sensitivity (range 0–100 %) and specificity (range 59–100 %) for overall EPTB [60]. For lymph node and pleural EPTB, the mean sensitivity was 64 % (range 28–92 %) and 46 % (range 29–63 %), respectively [60]. Misdiagnosis of EPTB is common in all countries and may, therefore, result in unnecessary treatment if falsely diagnosed, or greater morbidity and mortality if the diagnosis is missed, especially in HIV-infected patients [59].
Nevertheless, the association between EPTB and HIV documented in the current study is consistent with presumed pathogenesis and clinical rationale [54, 55], which reinforced our confidence in effect estimation.
Improved recognition of EPTB risk factors may facilitate earlier case detection and treatment, resulting in subsequent reduction in EPTB-associated morbidity and mortality. The positive association between EPTB and HIV documented in the current study implies that TB control program might benefit from a focus on interventions designed to reduce HIV infection and vice versa. Improved understanding of risk factors for EPTB is important to the goal of TB elimination and would enable clinicians to apply a high index of suspicion to the HIV-infected population at risk for EPTB. Also, a positive diagnosis of EPTB should alert the clinician of a possible HIV-positive status. Similar to earlier studies, the present study acknowledges that HIV and M. tuberculosis infections are different and far more complex in coinfected compared with monoinfected patients.
In conclusion, the present meta-analysis indicates that there is evidence for the association between HIV and EPTB based on the case control studies. Further studies to understand the mechanisms of interaction of the two pathogens are recommended.
References
WHO (World Health Organization) (2011) Global tuberculosis control: WHO report, Geneva. http://www.who.int/tb/publications/global_report/en/. Accessed 2 Aug 2012
Pawlowski A, Jansson M, Sköld M, Rottenberg ME, Källenius G (2012) Tuberculosis and HIV co-infection. PLoS Pathog 8(2):e1002464
Barnes PF, Bloch AB, Davidson PT, Snider DE Jr (1991) Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med 324:1644–1650
WHO (World Health Organization) (2007) Global tuberculosis control: surveillance, planning, financing. WHO report 2007. Geneva http://www.who.int/tb/publications/global_report/2007/pdf/full.pdf. Accessed 10 Nov 2011
Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR (2009) Epidemiology of extrapulmonary tuberculosis in the United States, 1993–2006. Clin Infect Dis 49:1350–1357
Al-Otaibi F, El Hazmi MM (2010) Extra-pulmonary tuberculosis in Saudi Arabia. Indian J Pathol Microbiol 53:227–231
MDG (The Millennium Development Goals) 6: combat HIV/AIDS, malaria and other diseases. http://www.who.int/topics/millennium_development_goals/diseases/en/index.html. Accessed 2 Aug 2012
Greenland S, Robins JM (1985) Estimation of a common effect parameter from sparse follow-up Data. Biometrics 41:55–68
Higgins JPT, Green S (2009) Principles of meta-analysis. In: Higgins JPT, Green S (eds) Cochrane handbook for systemic reviews of interventions. Cochrane book series. Wiley, West Sussex, pp 419–429
Buskin SE, Gale JL, Weiss NS, Nolan CM (1994) Tuberculosis risk factors in adults in King County, Washington, 1988 through 1990. Am J Public Health 84:1750–1756
Chang KC, Leung CC, Yew WW, Ho SC, Tam CM (2004) A nested case-control study on treatment-related risk factors for early relapse of tuberculosis. Am J Respir Crit Care Med 170:1124–1130
Chen CH, Lian JD, Cheng CH, Wu MJ, Lee WC, Shu KH (2006) Mycobacterium tuberculosis infection following renal transplantation in Taiwan. Transpl Infect Dis 8:148–156
Harstad I, Jacobsen GW, Heldal E et al (2010) The role of entry screening in case finding of tuberculosis among asylum seekers in Norway. BMC Public Health 10:670
Lorent N, Mugwaneza P, Mugabekazi J et al (2008) Risk factors for delay in the diagnosis and treatment of tuberculosis at a referral hospital in Rwanda. Int J Tuberc Lung Dis 12:392–396
Shetty N, Shemko M, Vaz M, D’Souza G (2006) An epidemiological evaluation of risk factors for tuberculosis in South India: a matched case control study. Int J Tuberc Lung Dis 10:80–86
Sterling TR, Dorman SE, Chaisson RE et al (2001) Human immunodeficiency virus-seronegative adults with extrapulmonary tuberculosis have abnormal innate immune responses. Clin Infect Dis 33:976–982
Wasif GS, Sulaiman SAS, Ali JA (2011) Clinical presentation and association among tuberculosis patients; cohort comparison between smokers versus never-smoke in Penang, Malaysia. IRJP 2:66–75
Cagatay AA, Caliskan Y, Aksoz S et al (2004) Extrapulmonary tuberculosis in immunocompetent adults. Scand J Infect Dis 36:799–806
Hesselink DA, Yoo SM, Verhoeven GT, Brouwers JW, Smit FJ, van Saase JL (2003) A high prevalence of culture-positive extrapulmonary tuberculosis in a large Dutch teaching hospital. Neth J Med 61:65–70
Hou CL, Tsai YC, Chen LC, Huang JL (2008) Tuberculosis infection in patients with systemic lupus erythematosus: pulmonary and extrapulmonary infection compared. Clin Rheumatol 27:557–563
Young F, Wotton CJ, Critchley JA, Unwin NC, Goldacre MJ (2010) Increased risk of tuberculosis disease in people with diabetes mellitus: record-linkage study in a UK population. J Epidemiol Community Health. doi:10.1136/jech.2010.114595
Maltezou HC, Spyridis P, Kafetzis DA (2000) Extra-pulmonary tuberculosis in children. Arch Dis Child 83:342–346
Ullah S, Shah SH, Aziz-ur-Rehman, Kamal A, Begum N, Khan G (2008) Extrapulmonary tuberculosis in Lady Reading Hospital Peshawar, NWFP, Pakistan: survey of biopsy results. J Ayub Med Coll Abbottabad 20:43–46
Yoon HJ, Song YG, Park W, Choi JP, Chang KH, Kim JM (2004) Clinical manifestations and diagnosis of extrapulmonary tuberculosis. Yonsei Med J 45:453–461
Leeds IL, Magee MJ, Kurbatova EV et al (2012) Site of extrapulmonary tuberculosis is associated with HIV infection. Clin Inf Dis 55:75–81
Forssbohm M, Zwahlen M, Loddenkemper R, Rieder HL (2008) Demographic characteristics of patients with extrapulmonary tuberculosis in Germany. Eur Respir J 31:99–105
Kruijshaar ME, Abubakar I (2009) Increase in extrapulmonary tuberculosis in England and Wales 1999–2006. Thorax 64:1090–1095
Manangan L, Elmore K, Lewis B et al (2009) Disparities in tuberculosis between Asian/Pacific Islanders and non-Hispanic Whites, United States, 1993–2006. Int J Tuberc Lung Dis 13:1077–1085
Mehta JB, Dutt A, Harvill L, Mathews KM (1991) Epidemiology of extrapulmonary tuberculosis. A comparative analysis with pre-AIDS era. Chest 99:1134–1138
Ramos JM, Reyes F, Tesfamariam A (2010) Childhood and adult tuberculosis in a rural hospital in Southeast Ethiopia: a ten-year retrospective study. BMC Public Health 10:215
Wang JY, Hsueh PR, Jan IS et al (2007) The effect of smoking on tuberculosis: different patterns and poorer outcomes. Int J Tuberc Lung Dis 11:143–149
Gunal S, Yang Z, Agarwal M, Koroglu M, Arici ZK, Durmaz R (2011) Demographic and microbial characteristics of extrapulmonary tuberculosis cases diagnosed in Malatya, Turkey, 2001–2007. BMC Public Health 11:154
Musellim B, Erturan S, Duman ES, Ongen G (2005) Comparison of extra-pulmonary and pulmonary tuberculosis cases: factors influencing the site of reactivation. Int J Tuberc Lung Dis 9:1220–1223
Nur N, Ozsahin SL, Sumer H (2009) An evaluation of gender differences in the epidemiology of tuberculosis. Health Med 3:352–358
Antony SJ, Harrel V, Christie JD, Adams HG, Rumley RL (1995) Clinical differences between pulmonary and extrapulmonary tuberculosis: a 5-year retrospective study. J Natl Med Assoc 87:187–192
Fiske CT, Griffin MR, Erin H et al (2010) Black race, sex, and extrapulmonary tuberculosis risk: an observational study. BMC Infect Dis 10:16
Gonzalez OY, Adams G, Teeter LD, Bui TT, Musser JM, Graviss EA (2003) Extrapulmonary manifestations in a large metropolitan area with a low incidence of tuberculosis. Int J Tuberc Lung Dis 7:1178–1185
Kipp AM, Stout JE, Hamilton CD, Van Rie A (2008) Extrapulmonary tuberculosis, human immunodeficiency virus, and foreign birth in North Carolina, 1993–2006. BMC Public Health 8:107
Ong A, Creasman J, Hopewell PC et al (2004) A molecular epidemiological assessment of extrapulmonary tuberculosis in San Francisco. Clin Infect Dis 38:25–31
Yang Z, Kong Y, Wilson F, Foxman B, Fowler AH, Marrs CF et al (2004) Identification of risk factors for extrapulmonary tuberculosis. Clin Infect Dis 38:199–205
Castilla J, Gutierrez A, Guerra L et al (1997) Pulmonary and extrapulmonary tuberculosis at AIDS diagnosis in Spain: epidemiological differences and implications for control. AIDS 11:1583
García-Rodríguez JF, Álvarez-Díaz H, Lorenzo-García MV, Mariño-Callejo A, Fernández-Rial Á, Sesma-Sánchez P (2011) Extrapulmonary tuberculosis: epidemiology and risk factors. Enferm Infect Microbiol Clin 29:502–509
Malkin JE, Prazuck T, Simonnet F et al (1997) Tuberculosis and human immunodeficiency virus infection in West Burkina Faso: clinical presentation and clinical evolution. Int J Tuberc Lung Dis 1:68–74
Gupta S, Shenoy VP, Bairy I, Srinivasa H, Mukhopadhyay C (2011) Diabetes mellitus and HIV as co-morbidities in tuberculosis patients of rural south India. J Infect Public Health 4:140–144
Lin HP, Deng CY, Chou P (2009) Diagnosis and treatment delay among pulmonary tuberculosis patients identified using the Taiwan reporting enquiry system, 2002–2006. BMC Public Health 9:55
Cailhol J, Decludt B, Che D (2005) Sociodemographic factors that contribute to the development of extrapulmonary tuberculosis were identified. J Clin Epidemiol 58:1066–1071
Noertjojo K, Tam CM, Chan SL, Chan-Yeung MMW (2002) Extra-pulmonary and pulmonary tuberculosis in Hong Kong. Int J Tuberc Lung Dis 6:879–886
Sreeramareddy CT, Panduru KV, Verma SC, Joshi HS, Bates MN (2008) Comparison of pulmonary and extrapulmonary tuberculosis in Nepal: a hospital-based retrospective study. BMC Infect Dis 8:8
Te Beek LA, van der Werf MJ, Richter C, Borgdorff MW (2006) Extrapulmonary tuberculosis by nationality, the Netherlands, 1993–2001. Emerg Infect Dis 12:1375–1382
Kherad O, Hermann FR, Zellweger JP, Rochat T, Janssens JP (2009) Clinical presentation, demographics and outcome of tuberculosis (TB) in a low incidence area: a 4-year study in Geneva. Switzerland. BMC Infect Dis 9:217
Madhi SA, Huebner RE, Doedens L, Aduc T, Wesley D, Cooper PA (2000) HIV-1 co-infection in children hospitalized with tuberculosis in South Africa. Int J Tuberc Lung Dis 4:448–454
Kingkaew N, Sangtong B, Amnuaiphon W et al (2009) HIV-associated extrapulmonary tuberculosis in Thailand: epidemiology and risk factors for death. Int J Infect Dis 13:722–729
Orme IM (2011) Development of new vaccines and drugs for TB: limitations and potential strategic errors. Future Microbiol 6:161–177
Lawn SD, Alimuddin I, Zumla AI (2011) Tuberculosis. Lancet 378:57–72. doi:10.1016/S0140-6736(10)62173-3
Sharma SK, Mohan A, Kadhiravan T (2005) HIV-TB co-infection: epidemiology, diagnosis & management. Indian J Med Res 121:550–567
Cowie RL, Sharpe JW (1998) Tuberculosis among immigrants: interval from arrival in Canada to diagnosis. A 5-year study in southern Alberta. CMAJ 158:599–602
Harries AD, Parry C, Nyongonya ML et al (1997) The pattern of tuberculosis in Queen Elizabeth Central Hospital, Blantyre, Malawi: 1986–1995. Int J Tuberc Lung Dis 1:346–351
Steingart KR, Henry M, Laal S et al (2007) Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: a systematic review. PLoS Med 4:e202 (Erratum, 2007;4(8):e254)
Steingart KR, Dendukuri N, Henry M et al (2009) Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta-analysis. Clin Vaccine Immunol 16:260–276
Steingart KR, Flores LL, Dendukuri N et al (2011) Commercial serological tests for the diagnosis of active pulmonary and extrapulmonary tuberculosis: an updated systematic review and meta-analysis. PLoS Med 8:e1001062
Acknowledgments
The authors are grateful to the researchers and participants of the primary studies identified for the present analysis. They thank anonymous reviewers for the comments provided and the helpful input. International Medical University, Malaysia (Project ID: BMSc I-01/2011(01)).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naing, C., Mak, J.W., Maung, M. et al. Meta-Analysis: The Association Between HIV Infection and Extrapulmonary Tuberculosis. Lung 191, 27–34 (2013). https://doi.org/10.1007/s00408-012-9440-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-012-9440-6