Abstract
The reversed halo sign (RHS) is a chest computed tomography (CT) pattern defined as a focal round area of ground-glass attenuation surrounded by a crescent or ring of consolidation. The RHS was first described as being relatively specific for cryptogenic organizing pneumonia but was later observed in several other infectious and noninfectious diseases. Although the presence of the RHS on CT may help narrow the range of diseases considered in differential diagnoses, final diagnoses should be based on correlation with the clinical scenario and the presence of additional disease-specific CT findings. However, frequently a biopsy may be needed to establish the diagnosis. Organizing pneumonia is the most frequent cause of the RHS. This is a distinct clinical and pathologic entity that can be cryptogenic or secondary to other known causes. Morphologic aspects of the halo, particularly the presence of small nodules in the wall or inside the lesion, usually indicate an active granulomatous disease (tuberculosis or sarcoidosis) rather than organizing pneumonia. Immunocompromised patients presenting with the RHS on CT examination should be considered to have an infection until further analyses prove otherwise. Pulmonary zygomycosis and invasive pulmonary aspergillosis are typically seen in patients with severe immunosuppression, most commonly secondary to hematological malignancies. Other causes of the RHS include noninvasive fungal infections such as paracoccidioidomycosis, histoplasmosis, and Pneumocystis jiroveci pneumonia. Furthermore, Wegener’s granulomatosis, radiofrequency ablation, and lymphomatoid granulomatosis may also lead to this finding. Based on a search of the PubMed and Scopus databases, we review the different diseases that can manifest with the RHS on CT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The computed tomography (CT) finding currently known as the reversed halo sign (RHS) was initially described in 1996 by Voloudaki et al. [1], who reported two cases of cryptogenic organizing pneumonia (COP; formerly known as bronchiolitis obliterans organizing pneumonia) that manifested on CT as “crescentic and ring-shaped opacities, surrounding areas of ground-glass attenuation.” The RHS was described as a new CT finding that could be a characteristic feature for the diagnosis of COP. The central areas of ground-glass attenuation within the lesions were shown to correspond to alveolar septal inflammation, and the denser peripheral area corresponded to a granulomatous process [1].
In 1999, Zompatori et al. [2] described similar CT findings in one patient with COP. They used the term “atoll sign” because of its resemblance to the “ring-shaped coral reefs of the tropics that enclose a lagoon with shallow waters.” They also noted that this CT presentation resembled the photographic negative of the “halo sign” often found in angioinvasive aspergillosis. The authors suggested that this CT sign should be considered highly specific for COP.
In a review of the CT scans of 31 patients with COP in 2003, Kim et al. [3] identified six patients with a central ground-glass opacity surrounded by a crescentic or ring-shaped denser consolidation, similar to the findings described by Voloudaki et al. [1] and Zompatori et al. [2]. Kim et al. [3] named this finding the RHS and, like previous authors, considered it to be relatively specific for COP.
The Fleischner Society [4] currently defines the RHS as “a focal, rounded area of ground-glass opacity surrounded by a more or less complete ring of consolidation” observed on CT images. Although some authors [5, 6] still prefer the term “atoll sign,” the term RHS should be preferred to avoid confusion and to standardize the keywords used in literature searches [7].
Until 2005/2006, the RHS was considered exclusively diagnostic of organizing pneumonia (OP), as claimed by Polverosi et al. [6]: “This sign is typical of OP, as OP is the only disease in which it has been reported.” However, in 2005, Gasparetto et al. [8] reported that they had observed the RHS in 15 patients with paracoccidioidomycosis, which indicated that the RHS could be seen in patients with active infection and was therefore not specific for COP. Subsequently, various authors have demonstrated the presence of this sign in a wide spectrum of diseases, including infectious and noninfectious pulmonary disorders, confirming the nonspecific nature of the RHS [9–12].
We performed searches of the US National Library of Medicine (PubMed) and Scopus databases using the keywords “reversed halo sign,” “reverse halo sign,” “atoll sign,” and “fairy ring” (another term associated with the RHS) to identify cases in which the RHS had been described. We identified 42 English-language reports (full-length articles, case reports, and letters) published between 1996 and 2011. Based on these data, in this article we review the infectious diseases and noninfectious conditions that can manifest with the RHS on CT.
Organizing Pneumonia
OP is the most frequent cause of the RHS [10, 12–14] (Fig. 1). The described incidence of the RHS in patients with OP ranges from 12 to 19 % [3, 15]. OP is a distinct clinical and pathologic entity that can be cryptogenic or secondary to other known causes. COP is classified as an idiopathic interstitial pneumonia, whereas secondary OP is associated with a variety of diseases presenting with OP clinical syndrome and the characteristic pathologic pattern. These entities include connective tissue diseases, infections, malignancies, drugs, radiation injury, organ transplantation, hypersensitivity pneumonitis, chronic eosinophilic pneumonia, diffuse alveolar damage, and aspiration, among others [15–17]. COP is diagnosed in the appropriate clinical, radiographic, and pathologic setting after excluding diseases associated with secondary OP. The clinical presentation and radiographic findings of COP and secondary OP are similar. The distinction between COP and secondary OP is clinically important because the management of patients with secondary OP includes not only treatment of OP, but also treatment of the underlying disease and avoidance of any known offending agents. To date, it remains unclear whether COP and secondary OP represent two distinct clinical entities or whether they are a single entity of nonspecific lung injury and repair [16].
Although several publications have attributed the RHS directly to different diseases (dermatomyositis [18], lipoid pneumonia [19], radiotherapy [17], nonspecific interstitial pneumonia [20]), descriptions of the pathologic findings in these cases show evidence of secondary OP (e.g., presence of polypoid granulation tissue).Thus, the RHS most likely represented secondary OP as a response to the primary disease in these cases. In other cases, such as in one patient with pneumococcal pneumonia [21], the RHS appeared during the resolution phase of the disease, days or weeks after the diagnosis of pulmonary infection had been made. This timing suggests that the RHS probably represented secondary OP, although no histopathologic sample was obtained.
Sarcoidosis and Tuberculosis: The Nodular Reversed Halo Sign
In 1999, K Marlow et al. [22] reported a peculiar CT finding in a patient with pulmonary sarcoidosis: a ring of granulomatous tissue extending concentrically from a specific point in the lung. The central area of the lesion was composed of normal lung tissue, and the peripheral opacity had a nodular border. The authors named this lesion the “fairy ring” sign, based on Celtic mythology [23]. This pattern can be considered a variant of the RHS.
Later, Kumazoe et al. [24] reported the RHS in a case of sarcoidosis in which high-resolution computed tomography (HRCT) images showed multiple, central ground-glass opacities surrounded by crescentic or ring-shaped areas of consolidation in both lungs. The authors also noted the presence of miliary nodules within the central ground-glass opacities and around the outer areas of consolidation [23, 24]. Several other authors subsequently emphasized the need to include the RHS within the spectrum of HRCT findings of pulmonary sarcoidosis [25–27].
Kumazoe et al. [24] reported that the mechanism of RHS formation in pulmonary sarcoidosis was unclear but was possibly related to secondary OP or noncaseating granulomatous inflammation. However, subsequent studies with pathologic confirmation have shown that the RHS is caused in most cases by the presence of non-necrotizing granulomas [11, 28].
Two initial case reports described the RHS in patients with tuberculosis [29, 30]; both had anatomopathologic confirmation showing multiple caseating granulomas and positive cultures for Mycobacterium tuberculosis. Recently, a study comparing 12 cases of the RHS in patients with tuberculosis and 10 in patients with COP [31] showed that all patients with tuberculosis presented the RHS with nodular walls and that small nodules were also observed inside the ground-glass component of the RHS in 83 % of these cases. No patient with COP presented the RHS with nodular walls or central nodules. The authors concluded that although COP is considered the most frequent cause of the RHS, the presence of nodular walls or nodules inside the RHS strongly favors the diagnosis of active pulmonary tuberculosis instead of COP.
The nodular aspect of the RHS (Fig. 2) usually corresponds to the presence of granulomatous disease and most likely represents active pulmonary sarcoidosis or a granulomatous infection, particularly tuberculosis. Histopathologic analysis of such specimens has revealed the presence of granulomas within the ring portion of the RHS and/or inside the RHS [12, 27, 31, 32].
Invasive Fungal Infections Associated with the Reversed Halo Sign in Immunocompromised Hosts
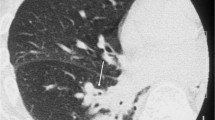
Although invasive pulmonary aspergillosis (IPA) is the most common type of invasive fungal infection (IFI), other angioinvasive molds such as Fusarium and Zygomycetes species are increasingly encountered in severely immunocompromised hosts [33–35]. In immunosuppressed patients, the presence of the RHS (Fig. 3) on CT should be considered an indication of IFI until proven otherwise. Wahba et al. [33] found the RHS in 4 % of immunocompromised patients with IFI. It is an early sign that is more frequently seen in patients with pulmonary zygomycosis (PZ) than in those with IPA [35]. The RHS was seen in 19 % of patients with PZ, in <1 % of patients with IPA, and in no patient with fusariosis [36]. Histopathologic correlation showed that the RHS was associated with infarcted lung tissue, with a greater amount of hemorrhage at the periphery than in the center [33].
Chest computed tomography image of a 24-year-old woman with leukemia and pulmonary zygomycosis, showing a focal round area of ground-glass attenuation surrounded by a ring of consolidation in the right lung, consistent with the reversed halo sign. The mediastinal windows (not shown) also demonstrated a right pleural effusion
It is important to distinguish PZ from IPA because the therapy for presumed fungal pneumonia in this population is often aimed at IPA due to its higher incidence, and because the preferred antifungal agent for IPA is voriconazole, which is not effective against PZ. The presence of the RHS, in the appropriate context, should prompt the consideration of PZ and the selection of an antifungal therapy with coverage against Zygomycetes species [33–35, 37].
Paracoccidioidomycosis and Other Noninvasive Fungal Infections
Gasparetto et al. [8] reviewed the CT scans of 148 patients with paracoccidioidomycosis and observed the RHS in 10 % of the cases (Fig. 4). Open lung biopsy specimens from three of these patients showed that the central area of ground-glass attenuation of the RHS on CT corresponded to inflammatory infiltrate involving mainly the alveolar septa, with relative preservation of the alveolar spaces. The peripheral regions of consolidation on CT consisted of dense and homogeneous intra-alveolar inflammatory infiltrates. No evidence of OP was found. Grocott-Gomori methenamine-silver stain confirmed the presence of the fungus Paracoccidioides brasiliensis in the alveolar septa and within the air spaces [8, 38].
A few isolated cases of other noninvasive fungal infections presenting with the RHS have been described, including histoplasmosis [10] and Pneumocystis jiroveci pneumonia [39], the latter occurring in immunocompromised patients with acquired immune deficiency syndrome (AIDS).
Other Diseases Presenting with the Reversed Halo Sign
The RHS was reported in two cases of Wegener’s granulomatosis [40, 41], both showing multiple lesions. In one case [40], histopathologic examination with elastic staining for the evaluation of vascular structures revealed necrotizing vasculitis, confirming the diagnosis of Wegener’s granulomatosis. In the other case [41], no pulmonary biopsy was performed. However, a renal biopsy was suggestive of necrotizing vasculitis with crescentic glomerulonephritis; immunofluorescence microscopy revealed pauci-immune immunoglobulin G deposits. Antineutrophil cytoplasmic antibodies, detected by indirect immunofluorescence, displayed a diffuse granular cytoplasmic pattern. A final diagnosis of Wegener’s granulomatosis was made based on these findings.
The RHS has also been described following radiofrequency ablation (RFA) of a lung nodule [42]. Although pathologic correlation was not available, imaging up to 13 months after treatment demonstrated a gradual reduction of lesion size, consistent with successful ablation. The authors postulated that the central ground-glass opacity of the RHS corresponded to coagulation necrosis of the tumor and adjacent pulmonary parenchyma, whereas the peripheral consolidative component of the RHS might have represented fibrotic tissue with inflammatory cells. This presumption was based on histopathologic findings described in animal models after RFA.
Lymphomatoid granulomatosis was also reported as a cause of RHS. Benamore et al. [43] described a patient who presented with multiple RHSs. Histopathologic analysis was compatible with grade 1 lymphomatoid granulomatosis.
Final Considerations
The RHS is not as specific as initially reported. Although the presence of the RHS on CT may help narrow the range of diseases considered in differential diagnoses, final diagnoses should be based on correlation with the clinical scenario and the presence of additional disease-specific CT findings. When the RHS is the sole abnormality on CT, the correlation with clinical findings is essential for the final diagnosis [11, 12]. Although a biopsy may be needed to establish the diagnosis of many of the diseases that can present with the RHS, in certain scenarios a biopsy can be avoided.
The interpretation of the RHS should be based on information from the patient’s history, clinical examination, and other radiologic findings. The clinical context of the patient is crucial. Any extrapulmonary symptom or sign that is not compatible with COP should suggest other diagnoses [21]. The patient’s immune status is the most relevant clinical information in the differential diagnosis of infectious diseases. Immunocompromised patients presenting with the RHS on CT examination should be considered to have an infection until further analyses prove otherwise. PZ and IPA are typically seen in patients with severe immunosuppression, most commonly secondary to hematological malignancies. Pneumocystis jiroveci pneumonia is more prevalent in patients with AIDS. The patient’s epidemiological history is also very important in differential diagnoses. For example, residence in or recent travel to South America should lead the clinician to suspect paracoccidioidomycosis. Patients from endemic areas or those who have had contact with soil contaminated with bird or bat guano are at increased risk for histoplasmosis [12]. In addition to pertinent clinical findings, the presence of small nodules in the wall or within the RHS usually indicates an active granulomatous disease, either granulomatous infection or sarcoidosis [12].
In conclusion, the term RHS should be favored over other previously described similar terms to maintain a standardized approach. The RHS should be considered a nonspecific CT sign seen in a variety of pulmonary diseases, which, in conjunction with the clinical findings, may help to narrow the differential diagnosis.
References
Voloudaki AE, Bouros DE, Froudarakis ME, Datseris GE, Apostolaki EG, Gourtsoyiannis NC (1996) Crescentic and ring-shaped opacities. CT features in two cases of bronchiolitis obliterans organizing pneumonia (BOOP). Acta Radiol 37:889–892
Zompatori M, Poletti V, Battista G, Diegoli M (1999) Bronchiolitis obliterans with organizing pneumonia (BOOP), presenting as a ring-shaped opacity at HRCT (the atoll sign). A case report. Radiol Med 97(4):308e10
Kim SJ, Lee KS, Ryu YH, Yoon YC, Choe KO, Kim TS, Sung KJ (2003) Reversed halo sign on high-resolution CT of cryptogenic organizing pneumonia: diagnostic implications. AJR Am J Roentgenol 180(5):1251–1254
Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J (2008) Fleischner Society: glossary of terms for thoracic imaging. Radiology 246(3):697–722
Walsh SL, Roberton BJ (2010) The atoll sign. Thorax 65(11):1029–1030
Polverosi R, Maffessanti M, Dalpiaz G (2006) Organizing pneumonia: typical and atypical HRCT patterns. Radiol Med 111:202–212
Marchiori E, Irion KL, Zanetti G, Hochhegger B (2011) Atoll sign or reversed halo sign? Which term should be used? Thorax 66(11):1009–1010
Gasparetto EL, Escuissato DL, Davaus T, de Cerqueira EM, Souza AS Jr, Marchiori E, Müller NL (2005) Reversed halo sign in pulmonary paracoccidioidomycosis. AJR Am J Roentgenol 184(6):1932–1934
Georgiadou SP, Sipsas NV, Marom EM, Kontoyiannis DP (2011) The diagnostic value of halo and reversed halo signs for invasive mold infections in compromised hosts. Clin Infect Dis 52(9):1144–1155
Marchiori E, Melo SM, Vianna FG, Melo BS, Melo SS, Zanetti G (2011) Pulmonary histoplasmosis presenting with the reversed halo sign on high-resolution CT scan. Chest 140(3):789–791
Marchiori E, Zanetti G, Meirelles GS, Escuissato DL, Souza AS Jr, Hochhegger B (2011) The reversed halo sign on high-resolution CT in infectious and noninfectious pulmonary diseases. AJR Am J Roentgenol 197(1):W69–W75
Marchiori E, Zanetti G, Escuissato DL, Souza AS Jr, Meirelles GD, Fagundes J, Souza CA, Hochhegger B, Marom EM, Godoy MC (2012) Reversed halo sign: high-resolution CT findings in 79 patients. Chest. doi:10.1378/chest.11-1050
Walker CM, Mohammed TL, Chung JH (2011) Reversed halo sign. J Thorac Imaging 26(3):W80
Maimon N (2010) A 47-year-old female with shortness of breath and “reversed halo sign”. Eur Respir Rev 19(115):83–85
Bravo Soberón A, Torres Sánchez MI, García Río F, Sánchez Almaraz C, Parrón Pajares M, Pardo Rodríguez M (2006) High resolution computed tomography patterns of organizing pneumonia. Arch Bronconeumol 42(8):413–416
Drakopanagiotakis F, Paschalaki K, Abu-Hijleh M, Aswad B, Karagianidis N, Kastanakis E, Braman SS, Polychronopoulos V (2011) Cryptogenic and secondary organizing pneumonia: clinical presentation, radiographic findings, treatment response and prognosis. Chest 139(4):893–900
Gudavalli R, Diaz-Guzman E, Arrossi AV, Chapman JT, Mehta AC (2011) Fleeting alveolar infiltrates and reversed halo sign in patients with breast cancer treated with tangential beam irradiation. Chest 139(2):454–459
Tokuyasu H, Isowa N, Shimizu E, Yamadori I (2010) Reversed halo sign associated with dermatomyositis. Intern Med 49(15):1677–1678
Kanaji N, Bandoh S, Nagamura N et al (2007) Lipoid pneumonia showing multiple pulmonary nodules and reversed halo sign. Respir Med Extra 3(3):98–101
Hong SH, Kang EY, Shin BK, Shim JJ (2011) Reversed halo sign on thin-section CT in a patient with non-specific interstitial pneumonia. Br J Radiol 84(1001):e103–e105
Tzilas V, Bastas A, Provata A, Koti A, Tzouda V, Tsoukalas G (2010) The “reversed halo” sign in pneumonococcal pneumonia: a review with a case report. Eur Rev Med Pharmacol Sci 14(5):481–486
Marlow TJ, Krapiva PI, Schabel, Judson MA (1999) The “fairy ring”: a new radiographic finding in sarcoidosis. Chest 115(1):275–276
Marchiori E, Zanetti G, Barreto MM, Azeredo F, Rodrigues RS (2011) Atypical distribution of small nodules on high resolution CT studies: patterns and differentials. Respir Med 105:1263–1267
Kumazoe H, Matsunaga K, Nagata N, Komori M, Wakamatsu K, Kajiki A, Nakazono T, Kudo S (2009) “Reversed halo sign” of high-resolution computed tomography in pulmonary sarcoidosis. J Thorac Imaging 24(1):66–68
Marchiori E, Zanetti G, Barreto MM, Rodrigues RS (2011) Pulmonary sarcoidosis: still more aspects of the “great pretender”. Clin Radiol 66:484–487
Marchiori E, Zanetti G, Hochhegger B, Carvalho J (2011) Sarcoid cluster sign and the reversed halo sign: extending the spectrum of radiographic manifestations in sarcoidosis. Eur J Radiol 80(2):567–568
Marchiori E, Irion KL, Zanetti G, Hochhegger B (2011) Sarcoidosis and the reversed halo sign. Radiographics 31(3):892–893 author reply 893
Marchiori E, Zanetti G, Mano CM, Hochhegger B, Irion KL (2010) The reversed halo sign: another atypical manifestation of sarcoidosis. Korean J Radiol 11(2):251–252
Ahuja A, Gothi D, Joshi JM (2007) A 15-year-old boy with “reversed halo”. Indian J Chest Dis Allied Sci 49(3):99–101
Marchiori E, Grando RD, Simões Dos Santos CE, Maffazzioli Santos Balzan L, Zanetti G, Mano CM, Gutierrez RS (2010) Pulmonary tuberculosis associated with the reversed halo sign on high-resolution CT. Br J Radiol 83(987):e58–e60
Marchiori E, Zanetti G, Irion KL, Nobre LF, Hochhegger B, Mançano AD, Escuissato DL (2011) Reversed halo sign in active pulmonary tuberculosis: criteria for differential diagnosis from cryptogenic organizing pneumonia. AJR Am J Roentgenol 197:1324–1327
Marchiori E, Zanetti G, Hochhegger B, Irion KL (2010) Reversed halo sign: nodular wall as criteria for differentiation between cryptogenic organizing pneumonia and active granulomatous diseases. Clin Radiol 65(9):770–771
Wahba H, Truong MT, Lei X, Kontoyiannis PD, Marom EM (2008) Reversed halo sign in invasive pulmonary fungal infections. Clin Infect Dis 46(11):1733–1737
Chung JH, Godwin JD, Chien JW, Pipavath MSSJ (2010) Pulmonary mucormycosis. Radiology 256(2):667–670
Godoy MC, Marom EM (2011) Reversed halo sign in pulmonary zygomycosis. Thorax 66(6):544
Marom EM, Kontoyiannis DP (2011) Imaging studies for diagnosing invasive fungal pneumonia in immunocompromised patients. Curr Opin Infect Dis 24(4):309–314
Busca A, Limerutti G, Locatelli F, Barbui A, De Rosa FG, Falda M (2011) The reversed halo sign as the initial radiographic sign of pulmonary zygomycosis. Infection 40(1):77–80
Souza AS Jr, Gasparetto EL, Davaus T, Escuissato DL, Marchiori E (2006) High-resolution CT findings of 77 patients with untreated pulmonary paracoccidioidomycosis. AJR Am J Roentgenol 187(5):1248–1252
Otera H, Tada K, Sakurai T, Hashimoto K, Ikeda A (2010) Reversed halo sign in pneumocystis pneumonia: a case report. BMC Med Imaging 10:26
Choi YH, Im JG, Park CK (2002) Notes from the 2001 Annual Meeting of the Korean Society of Thoracic Radiology. J Thorac Imaging 17(2):170–175
Agarwal R, Agarwal AN, Gupta D (2007) Another cause of reverse halo sign: Wegener’s granulomatosis. Br J Radiol 80(958):849–850
Mango VL, Naidich DP, Godoy MC (2011) Reversed halo sign after radiofrequency ablation of a lung nodule. J Thorac Imaging 26(4):W150–W152
Benamore RE, Weisbrod GL, Hwang DM, Bailey DJ, Pierre AF, Lazar NM, Maimon N (2007) Reversed halo sign in lymphomatoid granulomatosis. Br J Radiol 80(956):E162–E166
Conflict of interest
Edson Marchiori, Gláucia Zanetti, Bruno Hochhegger, Klaus L Irion, Antonio Carlos Pires Carvalho, and Myrna C. B. Godoy have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marchiori, E., Zanetti, G., Hochhegger, B. et al. Reversed Halo Sign on Computed Tomography: State-of-the-Art Review. Lung 190, 389–394 (2012). https://doi.org/10.1007/s00408-012-9392-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-012-9392-x