Abstract
The objective of this prospective cohort study was to see the effect of the implementation of a Sepsis Intervention Program on the standard processes of patient care using a collaborative approach between the Emergency Department (ED) and Medical Intensive Care Unit (MICU). This was performed in a large urban tertiary-care hospital, with no previous experience utilizing a specific intervention program as routine care for septic shock and which has services and resources commonly available in most hospitals. The study included 106 patients who presented to the ED with severe sepsis or septic shock. Eighty-seven of those patients met the inclusion criteria for complete data analysis. The ED and MICU staff underwent a 3-month training period followed by implementation of a protocol for sepsis intervention program over 6 months. In the first 6 months of the program’s implementation, 106 patients were admitted to the ED with severe sepsis and septic shock. During this time, the ED attempted to initiate the sepsis intervention protocol in 76% of the 87 septic patients who met the inclusion criteria. This was assessed by documentation of a central venous catheter insertion for continuous SvO2 monitoring in a patient with sepsis or septic shock. However, only 48% of the eligible patients completed the early goal-directed therapy (EGDT) protocol. Our data showed that the in-hospital mortality rate was 30.5% for the 87 septic shock patients with a mean APACHE II score of 29. This was very similar to a landmark study of EGDT (30.5% mortality with mean APACHE II of 21.5). Data collected on processes of care showed improvements in time to fluid administration, central venous access insertion, antibiotic administration, vasopressor administration, and time to MICU transfer from ED arrival in our patients enrolled in the protocol versus those who were not. Further review of our performance data showed that processes of care improved steadily the longer the protocol was in effect, although this was not statistically significant. There was no improvement in secondary outcomes, including total length of hospital stay, MICU days, and mortality. Implementation of a sepsis intervention program as a standard of care in a typical hospital protocol leads to improvements in processes of care. However, despite a collaborative approach, the sepsis intervention program was underutilized with only 48% of the patients completing the sepsis intervention protocol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years there have been several trials that have demonstrated a survival benefit of new interventions for severe sepsis and septic shock [1–3]. One of the most dramatic studies was a trial of early goal-directed therapy (EGDT) in patients with severe sepsis and evidence of hypoperfusion who were admitted from the emergency department (ED) [4]. In the original study, multiple interventions were coalesced into a protocol that focused on therapy directed by specific physiologic goals and also on prompt attainment of those goals. In this landmark study, a significant mortality benefit was found for patients treated with this EGDT protocol when compared with patients who received standard care.
In the years since the publication of these data many institutions have recreated the protocols and treatments used in that study with good success [5–9]. That study has led many clinicians to suggest that early, aggressive resuscitation should become the standard of care for patients with severe sepsis and septic shock [10–14]. EGDT has become one of the cornerstones of the Surviving Sepsis Campaign (SSC) guidelines that represent an international collaboration to reduce mortality due to sepsis [15, 16].
The purpose of our study was to see if a sepsis intervention program based on the tenets of EGDT could be established as the standard of care in a typical urban hospital setting and assess how its implementation affects the care of patients presenting to the ED with septic shock. The introduction of a sepsis intervention program necessitated the use and insertion of more invasive and sophisticated monitoring equipment than is routinely utilized in our ED for the care of patients with septic shock. This included the monitoring of hemodynamic parameters such as central venous pressure, arterial pressure, and continuous venous oxygen saturation. We established a collaborative model combining training and resources from the medical intensive care unit (MICU) staff and the ED staff to adopt the sepsis intervention program. Our model called for training of the ED staff, use of central venous pressure (CVP) monitoring, physiologic goal-directed treatment, and enhanced communication between the ED and MICU staff to monitor patients and speed up transfer to the MICU. For a 6-month observation period we tracked the impact of our sepsis intervention program on the processes of care such as time to fluid administration, vasopressor administration, catheter insertion, transfer to MICU, and time to initial antibiotics, as well as traditional outcomes such as mortality and length of stay in the MICU.
Methods
Approval of Study Design
This prospective cohort study was approved by the institutional review board of Rhode Island Hospital and conducted under the auspices of an independent data and safety monitor. Based on the previously published data, the protocol was introduced as a change in the standard of care offered to all patients admitted to the ED with severe sepsis and/or hypotension. As a quality improvement study, informed consent was waived.
Preimplementation
Prior to implementing the sepsis protocol, meetings were held between the directors of all MICUs and ED. All directors agreed that the treatments dictated by the protocols were appropriate. Over a period of 3 months a program of training sessions was begun that involved critical care staff teaching ED residents, attendings, and nurses how to identify sepsis and the rationale behind the resuscitation protocol. In addition, a collaborative treatment model was established between the critical care staff and the ED that included the following: (1) early consultation of the critical care staff, (2) enhanced communication through a dedicated “sepsis beeper” carried by a member of the on-call critical care team, and (3) improvement in patient transfer by predetermining that all patients with severe sepsis for whom the early resuscitation protocol is initiated would be automatically admitted to the MICU. Training in the physiologic concepts and practical logistics of the resuscitation protocol was conducted in both the ED and the MICU. Training sessions were held for both nurses and physicians in both units. In addition, in the first 3 months of implementation of the sepsis intervention protocol a dedicated MICU research fellow was available to aid with central venous line insertion at the request of the ED.
Eligibility and Resuscitation Protocol
Any patient presenting to the ED with a real or suspected infection with either hypotension after 30 cc/kg resuscitation with a crystalloid fluid or a lactate of more than 4 mmol/l was eligible for entry into the protocol. After meeting the criteria for suspected sepsis, all patients had a Presep (Edwards Lifescience, Irvine, CA), central venous catheter inserted. This catheter enabled fluid replacement, CVP monitoring, and continuous monitoring of central venous oxygen saturation (ScvO2). The resuscitation protocol was similar to that published in the study by Rivers et al. [4] (Fig. 1). The protocol was initiated in the ED by the ED team and then continued during and after transfer to the MICU.
Study Design
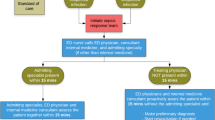
Over the course of the study period, all patients admitted to the ED with severe sepsis and hypotension or elevated lactate level were eligible for the protocol. Patients were excluded if they (1) refused central line insertion or had a documented contraindication to central line insertion (e.g., coagulopathy), (2) did not survive long enough to undergo 6 h of EGDT, or (3) were not candidates for aggressive treatment (made comfort measures only, advance directive or pre-existing diagnosis) (Fig. 2). The patients were subsequently divided into two groups. (1) Completed protocol: attempts to reach all the goals of the resuscitation protocol (Fig. 1) were documented in the patient record, e.g., CVP, MAP, and ScvO2 measurements had to be recorded where appropriate according to the protocol (Fig. 1). Patients were included in this group even if all the target goals were not achieved within the 6-h window therapeutic of EGDT, e.g., ScvO2 was still not greater than 70% despite receiving adequate fluid resuscitation, transfusion, and inotropes. This was not viewed as failure to complete. (2) Failed-to-complete protocol: failure to either initiate or complete the protocol. The reasons for no enrollment included ED physician preference, catheter insertion but no protocol started, or patient sent to the ICU without the catheter placed despite the patient having no contraindication to catheter insertion. Failure to complete also included no documentation of CVP, MAP, or ScvO2 measurement where appropriate according to the protocol. Adherence to the protocol was assessed in an all-or-none fashion; that is, if there was noncompliance with one element of the protocol, then there was noncompliance with the entire protocol. Therefore, a single violation of protocol was assessed as failure to complete the protocol. This group of patients served as a comparative group with the patients who did complete the sepsis intervention protocol.
Any deficiencies regarding the implementation of the sepsis intervention protocol were identified through monthly audits and relayed to the ED/MICU as areas that required improvement. Also, during the final 3 months the ED were responsible for instituting the sepsis intervention protocol without any assistance from the MICU research fellow in central line insertion.
Outcome Variables
All patients enrolled in the sepsis intervention protocol were tracked for resource utilization. Our primary outcome variables were time from admission to the ED to catheter insertion; time to fluid administration, vasopressors, and antibiotics; and time to transfer from the ED to the MICU. The baseline time (T 0) for all measured outcomes was time of arrival (registration) in the ED. A 6-month analysis was performed to determine if the protocol group exhibited diminishing times to therapeutic actions postintervention and to see how this compared to the nonprotocol group. There was a statistically significant increase in the difference of the APACHE II scores of the protocol and nonprotocol groups over 6 months (Table 1). As a consequence of this confounder, the differences in secondary outcomes, including total length of hospital stay, MICU days, and mortality, were not calculated between these groups.
A further analysis was performed using only the patients in the final 3 months of the study, comparing the protocol group with the nonprotocol group. This was motivated by the fact that early in the study many patients were started on the protocol but did not continue to receive care as per protocol (e.g., continuous monitoring of ScvO2 was not performed, CVP pressure measurements were not performed). These “shortcuts” could have led to the conclusion of falsely improved processes of care by reducing the time spent managing the central line (a possible obstacle to institution of a sepsis intervention program). After the first 3 months complete adherence to the sepsis intervention protocol had improved. For this reason, analysis comparing the median times of the protocol and nonprotocol groups in the processes of care, namely, time to fluid administration, central venous access insertion, antibiotic administration, pressor administration, and time to MICU transfer from ED arrival, was performed using the data of only those patients in the final 3 months of the study. An analysis of the differences in secondary outcomes of these two groups, including total length of stay, MICU days, and mortality, also was performed. We also measured rate of initiation of sepsis intervention protocol and compliance with protocol over the 6-month period of the study. Furthermore, we measured how many septic patients had lactate levels drawn in the 6 months of sepsis intervention protocol and compared that number to that of the 6-month period prior to sepsis intervention protocol.
Statistical Methods
We compared mean age, lactate level, and APACHE II score between the protocol and nonprotocol groups using the independent-samples t test over both the 3-month and the 6-month period. In the 6-month analysis we regressed—separately for the protocol and nonprotocol groups—each of the time-to-therapy variables (as listed above) on the number of months postintervention to determine if the protocol group exhibited diminishing times to therapeutic actions postintervention. For these analyses we employed median regression to address right-tail outliers. For comparing the protocol versus the nonprotocol group, we used a p value based on an independent-samples t test to assess if there was a statistically significant difference between the two groups. In the analysis of the patients in the final 3 months of the study, we compared median time intervals (time to fluid administration, vasopressor administration, antibiotic administration, catheter insertion, and transfer to MICU), length of stay, ICU days, and total hospital cost between those groups using the Wilcoxon rank-sum test. The χ2 test was used to test for group differences in gender and discharge disposition. We established an α value of 0.05 as indicating statistical significance in two-tailed comparisons. All statistics were performed using Stata ver. 8 (Stata Corp., College Station, TX).
Results
One hundred six patients with sepsis or septic shock presented to the ED in the 6-month study period. Of the 106 patients, 87 met the inclusion criteria for further data analysis. Overall, 82 (66%) of the patients who presented to the ED had the sepsis intervention protocol initiated. However, the sepsis intervention was only initiated in 66 of 87 (76%) patients with sepsis shock who were deemed eligible for complete data analysis according to the a priori exclusion criteria (Fig. 2). The eligible patients had an overall in-hospital mortality rate of 30.5% with a mean APACHE II score of 29. The overall compliance rate compares favorably to that in the initial 3 months when implementation rates were only (26/47) 55% of the eligible population. However, only 42 of 87 patients (48%) completely complied with the protocol over the 6-month period. The compliance rate increased to 50% (20/40) in the last 3 months compared to 42% (20/47) in the first 3 months.
There was a statistically significant increase in the APACHE II score between the protocol and nonprotocol groups over 6 months (Table 1). As a consequence of this confounder, the differences in secondary outcomes, including total length of stay, MICU days, and mortality, were not calculated between these groups. In the analysis of the data of the 3-month period, there were no statistically significant differences between the protocol and nonprotocol groups with respect to the baseline characteristics tested, although we are underpowered to conclude that there is no difference between the groups on gender (Table 2).
For each of the five time-to-therapy variables tested as our primary outcomes, the median interval was shorter in the protocol group than the nonprotocol group, and the difference was statistically significant for the time to fluid administration and the time to catheter insertion (all other p values were less than 0.2) (Table 3). There were no significant group differences exhibited for our secondary outcomes (Table 3). The coefficients of each of the five time-to-therapy (dependent) variables for the protocol group, though not statistically significant, were negative, providing further evidence that the sepsis intervention program was effective in reducing therapy intervals (Table 4). Coefficients were positive for all but one (time to catheter insertion) of the time variables for the nonprotocol group, which suggests that factors other than the intervention were not at play in explaining the diminished times exhibited for the protocol subjects (Table 4). This is supported by the fact that the coefficients of the five time-to-therapy (dependent) variables for the protocol group were statistically improved compared to those of the nonprotocol group (Table 4). Over the 6-month period, the introduction of this protocol led to an increase of 32% in the rate at which lactate levels were obtained in patients with sepsis who presented to the ED, suggesting increasing awareness of the protocol. Lactate levels were measured in 90 of the 106 patients (85%) during the 6 months of sepsis intervention protocol. This represents an absolute increase of 32% compared to the preceding observational 6 months where it was measured in 60 of 113 patients (53%).
Discussion
The physiologic rationale for the use of early, aggressive resuscitation of patients with severe sepsis or septic shock is based on earlier studies in which increased oxygen delivery was found to be associated with improved survival [17, 18]. In the original trial that evaluated the impact of early goal-directed therapy on patients with severe sepsis or septic shock, Rivers et al. [4] conducted the protocol over a 6-h period, entirely in the ED. This protocol led to an absolute mortality reduction of 16%. Several observational studies have validated the effectiveness of protocol-directed resuscitation [19–23] and its use has been advocated in practice-based guidelines for sepsis management [15, 16]. More institutions have adopted a formal protocol for the delivery of sepsis therapy [24–26]. Despite accumulating clinical evidence of the value of a standardized approach to the treatment of sepsis, these interventions remain underutilized [27–29]. The failure to translate evidence into practice has been identified as one of the great challenges of modern medicine [30–32]. In institutions that have adopted protocol-based resuscitation, compliance ranges from 50 to 60% [20, 33, 34]. Difficulty in recognizing patients with early severe sepsis in busy EDs, the complex nature of the intervention, and resource utilization may partly account for the slow rate at which this important intervention for septic patients has been adopted into routine clinical practice [27, 35]. Because there is no physiologic reason why a sepsis intervention protocol must be conducted solely in the ED, the purpose of this trial was to evaluate whether a collaborative protocol between the ED and MICU could be developed that would facilitate the initiation of sepsis intervention program in the ED.
Our primary goal was to assess whether it would be possible to develop a collaborative approach (ED/MICU) in the implementation of a sepsis intervention program in a large ED (approximately 100,000 visits/year) of a teaching hospital that had no previous experience with the routine use of a treatment protocol for septic patients. Our aim was to develop a protocol that would allow one to identify septic patients in the ED, obtain the necessary physiologic measurements, start a sepsis intervention protocol, and facilitate transfer to the MICU. This protocol involved early communication between ED and MICU personnel and early identification of patients with severe sepsis and hypoperfusion as evidenced by hypotension or elevated lactate levels. Overall, 66% (82 patients) of our 106 patients with sepsis or septic shock had the sepsis intervention protocol initiated. This represents 76% of the eligible patients according to the a priori exclusion criteria. This number is difficult to interpret as sepsis care was not administered systematically prior to the initiation of the protocol. However, this compares favorably to the initial 3 months the when implementation rate in eligible patients was only 55%. This is also supported by the reduction in catheter insertion times that occurred over the 6 months, suggesting an effect of increasing experience on the use of sepsis intervention protocol. Furthermore, over the 6-month period, the introduction of this protocol led to an increase of 32% in the rate at which lactate levels were obtained in patients who presented with sepsis in the ED, suggesting increasing awareness of the protocol.
In this study we chose to analyze separately the patients enrolled in the final 3 months of our protocol in order to better compare the group of patients who were eligible for the sepsis intervention program and those who actually received the therapy. Early in the study, many patients were started on the protocol after 6 h or had the catheter inserted but did not adhere to the initial phase of the protocol, e.g., continuous ScvO2 monitoring was not initiated. Therefore, the population chosen for analysis included the patients in the final 3 months of the study with the hope that the formal feedback mechanisms would limit similar types of training effects.
The patients who received the sepsis intervention protocol were compared to a group of patients with severe sepsis who were eligible for the protocol but did not receive the therapy. This concurrently collected group of patients, while not a prospectively identified nonprotocol group, consisted of patients who were not started on the protocol for unclear reasons. It should be acknowledged that this comparison is, in fact, the very essence of a selection bias or “confounding by indication.” This is a fundamental flaw of all retrospective studies and any prospective study that does not undergo randomization [36]. Even propensity matching cannot account for unseen biases [37]. In its essence, “confounding by indication” is based on the assumption that no physician would withhold a therapy that was thought to be beneficial for a patient [38]. This bias needs to be considered when evaluating our results. Certainly this selection bias may have influenced the secondary outcomes, i.e., total length of stay, ICU days, and mortality. However, we feel that processes of care such as time to fluid administration, vasopressor administration, catheter insertion, transfer to MICU, and time to initial antibiotics should be not be influenced by physician selection bias if the fundamental assumption of “confounding by indication” holds true, that is, no physician would withhold beneficial therapy. This is particularly relevant considering that we applied stringent criteria to ensure that patients not appropriate for aggressive measures were excluded from receiving the sepsis intervention program (Fig. 1).
Our data suggest that the use of a collaborative protocol for sepsis intervention may decrease the time to initiation of resuscitation for patients admitted to the ED with severe sepsis and decrease the time to transfer to the MICU. In the last 3 months of the protocol study, there was a statistically significant decrease in time to initial fluid administration and time to catheter insertion in the ED. In addition, there were trends toward decreased time for administration of vasopressors and antibiotics and transfer time to the MICU. While not statistically significant, by regression analysis, all time variables—fluids, vasopressors, antibiotics, and transfer to the MICU—showed negative coefficients, indicating decreased time for all variables over the 6-month study period. Also, adherence to the protocol over the 6 months significantly improved for four of the five processes of care compared to patients in which the protocol was not completed, namely, central venous access, antibiotics, vasopressor administration, and time to MICU transfer from ED arrival. Transfer from the ED to the MICU is particularly important since our data suggest that introducing a collaborative protocol between the ED and the MICU decreases the amount of time that these patients with severe sepsis and evidence of hypoperfusion spend in the ED. This is an important benefit of a standard approach to sepsis care as it may encourage clinicians to adopt a strategy that has already been proven to confer a survival benefit on this critically ill population [31].
Another important and interesting aspect of our data is the impact of the collaborative protocol on the timing of initiating antibiotic therapy. Our sepsis intervention protocol did not mention antibiotic administration. The protocol addressed only initial resuscitation efforts with fluids and the possible use of red blood cell transfusions and the administration of inotropic therapy (dobutamine), as per the initial Rivers et al. trial [4]. It appears that as a result of the protocol, greater emphasis was placed on identifying and treating patients with sepsis and hypoperfusion in the ED. It is interesting to note that by simply increasing the attention paid to these critically ill patients, the time to antibiotic administration was reduced and thus the overall quality of care for these patients was improved. Our data lend support to the suggestions in the literature that specific efforts at quality improvement in patients with sepsis and septic shock may lead to general improvement in care [26, 39, 40].
There are several weaknesses of this study. First, the number of patients enrolled was small and the period of evaluation was short. The small number of patients meant that the likelihood of an effect of the protocol on any of the secondary outcomes, especially mortality, was very unlikely. It also needs to be reiterated that patients in this study were not randomized. However, we feel that the consistent outcomes seen in the regression analysis demonstrate a clear impact of the collaborative protocol, over the 6-month study period, on the timing of resuscitation in the ED and transfer to the MICU. We believe that these results support the benefit of making operational a sepsis intervention program with an ED/ICU collaborative approach.
Despite the obvious benefits to patients of a protocolized approach to sepsis care, many institutions have low compliance rates, suggesting that making a sepsis intervention protocol operational presents difficulties [28, 33, 41]. One possibility is that physicians are unduly influenced by the concern that the amount of time required to treat these patients in the ED will be increased [29]. Our data suggest that through collaboration between the ED and the MICU, the use of a protocolized approach can facilitate earlier transfer from the ED to the MICU. This should alleviate some of the pressures caused by limited personnel time in a busy ED. However, the collaborative approach and the use of a formal feedback mechanism did not overcome the problem of poor compliance rates with the sepsis intervention protocol. Interestingly, the use of “sepsis consultative teams” had similar rates of failure [33]. Low compliance rates appear to be common in clinical practice [20, 33, 34]. Clearly, the adoption of evidence-based guidelines needs to continue to be a focus of leaders in the field of septic shock to create an organizational commitment to quality improvement [42–44]. Through a program of nurse and physician education and regular communication, we were able to introduce the protocol and decrease the time to resuscitation for patients with severe sepsis or septic shock. However, barriers to universal implementation clearly persisted throughout the 6 months and demonstrated the challenges in translating evidence into clinical practice [30–32]. Our approach incorporated many of the core principles of quality control, namely, the sepsis intervention program was adequately described, continuously monitored, and improvements suggested based on feedback. It promoted improved coordination and collaboration between different departments in the hospital, but our success was limited due to a failure to create an organizational culture of a uniform approach to management of patients with sepsis.
In conclusion, the results of this study demonstrate that it is possible to introduce a collaborative protocol of sepsis care that may facilitate transfer of patients from the ED to intensive care areas. We believe that these results should encourage physicians to introduce collaborative protocols for patients who present to the emergency department with evidence of sepsis and hypoperfusion as evidenced by a lactate of greater than 4 and/or hypotension.
References
(2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 342(18):1301–1308
Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ Jr, Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) Study Group (2001) Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 344(10):699–709
Han YY, Carcillo JA, Dragotta MA, Bills DM, Watson RS, Westerman ME, Orr RA (2003) Early reversal of pediatric-neonatal septic shock by community physicians is associated with improved outcome. Pediatrics 112(4):793–799
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M, Early Goal-Directed Therapy Collaborative Group (2001) Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345(19):1368–1377
He ZY, Gao Y, Wang XR, Hang YN (2007) [Clinical evaluation of execution of early goal directed therapy in septic shock]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 19(1):14–16
Kortgen A, Niederprum P, Bauer M (2006) Implementation of an evidence-based “standard operating procedure” and outcome in septic shock. Crit Care Med 34(4):943–949
Sebat F, Johnson D, Musthafa AA, Watnik M, Moore S, Henry K, Saari M (2005) A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients. Chest 127(5):1729–1743
Shapiro NI, Howell MD, Talmor D, Lahey D, Ngo L, Buras J, Wolfe RE, Weiss JW, Lisbon A (2006) Implementation and outcomes of the Multiple Urgent Sepsis Therapies (MUST) protocol. Crit Care Med 34(4):1025–1032
Trzeciak S, Dellinger RP, Abate NL, Cowan RM, Stauss M, Kilgannon JH, Zanotti S, Parrillo JE (2006) Translating research to clinical practice: a 1-year experience with implementing early goal-directed therapy for septic shock in the emergency department. Chest 129(2):225–232
Bilkovski RN, Rivers EP, Horst HM (2004) Targeted resuscitation strategies after injury. Curr Opin Crit Care 10(6):529–538
Chapman M, Gattas D, Suntharalingam G (2005) Why is early goal-directed therapy successful—is it the technology? Crit Care 9(4):307–308
Frey B, Macrae DJ (2005) Goal-directed therapy may improve outcome in complex patients—depending on the chosen treatment end point. Intensive Care Med 31(4):508–509
Gunn SR, Fink MP, Wallace B (2005) Equipment review: the success of early goal-directed therapy for septic shock prompts evaluation of current approaches for monitoring the adequacy of resuscitation. Crit Care 9(4):349–359
Rhodes A, Bennett ED (2004) Early goal-directed therapy: an evidence-based review. Crit Care Med 32(11 Suppl):S448–S450
Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, Ramsay G, Zimmerman JL, Vincent JL, Levy MM, Surviving Sepsis Campaign Management Guidelines Committee (2004) Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 32(3):858–873
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL, International Surviving Sepsis Campaign Guidelines Committee, American Association of Critical-Care Nurses, American College of Chest Physicians, American College of Emergency Physicians, Canadian Critical Care Society, European Society of Clinical Microbiology and Infectious Diseases, European Society of Intensive Care Medicine, European Respiratory Society, International Sepsis Forum, Japanese Association for Acute Medicine, Japanese Society of Intensive Care Medicine, Society of Critical Care Medicine, Society of Hospital Medicine, Surgical Infection Society, World Federation of Societies of Intensive and Critical Care Medicine (2008) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 36(1):296–327; erratum in: Crit Care Med 36(4):1394–1396
Boyd O, Grounds RM, Bennett ED (1993) A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. JAMA 270(22):2699–2707
Polonen P, Ruokonen E, Hippelainen M, Poyhonen M, Takala J (2000) A prospective, randomized study of goal-oriented hemodynamic therapy in cardiac surgical patients. Anesth Analg 90(5):1052–1059
Castro R, Regueira T, Aguirre ML, Llanos OP, Bruhn A, Bugedo G, Dougnac A, Castillo L, Andresen M, Hernández G (2008) An evidence-based resuscitation algorithm applied from the emergency room to the ICU improves survival of severe septic shock. Minerva Anestesiol 74(6):223–231
Gao F, Melody T, Daniels DF, Giles S, Fox S (2005) The impact of compliance with 6-hour and 24-hour sepsis bundles on hospital mortality in patients with severe sepsis: a prospective observational study. Crit Care 9(6):R764–R770
Jones AE, Focht A, Horton JM, Kline JA (2007) Prospective external validation of the clinical effectiveness of an emergency department-based early goal-directed therapy protocol for severe sepsis and septic shock. Chest 132(2):425–432
Lin SM, Huang CD, Lin HC, Liu CY, Wang CH, Kuo HP (2006) A modified goal-directed protocol improves clinical outcomes in intensive care unit patients with septic shock: a randomized controlled trial. Shock 26(6):551–557
Micek ST, Roubinian N, Heuring T, Bode M, Williams J, Harrison C, Murphy T, Prentice D, Ruoff BE, Kollef MH (2006) Before-after study of a standardized hospital order set for the management of septic shock. Crit Care Med 34(11):2707–2713
Cardoso T, Carneiro AH, Ribeiro O, Teixeira-Pinto A, Costa-Pereira A (2010) Reducing mortality in severe sepsis with the implementation of a core 6-hour bundle: results from the Portuguese community-acquired sepsis study (SACiUCI study). Crit Care 14(3):R83
Castellanos-Ortega A, Suberviola B, Garcia-Astudillo LA, Holanda MS, Ortiz F, Llorca J, Delgado-Rodríguez M (2010) Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three-year follow-up quasi-experimental study. Crit Care Med 38(4):1036–1043
Ferrer R, Artigas A, Levy MM, Blanco J, Gonzalez-Diaz G, Garnacho-Montero J, Ibáñez J, Palencia E, Quintana M, de la Torre-Prados MV, Edusepsis Study Group (2008) Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA 299(19):2294–2303
Huang DT, Clermont G, Dremsizov TT, Angus DC (2007) Implementation of early goal-directed therapy for severe sepsis and septic shock: a decision analysis. Crit Care Med 35(9):2090–2100
McIntyre LA, Hebert PC, Fergusson D, Cook DJ, Aziz A (2007) A survey of Canadian intensivists’ resuscitation practices in early septic shock. Crit Care 11(4):R74
Reade MC, Huang DT, Bell D, Coats TJ, Cross AM, Moran JL, Peake SL, Singer M, Yealy DM, Angus DC, British Association for Emergency Medicine, UK Intensive Care Society, UK Society for Acute Medicine, Australasian Resuscitation in Sepsis Evaluation Investigators, Protocolized Care for Early Septic Shock Investigators (2010) Variability in management of early severe sepsis. Emerg Med J 27(2):110–115
Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, Rubin HR (1999) Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 282(15):1458–1465
Kalil AC, Sun J (2008) Why are clinicians not embracing the results from pivotal clinical trials in severe sepsis? A Bayesian analysis. PLoS One 3(5):e2291
Kalil AC, Sun J (2008) How many patients with severe sepsis are needed to confirm the efficacy of drotrecogin alfa activated? A Bayesian design. Intensive Care Med 34(10):1804–1811
Mikkelsen ME, Gaieski DF, Goyal M, Miltiades AN, Munson JC, Pines JM, Fuchs BD, Shah CV, Bellamy SL, Christie JD (2010) Factors associated with non-adherence with early goal-directed therapy in the emergency department. Chest 138(3):551–558
Nguyen HB, Corbett SW, Steele R, Banta J, Clark RT, Hayes SR, Edwards J, Cho TW, Wittlake WA (2007) Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med 35(4):1105–1112
Wager GC (2005) Critical care physicians are proponents of early, aggressive, multifaceted therapy. Crit Care Med 33(3):702
Grobbee DE, Hoes AW (1997) Confounding and indication for treatment in evaluation of drug treatment for hypertension. BMJ 315(7116):1151–1154
Bosco JL, Silliman RA, Thwin SS, Geiger AM, Buist DS, Prout MN, Yood MU, Haque R, Wei F, Lash TL (2010) A most stubborn bias: no adjustment method fully resolves confounding by indication in observational studies. J Clin Epidemiol 63(1):64–74
Kalil AC (2010) Does recombinant activated protein C work in patients with severe sepsis? Crit Care Med 38(4):1217–1220
Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, Schorr C, Artigas A, Ramsay G, Beale R, Parker MM, Gerlach H, Reinhart K, Silva E, Harvey M, Regan S, Angus DC, Surviving Sepsis Campaign (2010) The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 38(2):367–374
Shapiro NI, Howell M, Talmor D (2005) A blueprint for a sepsis protocol. Acad Emerg Med 12(4):352–359
Sivayoham N (2007) Management of severe sepsis and septic shock in the emergency department: a survey of current practice in emergency departments in England. Emerg Med J 24(6):422
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ, ACCP/SCCM Consensus Conference Committee (2009) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. 1992. Chest 136(5 Suppl):e28
Marshall JC, Dellinger RP, Levy M (2010) The Surviving Sepsis Campaign: a history and a perspective. Surg Infect (Larchmt) 11(3):275–281
Townsend SR, Schorr C, Levy MM, Dellinger RP (2008) Reducing mortality in severe sepsis: the Surviving Sepsis Campaign. Clin Chest Med 29(4):721–733, x
Author information
Authors and Affiliations
Corresponding author
Additional information
B. Casserly and M. Baram contributed equally to this work.
Rights and permissions
About this article
Cite this article
Casserly, B., Baram, M., Walsh, P. et al. Implementing a Collaborative Protocol in a Sepsis Intervention Program: Lessons Learned. Lung 189, 11–19 (2011). https://doi.org/10.1007/s00408-010-9266-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-010-9266-z