Abstract
Nontuberculous mycobacteria (NTM) are resilient bacteria that grow in virtually any environment, especially those where competing microorganisms are destroyed, such as in chlorinated water. They have been discovered in soil, dust, food, water, and domestic and wild animals. Nontuberculous mycobacteria tend to infect individuals with local (e.g., damaged skin or lung) or systemic (e.g., HIV, drugs, malignancy) defects in host defence, and their incidence and prevalence have consistently increased in the last decade. Difficulty may arise in determining whether an isolated NTM from a microbiological sample is in fact a contaminant or a pathogenic organism. In this review, we discuss the important mycobacteria involved in lung disease, factors that predispose individuals to infection, and their diagnosis and treatment according to updated guidelines. English language publications in MEDLINE and references from relevant articles from January 1, 1990 to June 28, 2009 were reviewed. Keywords searched were “nontuberculous,” “mycobacteria,” “diagnosis,” and “treatment.”
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mycobacteria are a genus of aerobic, nonmotile bacteria with the unique property of being acid-alcohol fast due to their thick, resilient, hydrophobic cell wall and envelope which contains mycolic acids. In human medicine the most significant group of mycobacteria are the members of the Mycobacterium tuberculosis complex (consisting of Mycobacterium tuberculosis, Mycobacterium bovis, Mycobacterium africanum, Mycobacterium microti, Mycobacterium pinipendi, and Mycobacterium canettii) [1]. Together with Mycobacterium leprae, the bacillus responsible for leprosy, these mycobacteria cause the vast majority of mycobacterial disease in humans. These obligate pathogens are distinct from environmental mycobacteria, known as nontuberculous mycobacteria (NTM), which are found free living and ubiquitous in the environment and were formerly considered of minor clinical significance [2].
NTM infection can result in skin and lung infections, lymphadenitis, gastrointestinal disease, and, in severely immunocompromised individuals, disseminated disease. Cavitation of the upper lobes, similar to that seen in traditional tuberculosis (TB) patients, was the first described pulmonary manifestation of NTM disease [3, 4]. Symptoms of this NTM cavitary disease closely resembled those of TB and included fever, sweats, weight loss, productive cough, and haemoptysis [3]. Since then, fibronodular bronchiectasis and hypersensitivity pneumonitis have also been described [3].
NTM are not obligate pathogens and are not considered contagious, with human-to-human transmission never having been demonstrated [3]. Since these infections are not reportable, data on the extent of NTM disease as a public health problem have been limited. Nonetheless, there is evidence of a recent increase in NTM infections described by many groups in the developed world [5] in the face of a falling incidence of TB [1]. This increase may be contributed to by a heightened awareness of these pathogens and improvements in detecting these organisms in the laboratory [6].
As NTM organisms are found worldwide in a variety of habitats, including water supplies, and are rarely pathogenic, their presence in culture must be taken in clinical context, differentiating between colonisation or contamination and actual disease. Pseudo-outbreaks of NTM have been described as a result of contamination of hospital laboratories, water supplies, and instruments such as bronchoscopes [7]. For this reason, the American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) recommend that a diagnosis of NTM infection should be made only in the presence of clinical, radiological, and microbiological evidence of infection [3].
NTM have been grouped on the basis of speed of growth and pigment production. Runyon classified groups I–III as slow growers and group IV as rapid growers [8]. Following the development of 16S ribosomal DNA gene sequencing, species identification became more accurate, which led to the discovery of more mycobacterial species. Developments in laboratory techniques have led to a huge increase in our understanding and recognition of mycobacterial species and their role in disease. Traditionally, they have been classified on a phenotypic basis according to their behaviour in laboratory culture. Biochemical classification, including fastidiousness and antibiotic susceptibility, are also important differentiating features.
In recent years molecular and genotypic classifications have become possible, further increasing the number of recognised species [9]. Genotypic classification focuses on variable regions of genes common to all mycobacteria and identifies base pair variations within them. Analysis has focussed especially on hypervariable regions (A and B) in the 16S ribosomal DNA and on the 65-kDA heat shock protein gene. New target variable genes are also emerging [10]. Over 130 species of NTM have now been identified, although a minority of these are important lung pathogens (Table 1).
More Relevant Pulmonary NTM Pathogens
Mycobacterium avium Complex
The Mycobacterium avium complex (MAC) is the term used to describe a group of slow-growing, nonpigmented (may produce a yellow pigment when cultured in the absence of light) acid-fast bacilli. Individual species of the group cannot be differentiated on the basis of biochemical tests. Conventionally, MAC consists of at least two mycobacterial species; Mycobacterium avium and Mycobacterium intracellulare, with more members identified in the last decade [3, 10, 11]. MAC organisms are pervasive in nature and may be found in water (fresh or salt), soil, and house dust and have been found in municipal water supplies [11]. They are chlorine-resistant and can survive in water with a low concentration of organic matter at relatively high temperatures. This has led to clusters of infections in hot-water environments, including showers and hot tubs [12, 13]. They are found worldwide but are isolated more frequently in temperate regions, including the USA, Europe, Japan, and South Africa [11].
MAC is the most common cause of NTM infections and may cause pulmonary or disseminated disease [14–16]. This is of special significance in HIV-positive patients in whom disseminated MAC infection is an AIDS-defining illness and has been reported among AIDS patients in the developed and developing worlds [17]. In contrast to infection with TB in HIV-positive patients, those infected with MAC are more likely to have a positive acid and alcohol fast bacillus (AAFB) bacteraemia and a lower CD4+ count and are less likely to have a positive sputum smear for AAFB [11]. Symptoms of lung disease include cough, sputum production, fever, night sweats, haemoptysis, malaise, and fatigue. Fever and haemoptysis are more common in patients with smear-positive sputum [18]. Infection may be insidious, with diagnosis years after the onset of symptoms.
In HIV-seronegative patients, MAC may more commonly cause lung disease in two distinct patterns that are routinely detectable on HRCT thorax. The first is in individuals with underlying lung disease where MAC manifests as apical fibrocavitatory disease. The second is nodular bronchiectasis, also described as Lady Windermere Syndrome, an illness, typically in elderly women, characterised by lingula or middle-lobe interstitial nodular disease [19]. Hypersensitivity pneumonitis has also been described in hot-tub users exposed to this pathogen [20–22]. Pleural effusions are rare, but pleural thickening adjacent to localised infection has been described [23].
MAC is saprophytic, with cell walls relatively impenetrable to an array of chemicals that endows them with intrinsic resistance to many antimicrobials; e.g., isoniazid is 100 times less effective against MAC than against M. tuberculosis [24]. This makes treatment of infections more challenging, which is further compounded by the fact that in vitro susceptibility testing often fails to prove any predictive benefit in assessing clinical response to treatment, with the notable exception of testing for macrolide resistance [3].
Mycobacterium kansasii
This slow-growing, photochromic mycobacterium is commonly found to be the second-most frequent cause of NTM pulmonary disease around the world [14, 25]. In certain geographic areas, including parts of the UK and the central US, it causes lung disease more frequently than MAC or other NTMs [16]. M. kansasii lung disease often occurs in geographic clusters, commonly in urban areas, and associations with coal mining areas and areas with high HIV prevalence exist [12, 26–30]. Isolates are usually thought to be of clinical significance, unlike other NTMs which commonly cause environmental contamination. In contrast to other NTMs, M. kansasii cannot survive for long periods in soil, but it has shown to be able to survive in water for up to 12 months [12]. Moreover, it is detected almost exclusively in water systems, including treated water, such as tap water, shower heads, and water distribution plants.
M. kansasii is known to have several different subtypes, of which at least seven have been identified. Subtype I is the most common form isolated from infected humans and is rarely found in the environment [31]. The other subtypes are most commonly isolated from environmental samples, although subtype II is occasionally the cause of opportunistic infections in immunocompromised patients [31].
Risk factors for infection with M. kansasii include pre-existing pulmonary disease, including COPD, malignancy, pneumoconiosis, bronchiectasis, and previous mycobacterial disease, as well as a history of alcohol excess [32]. Although M. kansasii infection is more common in a HIV-positive than a HIV-negative population, the high pathogenicity of this mycobacterium means that the mean CD4 count of those infected is higher than those infected with MAC. Due to the degree of immune response, these HIV-positive patients are less likely to present with a disseminated bacteraemia than those infected with MAC, and more than half present with pulmonary disease [33]. M. kansasii is commonly responsible for NTM disease in renal transplant patients, in whom disseminated or cutaneous disease is more common than pulmonary disease [34].
Nonspecific symptoms, often with haemoptysis, are common presenting features of infection, typically in middle-aged to elderly men [3]. The spectrum of disease seen can range from a mild self-limiting disease to persistent infection resulting in chronic structural damage to the lung parenchyma [35].
Radiologically, infection typically presents with unilateral cavitating apical lung disease and can closely resemble classical TB infection, causing diagnostic difficulties. However, more detailed imaging may reveal cavities with thinner walls and increased pleural reaction than that seen with TB [36]. Less frequently, a fibrosing nodular pattern has been identified, similar to the appearances of MAC infection [36].
In contrast to some of the other common NTM, M. kansasii is known to be highly pathogenic and is rarely isolated from humans in the absence of disease [3]. In addition, M. kansasii is more susceptible to treatment than many of the other NTMs. Diagnosis of M. kansasii is possible using a variety of techniques, including microscopy, culture, and species identification using high-performance liquid chromatography (HPLC) analysis or probe assays. Bronchial washings have been shown to be more reliable for yielding this organism than sputum samples [37].
Mycobacterium malmoense
Although rarely found in isolates from the US [38], Mycobacterium malmoense is commonly found in northern Europe, particularly in the UK and Scandinavia [39]. One study has shown M. malmoense to be responsible for up to 48% of NTM lung disease [39]. It is known to survive in soil and natural collections of water with environmental contamination of samples recognised.
In recent years there has been an increased incidence of M. malmoense isolated from clinical samples [3]. This is likely to represent improved laboratory techniques for culturing and identifying this slow-growing, nonphotochromogenic species combined with increased physician awareness. Culture on standard solid media can be difficult and can take several weeks, but results are seen with liquid media within only 10–21 days [39]. Techniques including HPLC analysis and a variety of probe assays can also be used for the identification of this mycobacterium.
Clinical disease is normally seen in middle-aged men who have a history of smoking and underlying lung disease such as COPD [40]. Alcohol excess is also a significant risk factor for disease [40]. Pulmonary infection normally presents with nonspecific symptoms, including cough, weight loss, fever, and haemoptysis. While pulmonary disease is the usual manifestation of M. malmoense infection, this organism can also cause lymphadenitis, tenosynovitis, and cutaneous and soft tissue infections. Disseminated disease has been reported in the immunocompromised, including HIV patients [41].
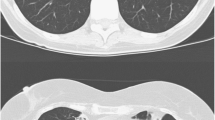
Radiologically, M. malmoense often causes a typical tuberculosis-like picture, including upper-lobe infiltrates and cavitation (Fig. 1), and sometimes nodular infiltrates. Large cavities, air-fluid levels, volume loss, and pneumonconiosis are seen more commonly with M. malmoense than with TB [42]. Hoefsloot et al. [43] recently examined patients who had M. malmoense isolated from their sputum and found that 80% of them fulfilled the ATS guidelines for diagnosis, indicating that this organism is a clinically highly relevant NTM. They also found that 78% of patients had pulmonary isolates and that cavitatory disease was most common (88%) [43]. Males with an average age of 56 years and a history of COPD presented most frequently [43]. Like M. kansasii, M. malmoense is thought to be highly pathogenic, and unlike many of the other NTMs, it is thought to be associated with active disease.
Mycobacterium xenopi
This scotochromogenic, thermophilic mycobacterium grows best at 45°C. Consequently, it has been found to colonize hot water tanks and cleaning systems, leading to contamination of laboratory samples and hospital equipment, with pseudoepidemics well recognised [44]. It is an important cause of NTM pulmonary disease in the UK, Europe, and areas of Canada but is rarely isolated in the US [45, 46].
In a retrospective study in Croatia, where M. xenopi is the most frequently isolated NTM, 60% of patients with M. xenopi isolates met the ATS/IDSA criteria for pulmonary disease [47]. All were immunocompetent but 90% had comorbidities, with COPD being the most common, carrying with it a worse treatment prognosis [47]. M. xenopi has only rarely been reported to cause extrapulmonary infection.
Mycobacterium szulgai
A slow-growing mycobacterium, recognized as a species since 1972, M. szulgai is genetically closest to M. malmoense but phenotypically unique [48]. It is an extremely rare pathogen but when isolated represents infection in the majority of instances. A recent study of all isolates of M. szulgai in the Netherlands over a 7-year period found that ATS/IDSA criteria for significant infection were met in 76% of cases [49]. A very limited number of cases have been reported, with most infections pulmonary, although it can manifest as infection of skin and renal tract, osteomyelitis, olecranon bursitis, and cervical lymphadenitis [50].
Presentation is clinically similar to tuberculosis, with upper-lobe cavitation, cachexia, and cough being common. The bulk of reported cases of pulmonary szulgai have been in middle-aged men, the majority of which had other risk factors such as COPD, smoking, alcohol abuse, and previous pulmonary tuberculosis [12]. A number of reported patients have also had HIV/AIDS. Immunosuppression is also a risk factor for extrapulmonary infection [49].
Mycobacterium scrofulaceum
Mycobacterium scrofulaceum is a slow-growing scotochromogenic NTM that can be definitively identified only by using molecular techniques. It is most renowned for causing lymphadenitis in children, although currently MAC is responsible for more cases [51]. Mycobacterium scrofulaceum is a rare cause of pulmonary disease that can present as a tuberculosis-like illness and it is associated with occupational dust exposure and a history of smoking [52]. It was responsible for pulmonary disease in 41 HIV-negative gold miners in South Africa between 1993 and 1996, of which 43% had evidence of previous lung disease on premorbid chest X-ray and 51% had previously received treatment for tuberculosis [27].
Mycobacterium simiae
Owing its name to its original isolation from rhesus monkeys in 1965, Mycobacterium simiae is a photochromogenic mycobacterium that grows best at 37°C. It is unique among NTM species in that it produces niacin, which can lead to a misdiagnosis of M. tuberculosis. It shares other biochemical features with MAC and M. scrofulaceum [53]. Conclusive identification of M. simiae may require genotypic analysis such as 16s RNA sequencing [54].
M. simiae is a rare cause of NTM disease in humans. It has been shown to be transmissible between monkeys in captivity [55]. M. simiae disease has been reported in France, Germany, and Thailand, but most cases have clustered in the southern states of the US, Cuba, and Israel [3, 56–58]. The majority of isolates do not represent significant infection as was demonstrated when a pseudo-outbreak in a Texas hospital was attributed to a contaminated hot-water system [59–61]. Disease occurs predominantly in patients with pre-existing pulmonary disease or immunosuppression, especially AIDS. Radiographical features include noncavitating infiltrates of the lower and middle lobes, and symptoms such as dyspnoea and productive cough are nonspecific [62].
Rapidly Growing Mycobacteria (RGM)
RGMs are classified as AAFB which form colonies on solid agar that are visible to the naked eye in 7 days or less [8]. There are three important species of RGM encountered in the context of human pulmonary disease, Mycobacterium abscessus, M. fortuitum, and M. chelonae. All of these species have been found in cold-water systems and they are relatively resistant to commonly used disinfecting chemicals [63, 64].
M. abscessus is the third most frequently isolated NTM pathogen in pulmonary disease in the US and is isolated four times more frequently than the other RGMs combined [65]. Two American studies of RGMs found M. abscessus to be the most frequently isolated RGM pathogen, with one of these studies demonstrating that 82% of RGM lung disease was due to M. abscessus [65, 66]. On the other hand, a Thai study did not find this to be the case, suggesting a regional variation in RGM species causing infection [67].
Disease usually manifests on HRCT as fibronodular bronchiectasis, although cavities and opacities have also been detected [68]. It is associated with gastro-oesophageal reflux and cystic fibrosis, where it is the second-most commonly isolated NTM after MAC [69, 70]. It rarely causes disseminated disease and is frequently resistant to antibiotic therapy, with localised surgery the only option to cure the disease in the most severe cases [71, 72].
M. fortuitum pulmonary infection has a better prognosis than infection with M. abscessus, although presentation is similar. Isolation less often represents a true infection and must be taken in clinical context [73]. M. fortuitum has been shown to possess an rRNA methylase gene known as erm which confers intrinsic macrolide resistance by impairing drug binding to the ribosome [74]. Therefore, macrolide monotherapy should never be used in treating infection. More recently, the erm gene has been detected in M. abscessus [75].
M. chelonae is resistant to disinfectants and can become resistant to gluteraldehyde, rendering it strong enough to survive endoscope cleaning [75, 76]. It is most frequently associated with pseudo-epidemics and specimen contamination. However, it has been reported to cause pulmonary disease, typically diffuse bronchiectasis and lung nodules, although empyema and bronchopleural fistula have been described [77, 78]. It is more commonly a pathogen of soft tissue and skin, particularly in immunocompromised patients or those treated with corticosteroids, and overall it is an unusual cause of NTM pulmonary disease where it can be very resistant to antibiotic therapy [79, 80].
Underlying Lung Disease as a Predisposing Factor
NTM isolates were originally discovered in individuals with underlying lung disease. Investigators wondered whether isolated NTM was a pathogen, coloniser, or contaminant. As time passed it became clear that certain underlying lung conditions, including pneumoconiosis, cystic fibrosis (CF), silicosis, tuberculosis, and pulmonary alveolar proteinosis, were associated with NTM infection [6]. In particular, studies on NTM infection in coal workers revealed a close association [27, 28].
While NTM is more common in bronchiectasis, infection with the pathogen itself can result in bronchiectasis. NTM may colonise and have a slow-burning course or an aggressive course in this group of patients. In 2006, Fowler et al. [81] found that 10% of their bronchiectasis patients had one random sputum culture positive for NTM and 3% of their patients had actual NTM lung disease. There was no difference in sex, age, and spirometry readings between infected and noninfected cases. M. abscessus and MAC are well-described pathogens in this group and can cause cavitation and worsening bronchiectasis [82].
Prevalence studies on CF patients have found that 4–20% of patients are positive for NTM [72, 83, 84]. In the US, a multicentre trial revealed that in CF patients with sputum positive for NTM, M. abscessus and MAC were present in 16 and 72%, respectively [72]. In Israel, Mussaffi et al. [85] found that CF patients treated with systemic steroids or who had concomitant allergic bronchopulmonary aspergillosis were more likely to have NTM cultured from their sputum.
Musculoskeletal abnormalities, including pectus excavatum and scoliosis, are also associated with MAC disease, with studies showing higher disease rates when compared to healthy controls [86].
When Henry et al. [87] looked at NTM infection in non-HIV patients, they also found that underlying lung disease was a significant contributing factor. For example, in the case of M. malmoense infection, eight patients were predisposed with COPD, one with bronchiectasis, one with cryptogenic fibrosing alveolitis (CFA), two with previous TB disease, and one with lung cancer. Another study revealed a close association between M. malmoense and coal worker pneumoconiosis [88].
Gastro-oesophageal reflux disease (GORD) is also associated with the development of NTM disease but it is difficult to ascertain the exact relationship. Does reflux cause lung disease that predisposes to NTM or does reflux expose the lung to more NTM? A recent study found that patients with MAC lung disease had a stronger association with GORD than age-matched controls [89]. Koh et al. [90] demonstrated that 26% of their NTM lung disease study group showed evidence of GORD when investigated with 24-h pH monitoring. CT thorax was more likely to show more widespread bronchiectasis and the sputum was more likely to be smear positive in these individuals. Surprisingly, only 27% of NTM lung disease patients have symptoms of reflux [89].
Acquired Immunosuppression as a Predisposing Factor
In HIV disease, individuals are generally predisposed to NTM infection once the CD4+ count is below 50/μl, with MAC the most prevalent pathogen [91, 92]. This group of patients tends to present with disseminated disease, with symptoms of weight loss, fever, night sweats, and anorexia. Clinical examination may reveal evidence of hepatosplenomegaly, lymphadenopathy, and anaemia [91]. These patients rarely experience significant NTM lung disease [93], and those that do tend to do so in the absence of dissemination, a phenomenon seen with M. kansasaii in particular [94]. Research in gold miners from South Africa demonstrated that the average CD4+ count associated with M. kansasaii infection was 381 × 106/l [27, 28]. Patients infected with M. kansasii and HIV who had a low CD4+ count and positive sputum smear had a poor prognosis. Treatment targeting M kansasii infection in combination with highly active antiretroviral therapy (HAART) improved survival [95].
In HIV patients, treatment of NTM infection can lead to important drug interactions between rifampicin and HAART. First, rifampicin accelerates the clearance of HAART thereby reducing its potency and effect in the treatment of HIV. Second, HAART may inhibit rifampicin metabolism resulting in toxic circulating levels of the drug and its associated side effects [3].
HARRT reduces HIV RNA levels with a concomitant increase in CD4+ lymphocyte levels which improves defensive inflammatory reactions toward infection and may manifest itself as immune reconstitution inflammatory syndrome (IRIS), with clinical deterioration of the patient [96, 97]. MAC is the most common pathogen involved in NTM-related IRIS [98]. In Canada, Shelburne et al. [99] demonstrated that 3.5% of HIV patients who started HARRT experienced NTM-related IRIS. They also noted that NTM-related IRIS occurred more commonly in patients who had a rapid improvement in CD4+ levels with therapy and in those who experienced early opportunistic infections [99].
Immunosuppression due to solid-organ or stem-cell transplantation is also associated with NTM disease. MAC and M. abscessus are the most common pathogens detected [34]. Malouf et al. [100] observed that 9% of patients at their heart and lung transplant centre were infected with NTM. NTM infection in renal transplantation is rare, with disseminated disease occurring more frequently than lung disease. In one study from Spain, five cases of NTM lung disease were found in 1,261 renal transplant patients, all related to M. kansasaii [101]. Pulmonary infection with M. xenopi and M. chelonae has also been described in renal transplant patients [34]. Treating these infections can be hampered by the interactions between the antibiotics and immunosuppressive drugs.
Malignancy is associated with a slight increase in the incidence of NTM, although it is not known if this effect is due to deficiencies in cell-mediated immunity, corticosteroids, chemotherapy, or underlying lung disease. For example, a previous study showed that 2.5 cases of M. kansasaii disease occurred per 100,000 patients with malignancy, which is significantly greater than the normal rate of 1–1.8 per 100,000 in the normal population [102]. Leukaemia has a stronger association with NTM disease than solid-organ malignancy, with an incidence of 5% in hairy cell leukaemia [103, 104].
NTM disease has been associated with diabetes mellitus. Cases of skin infection with M. chelonae at insulin injection sites have been reported [105–108]. Pleuritis, thyroiditis, pneumonia, and tenosynovitis related to NTM have also been described in patients with diabetes [109–112].
NTM lung disease has been described in patients taking anti-TNFα therapy, an effective treatment for many inflammatory disorders such as Crohn’s disease and rheumatoid arthritis. In particular, MAC, M. xenopi, M. abscessus, and M. szulgai have occurred while taking etanercept [113–115].
Congenital and Familial Immune Characteristics as a Predisposing Factor
Research in individuals who experience recurrent NTM infections has found that certain patients are predisposed to NTM due to any number of genetic defects in the IL-12 and IFN-γ signalling cascade [116]. Mutations of the NF-κB gene lead to different types of infection, including those due to NTM [117]. Chronic granulomatous disease has also been associated with NTM disease [118]. HLA, DR6, DQ4, A33, and A26 have all been associated with MAC lung disease [119, 120]. There has also been an association between CFTR mutations and NTM lung disease. Ehrmantraut et al. [121] demonstrated that 24% of patients with NTM lung disease had at least one CFTR mutation. A study by Ryu et al. [122] on Toll-Like Receptor 2 (TLR-2) on peripheral monocytes from individuals exposed to MAC and controls led them to propose that a defect in TLR-2 conferred susceptibility to NTM disease.
Data on the predisposition to NTM disease due to vitamin D receptor or NRAMP1 (natural resistance-associated macrophage protein-1) polymorphisms is more controversial, with different groups showing positive and negative associations [120, 123].
Diagnosis
As NTM disease is relatively rare, clinical suspicion is an extremely important component of the diagnostic process. This element of suspicion is useful in patients with underlying lung disease, immunosuppression, or constitutional symptoms. The ATS/IDSA guidelines recommend that the minimum evaluation of a patient suspected of having NTM lung disease should include the following: (1) chest radiograph or, in the absence of cavitation, chest HRCT scan, (2) three or more sputum specimens for AAFB analysis, and (3) exclusion of other disorders such as TB and lung malignancy [3]. These guidelines also highlight that diagnosis can be made without bronchoscopy or lung biopsy in the majority of cases [3].
Fluorochrome staining is rapid and the preferred stain of choice for initial samples [124]. Specimens are also treated with sodium hydroxide and N-acetylcysteine, followed by oxalic acid to prevent overgrowth of Pseudomonas aeruginosa which is frequently present in CF and non-CF bronchiectasis sufferers [6]. The specimens are cultured in both solid and liquid media. It can take approximately 3–6 weeks for detectable growth to be observed on solid media. However, growth is detected after 1–2 weeks in liquid media [3]. TB is quickly distinguished from NTM using nucleic acid amplification techniques in both smear-positive and -negative disease [124]. In addition, TB can be differentiated from NTM because nitrate reductase, catalase, and niacin production in both pathogens are extremely different [3]. TB can be differentiated from NTM also by using the para-nitro-α acetylamino-β hydroxypropiophenone test [125]. NTM species identification normally takes an additional 3–6 weeks once sufficient growth is detected on solid media [124]. Modern techniques such as the use of DNA probes, restriction fragment length polymorphism analysis, amplification techniques, high-performance liquid chromatography (HPLC), and DNA sequencing have improved the rapid identification of NTM species [6].
Once the species has been identified, antibiotic susceptibility testing may be performed on clinically relevant isolates. This process is very useful in determining the optimal antibiotic combination therapy for the relevant pathogen; however, a number of important exceptions exist. In the case of MAC disease, in vitro results do not correlate with the clinical response to therapy (except in the case of clarithromycin) and, thus, other antimicrobial susceptibility testing is not routinely performed [124]. Susceptibility testing is particularly important in M. kansasaii disease, particularly with rifampicin, where resistance can predict therapy failure [124].
The ATS/IDSA guidelines of 2007 [3] lay down criteria for the diagnosis of NTM (Table 2). Diagnosis of pulmonary infection requires fulfilment of clinical and microbiological criteria. Both are required for diagnosis. Clinically, (1) pulmonary symptoms, nodular or cavitory opacities on chest radiograph, or a high-resolution computed tomography scan that shows multifocal bronchiectasis with multiple small nodules and (2) appropriate exclusion of other diagnoses are required. Microbiologically, only one of the listed criteria are required: (1) positive culture results from at least two separate expectorated sputum samples, (2) a positive culture result from at least one bronchial wash or lavage, or (3) transbronchial or other lung biopsy, with mycobacterial histopathologic features (granulomatous inflammation or AFB) and positive culture for NTM, or biopsy showing mycobacterial histopathologic features (granulomatous inflammation or AFB) and one or more sputum or bronchial washings that are culture positive [3].
Treatment
The treatment of nontuberculous mycobacteria remains largely undefined and cannot be viewed as definitive [1].
The microbiological finding of NTM does not always prompt the initiation of treatment. In cases of positive sputum cultures in the absence of disease, i.e., colonisation, the ATS/IDSA advise withholding treatment because of potential toxicities and the uncertain rate of progression [3]. The decision to treat is also dependent upon the risks and benefits for the individual patient [3], taking into account comorbidities and pre-existing lung disease. The treatment regimen itself is complicated, being dependent on the species of NTM identified, in vitro susceptibility testing of the isolates, and the extent of infection [3].
Some NTM such as M kansasii are relatively straightforward to treat, but others are extremely difficult, e.g., MAC [1, 126]. In general, treatment requires intense regimes involving multiple antimycobacterial drugs that are administered until sputum cultures (sampled monthly) have been negative for at least a year, which commonly equates to over 12 months of treatment [3]. This long duration of treatment, its side effects, and interactions impact on patient compliance; therefore, understandably, treatment outcome is variable and often poor [1, 126].
Mycobacterium avium complex (MAC)
The most important component of treatment regimens for MAC is a macrolide, either clarithromycin or azithromycin [3]. Current ATS/IDSA recommendations state that treatment for MAC pulmonary disease should utilise a macrolide plus rifamycin or rifampicin and ethambutol [3]. For nodular/bronchiectatic disease, a three times weekly regimen including clarithromycin (1 g) or azithromycin (500 mg), rifampicin (600 mg), and ethambutol (25 mg/kg) is recommended, whereas for fibrocavitary or severe nodular/bronchiectatic disease, a daily regimen that includes clarithromycin (500 mg–1 g) or azithromycin (250 mg), rifampicin (600 mg) or rifabutin (150–300 mg), and ethambutol (15 mg/kg) with the possible addition of three times weekly amikacin/streptomycin early in therapy is advocated [3]. It is advised that treatment continue for 12 months after sputum culture conversion. [126]. Conversely, the British Thoracic Society (BTS) does not recommend the addition of rifampicin based on several reports that have suggested that rifampicin reduces serum concentrations of clarithromycin [127]. Regimens containing clarithromycin, ethambutol, and clofazimine have similar sputum conversion and relapse rates but are much better tolerated and easier to administer [128]. The main limitation to treatment programmes is drug intolerance, especially gastrointestinal which can be improved by introducing the antibiotics gradually over several weeks [6, 126]. In the case of macrolide resistance, isoniazid, rifampicin or rifabutin, ethambutol and amikacin, or streptomycin (first 6 months) is recommended [3, 126].
When there is disseminated MAC, even more intensive therapy is recommended by ATS/IDSA, incorporating daily clarithromycin (1 g) or azithromycin (500 mg) as an alternative and ethambutol (15 mg/kg), and maybe the addition of rifabutin (150–350 mg) [3].
Hypersensitivity pneumonitis is treated first by avoiding the mycobacterial antigen. In cases of severe disease or respiratory failure, prednisolone 1–2 mg/kg/day tapered over 4–8 weeks is recommended [3]. In immunocompromised individuals with persistent disease despite removal of the mycobacterial antigen (±steroid therapy) or in bronchiectasis patients, therapy with the relevant antimicrobial drugs as per routine infection is recommended, albeit for a shorter duration (e.g., 3–6 months) [3].
M. kansasii
In general, this species is very responsive to treatment and has a good prognosis [1]. Current guidelines set out by the ATS/IDSA suggest daily isoniazid (300 mg/day), rifampicin (600 mg/day), and ethambutol (15 mg/kg/day) [3]. If the organism is rifampicin-sensitive, this regimen is highly effective with low failure or relapse rates [126]. If there is extensive disease, combination with an aminoglycoside, e.g., streptomycin has had good effect [3, 126]. If the species is resistant to rifampicin or the patient cannot tolerate the above regimen, then clarithromycin, which also has substantial in vitro activity against M. kansasii, can be substituted [126]. The regimen of daily high-dose isoniazid (900 mg), ethambutol (25 mg/kg), pyridoxine (50 mg), and sulfamethoxazole (1 g TDS) with streptomycin or amikacin daily or five times per week for the initial 2–3 months, followed by amikacin or streptomycin intermittently for a total of 6 months has also been used in rifampicin-resistant cases [3, 127]. Treatment should be given until 12 months of negative sputum results has been achieved [3]. Research has been carried out on shortening the length of treatment; however, although reported sputum conversion rates are excellent, the relapse rate is higher [126].
M. malmoense
There are variable results from susceptibility testing of this organism and these correlate poorly with clinical response [1, 40]. The BTS suggest that combinations of isoniazid, rifampicin, and ethambutol with or without macrolides and quinolones have been successful [40]. They also advise to initiate a course of rifampicin and ethambutol despite any in vitro susceptibility testing because of this poor clinical correlation with in vitro cultures. General health and comorbidities appear to be a major contributing factor to the outcome of this infection and thus optimising overall health should always be a priority [1].
M. abscessus
The ATS/IDSA states that no drug regimen is of proven or predictable efficacy [3], but when treatment is indicated, it should be guided by drug susceptibility testing. The following antibacterial agents should be included in the susceptibility testing: clarithromycin, amikacin, tobramycin, quinolones, sulfamethoxazole, doxycycline, and imipenem [126]. Treatment usually involves clarithromycin, amikacin, and imipenem [3]. Surgical resection combined with drug therapy is the best treatment for M. abscessus [3]. Drug treatment is often aimed not at eradication but at symptom control when surgical intervention is not possible. Overall treatment outcome has been described as poor [1].
M. chelonae
The optimal therapy for M. chelaonae lung disease is unknown [3]. In vitro susceptibility testing suggests clarithromycin with an adjunct dependent on specific susceptibility tests in vitro for the particular culture [3].
M. xenopi
The optimal regimen for treatment of this mycobacterium is yet to be established, and in vitro susceptibility testing can be difficult to interpret with little correlation with clinical response [3]. The ATS/IDSA suggests that the best treatment regimen combines clarithromycin, rifampicin, and ethambutol (with or without streptomycin) [3]. The adjunct of surgical resection has been very successful in cases of very poor response to antimycobacterials or relapse [1].
M. fortuitum
This NTM exhibits macrolide resistance conferred by an inducible erythromycin methylase erm gene [74]. M. fortuitum, however, is susceptible to a wide range of antibiotics and individual testing should be carried out for the optimal regimen. Testing should include sulfonamides, amikacin, fluoroquinolones, doxycycline, cefoxitin, and imipenem [68]. A combination of at least two agents is advised for M. fortuitum lung disease, depending on the in vitro susceptibility testing [3]. Again, treatment should continue for at least 12 months of negative sputum cultures.
M. simiae
M. simiae is an uncommon cause of clinical disease that remains difficult to treat due to the poor clinical predictability following in vitro susceptibility tests. The more positive reports involve clarithromycin-based regimens, e.g., clarithromycin, moxifloxacin, and trimethoprim/sulfamethoxazole [3]. However, resistance to all first-line antimycobacterials has been described [128].
M. scrofulaceum
Molecular methodology is necessary to differentiate this isolate from other NTMs and there is minimal information on in vitro susceptibility and treatment regimens [3].
M. szulgai
Optimal treatment protocols are yet to be fully established, but it is suggested that a three to four drug regimen (including rifampicin, ethambutol, isoniazid with or without pyrazinamide) continued until a year of negative sputum cultures is achieved would be adequate [3, 126]. Susceptibility testing for M. szulgai does correlate with clinical response and treatment regimens should be adjusted accordingly [1] (Table 3).
Conclusions
Numerous studies have demonstrated that NTM disease is increasing in incidence and prevalence [14, 87, 129]. Over time physicians will deal with increased positive microbiological reports of NTM and therefore it is important that colonisation, contamination, and actual pulmonary infection are differentiated properly. The ATS/IDSA updated their guidelines in 2007, relaxing the microbiological criteria for diagnosis but making the clinical criteria more stringent [130].
Despite advances in diagnostics and drug therapy, prevalence fails to fall. A contributing factor to this is the poor compliance with drug therapy due to its long duration, drug interactions, and side effects. Appropriate drug regimen prescription is extremely important. Henry et al. [87] found that patients who were prescribed the recommended treatment as per guidelines had far better outcomes than those who were not. They also found that almost all of the patients with pulmonary infection had underlying lung disease.
Clearly, additional work in the area of diagnosis and treatment is warranted, but in the meantime, continued vigilance among at-risk populations and strict adherence to the updated international treatment guidelines are key to controlling and eradicating pulmonary NTM disease.
Abbreviations
- AFB:
-
Acid fast bacilli
- AIDS:
-
Acquired immunodeficiency syndrome
- ATS:
-
American Thoracic Society
- BAL:
-
Bronchoalveolar lavage
- CD:
-
Cluster of differentiation
- CF:
-
Cystic fibrosis
- CFA:
-
Cryptogenic fibrosing alveolitis
- CFTR:
-
Cystic fibrosis transmembrane conductance regulator
- COPD:
-
Chronic obstructive pulmonary disease
- CT:
-
Computed tomography
- DNA:
-
Deoxyribonucleic acid
- GORD:
-
Gastroesophageal reflux disease
- HAART:
-
Highly active antiretroviral treatment
- HIV:
-
Human immunodeficiency virus
- HLA:
-
Human leukocyte antigen
- HPLC:
-
High-performance liquid chromatography
- HRCT:
-
High-resolution computed tomography
- IDSA:
-
Infectious Diseases Society of America
- IFN:
-
Interferon
- IL:
-
Interleukin
- IRIS:
-
Immune reconstitution inflammatory syndrome
- MAC:
-
Mycobacterium avium complex
- NFκB:
-
Nuclear factor κB
- NRAMP:
-
Natural resistance-associated macrophage protein
- NTM:
-
Nontuberculous mycobacteria
- RGM:
-
Rapidly growing mycobacteria
- RNA:
-
Ribonucleic acid
- TDS:
-
Three times daily
- TLR:
-
Toll-like receptor
- TNF:
-
Tumor necrosis factor
References
Piersimoni C, Scarparo C (2004) Pulmonary infections associated with non-tuberculous mycobacteria in immunocompetent patients. Lancet Infect Dis 8(5):323–334
Herdman AV, Steele JC Jr (2004) The new mycobacterial species—emerging or newly distinguished pathogens. Clin Lab Med 24(3):651–690, vi
Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K, ATS Mycobacterial Diseases Subcommittee, American Thoracic Society, Infectious Disease Society of America (2007) An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175(4):367–416
Crow HE, King CT, Smith CE, Corpe RF, Stergus I (1957) A limited clinical, pathologic, and epidemiologic study of patients with pulmonary lesions associated with atypical acid-fast bacilli in the sputum. Am Rev Tuberc 75(2):199–222
McGrath EE, Anderson PB (2007) Increased prevalence of non-tuberculous mycobacteria infection. Lancet 370(9581):28
Field SK, Cowie RL (2006) Lung disease due to the more common nontuberculous mycobacteria. Chest 129(6):1653–1672
Gubler JG, Salfinger M, von Graevenitz A (1992) Pseudoepidemic of nontuberculous mycobacteria due to a contaminated bronchoscope cleaning machine. Report of an outbreak and review of the literature. Chest 101(5):1245–1249
Runyon EH (1959) Anonymous mycobacteria in pulmonary disease. Med Clin North Am 43(1):273–290
Tortoli E (2003) Impact of genotypic studies on mycobacterial taxonomy: the new mycobacteria of the 1990 s. Clin Microbiol Rev 16(2):319–354
Tortoli E (2006) The new mycobacteria: an update. FEMS Immunol Med Microbiol 48(2):159–178
Inderlied CB, Kemper CA, Bermudez LE (1993) The Mycobacterium avium complex. Clin Microbiol Rev 6(3):266–310
Falkinham JO III (1996) Epidemiology of infection by nontuberculous mycobacteria. Clin Microbiol Rev 9(2):177–215
Sugita Y, Ishii N, Katsuno M, Yamada R, Nakajima H (2000) Familial cluster of cutaneous Mycobacterium avium infection resulting from use of a circulating, constantly heated bath water system. Br J Dermatol 142(4):789–793
Thomsen VO, Andersen AB, Miorner H (2002) Incidence and clinical significance of non-tuberculous mycobacteria isolated from clinical specimens during a 2-y nationwide survey. Scand J Infect Dis 34(9):648–653
Haverkort F (2003) National atypical mycobacteria survey, 2000. Commun Dis Intell 27(2):180–189
Martin-Casabona N, Bahrmand AR, Bennedsen J, Thomsen VO, Curcio M, Fauville-Dufaux M, Feldman K, Havelkova M, Katila ML, Köksalan K, Pereira MF, Rodrigues F, Pfyffer GE, Portaels F, Urgell JR, Rüsch-Gerdes S, Tortoli E, Vincent V, Watt B, Spanish Group for Non-Tuberculosis Mycobacteria (2004) Non-tuberculous mycobacteria: patterns of isolation. A multi-country retrospective survey. Int J Tuberc Lung Dis 8(10):1186–1193
Crump JA, van Ingen J, Morrissey AB, Boeree MJ, Mavura DR, Swai B, Thielman NM, Bartlett JA, Grossman H, Maro VP, van Soolingen D (2009) Invasive disease caused by nontuberculous mycobacteria, Tanzania. Emerg Infect Dis 15(1):53–55
Obayashi Y, Fujita J, Suemitsu I, Kamei T, Nii M, Takahara J (1998) Clinical features of non-tuberculous mycobacterial disease: comparisons between smear-positive and smear-negative cases, and between Mycobacterium avium and Mycobacterium intracellulare. Int J Tuberc Lung Dis 2(7):597–602
Reich JM, Johnson RE (1992) Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. The Lady Windermere syndrome. Chest 101(6):1605–1609
Embil J, Warren P, Yakrus M, Stark R, Corne S, Forrest D, Hershfield E (1997) Pulmonary illness associated with exposure to Mycobacterium-avium complex in hot tub water. Hypersensitivity pneumonitis or infection? Chest 111(3):813–816
Kahana LM, Kay JM, Yakrus MA, Waserman S (1997) Mycobacterium avium complex infection in an immunocompetent young adult related to hot tub exposure. Chest 111(1):242–245
Cappelluti E, Fraire AE, Schaefer OP (2001) A case of “hot tub lung” due to Mycobacterium avium complex in an immunocompetent host. Arch Intern Med 163(7):845–848
Tanaka D, Niwatsukino H, Oyama T, Nakajo M (2001) Progressing features of atypical mycobacterial infection in the lung on conventional and high resolution CT (HRCT) images. Radiat Med 19(5):237–245
Mdluli K, Swanson J, Fischer E, Lee RE, Barry CE III (1998) Mechanisms involved in the intrinsic isoniazid resistance of Mycobacterium avium. Mol Microbiol 27(6):1223–1233
Bittner MJ, Horowitz EA, Safranek TJ, Preheim LC (1996) Emergence of Mycobacterium kansasii as the leading mycobacterial pathogen isolated over a 20-year period at a midwestern Veterans Affairs hospital. Clin Infect Dis 22(6):1109–1110
Churchyard GJ, Kleinschmidt I, Corbett EL, Mulder D, De Cock KM (1999) Mycobacterial disease in South African gold miners in the era of HIV infection. Int J Tuberc Lung Dis 3(9):791–798
Corbett EL, Blumberg L, Churchyard GJ, Moloi N, Mallory K, Clayton T, Williams BG, Chaisson RE, Hayes RJ, De Cock KM (1999) Nontuberculous mycobacteria: defining disease in a prospective cohort of South African miners. Am J Respir Crit Care Med 160(1):15–21
Corbett EL, Churchyard GJ, Hay M, Herselman P, Clayton T, Williams B, Hayes R, Mulder D, De Cock KM (1999) The impact of HIV infection on Mycobacterium kansasii disease in South African gold miners. Am J Respir Crit Care Med 160(1):10–14
Corbett EL, Churchyard GJ, Clayton T, Herselman P, Williams B, Hayes R, Mulder D, De Cock KM (1999) Risk factors for pulmonary mycobacterial disease in South African gold miners. A case-control study. Am J Respir Crit Care Med 159(1):94–99
Corbett EL, Hay M, Churchyard GJ, Herselman P, Clayton T, Williams BG, Hayes R, Mulder D, De Cock KM (1999) Mycobacterium kansasii and M. scrofulaceum isolates from HIV-negative South African gold miners: incidence, clinical significance and radiology. Int J Tuberc Lung Dis 3(6):501–507
Taillard C, Greub G, Weber R, Pfyffer GE, Bodmer T, Zimmerli S, Frei R, Bassetti S, Rohner P, Piffaretti JC, Bernasconi E, Bille J, Telenti A, Prod’hom G (2003) Clinical implications of Mycobacterium kansasii species heterogeneity: Swiss National Survey. J Clin Microbiol 41(3):1240–1244
Ahn CH, Lowell JR, Onstad GD, Shuford EH, Hurst GA (1979) A demographic study of disease due to Mycobacterium kansasii or M. intracellulare-avium in Texas. Chest 75(2):120–125
Smith MB, Molina CP, Schnadig VJ, Boyars MC, Aronson JF (2003) Pathologic features of Mycobacterium kansasii infection in patients with acquired immunodeficiency syndrome. Arch Pathol Lab Med 127(5):554–560
Doucette K, Fishman JA (2004) Nontuberculous mycobacterial infection in hematopoietic stem cell and solid organ transplant recipients. Clin Infect Dis 38(10):1428–1439
Francis PB, Jay SJ, Johanson WG Jr (1975) The course of untreated Mycobacterium kansasii disease. Am Rev Respir Dis 111(4):477–487
Christensen EE, Dietz GW, Ahn CH, Chapman JS, Murry RC, Hurst GA (1979) Pulmonary manifestations of Mycobacterium intracellularis. AJR Am J Roentgenol 133(1):59–66
Lillo M, Orengo S, Cernoch P, Harris RL (1990) Pulmonary and disseminated infection due to Mycobacterium kansasii: a decade of experience. Rev Infect Dis 12(5):760–767
Buchholz UT, McNeil MM, Keyes LE, Good RC (1998) Mycobacterium malmoense infections in the United States, January 1993 through June 1995. Clin Infect Dis 27(3):551–558
Research Committee of the British Thoracic Society (2001) First randomised trial of treatments for pulmonary disease caused by M. avium intracellulare, M. malmoense, and M. xenopi in HIV negative patients: rifampicin, ethambutol and isoniazid versus rifampicin and ethambutol. Thorax 56(3):167–172
[No authors listed] (2003) Pulmonary disease caused by M. malmoense in HIV negative patients: 5-yr follow-up of patients receiving standardised treatment. Eur Respir J 21(3):478-482
Fakih M, Chapalamadugu S, Ricart A, Corriere N, Amsterdam D (1996) Mycobacterium malmoense bacteremia in two AIDS patients. J Clin Microbiol 34(3):731–733
Evans AJ, Crisp AJ, Colville A, Evans SA, Johnston ID (1993) Pulmonary infections caused by Mycobacterium malmoense and Mycobacterium tuberculosis: comparison of radiographic features. AJR Am J Roentgenol 161(4):733–737
Hoefsloot W, van Ingen J, de Lange WC, Dekhuijzen PN, Boeree MJ, van Soolingen D (2009) Clinical relevance of Mycobacterium malmoense isolation in The Netherlands. Eur Respir J 34(4):926–931
Wallace RJ Jr, Brown BA, Griffith DE (1998) Nosocomial outbreaks/pseudo-outbreaks caused by nontuberculous mycobacteria. Annu Rev Microbiol 52:453–490
Simor AE, Salit IE, Vellend H (1984) The role of Mycobacterium xenopi in human disease. Am Rev Respir Dis 129(3):435–438
Jenkins PA, Campbell IA (2003) Pulmonary disease caused by Mycobacterium xenopi in HIV-negative patients: five year follow-up of patients receiving standardised treatment. Respir Med 97(4):439–444
Marusić A, Katalinić-Janković V, Popović-Grle S, Janković M, Mazuranić I, Puljić I, Sertić Milić H (2009) Mycobacterium xenopi pulmonary disease—epidemiology and clinical features in non-immunocompromised patients. J Infect 58(2):108–112
Marks J, Jenkins PA, Tsukamura M (1972) Mycobacterium szulgai—a new pathogen. Tubercle 53(3):210–214
van Ingen J, Boeree MJ, de Lange WC, de Haas PE, Dekhuijzen PN, van Soolingen D (2008) Clinical relevance of Mycobacterium szulgai in The Netherlands. Clin Infect Dis 46(8):1200–1205
Tortoli E, Besozzi G, Lacchini C, Penati V, Simonetti MT, Emler S (1998) Pulmonary infection due to Mycobacterium szulgai, case report and review of the literature. Eur Respir J 11(4):975–977
Wolinsky E (1995) Mycobacterial lymphadenitis in children: a prospective study of 105 nontuberculous cases with long-term follow-up. Clin Infect Dis 20(4):954–963
Emori M, Kajiki A, Ikedo Y, Ochiai S, Iwata Y, Harada Y, Kitahara Y (2007) 15 cases of pulmonary Mycobacterium scrofulaceum infection. Kekkaku 82(3):173–178
Hawkins JE (1977) Scotochromogenic mycobacteria which appear intermediate between Mycobacterium avium-intracellulare and Mycobacterium scrofulaceum. Am Rev Respir Dis 116(5):963–964
Rynkiewicz DL, Cage GD, Butler WR, Ampel NM (1998) Clinical and microbiological assessment of Mycobacterium simiae isolates from a single laboratory in southern Arizona. Clin Infect Dis 26(3):625–630
Weiszfeiler JG, Karasseva V, Karczag E (1981) Mycobacterium simiae and related mycobacteria. Rev Infect Dis 3(5):1040–1045
Levy-Frebault V, Pangon B, Buré A, Katlama C, Marche C, David HL (1987) Mycobacterium simiae and Mycobacterium avium-M. intracellulare mixed infection in acquired immune deficiency syndrome. J Clin Microbiol 25(1):154–157
Peters M, Schürmann D, Mayr AC, Heterzer R, Pohle HD, Ruf B (1989) Immunosuppression and mycobacteria other than Mycobacterium tuberculosis: results from patients with and without HIV infection. Epidemiol Infect 103(2):293–300
Sriyabhaya N, Wongwatana S (1981) Pulmonary infection caused by atypical mycobacteria: a report of 24 cases in Thailand. Rev Infect Dis 3(5):1085–1089
El Sahly HM, Septimus E, Soini H, Septimus J, Wallace RJ, Pan X, Williams-Bouyer N, Musser JM, Graviss EA (2002) Mycobacterium simiae pseudo-outbreak resulting from a contaminated hospital water supply in Houston, Texas. Clin Infect Dis 35(7):802–807
Conger NG, O’Connell RJ, Laurel VL, Olivier KN, Graviss EA, Williams-Bouyer N, Zhang Y, Brown-Elliott BA, Wallace RJ Jr (2004) Mycobacterium simae outbreak associated with a hospital water supply. Infect Control Hosp Epidemiol 25(12):1050–1055
Porteous NB, Redding SW, Jorgensen JH (2004) Isolation of non-tuberculosis mycobacteria in treated dental unit waterlines. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 98(1):40–44
Maoz C, Shitrit D, Samra Z, Peled N, Kaufman L, Kramer MR, Bishara J (2008) Pulmonary Mycobacterium simiae infection: comparison with pulmonary tuberculosis. Eur J Clin Microbiol Infect Dis 27(10):945–950
McGrath EE, Qureshi N (2007) Mycobacterium chelonei: friend or foe? Eur Respir J 30(2):397
De Groote MA, Huitt G (2006) Infections due to rapidly growing mycobacteria. Clin Infect Dis 42(12):1756–1763
Griffith DE, Girard WM, Wallace RJ Jr (1993) Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis 147(5):1271–1278
Han XY, De I, Jacobson KL (2007) Rapidly growing mycobacteria: clinical and microbiologic studies of 115 cases. Am J Clin Pathol 128(4):612–621
Phowthongkum P, Prasanthai V, Udomsantisook N, Suankratay C (2005) Rapidly growing mycobacteria in King Chulalongkorn Memorial Hospital and review of the literature in Thailand. J Med Assoc Thai 88(8):1153–1162
Daley CL, Griffith DE (2002) Pulmonary disease caused by rapidly growing mycobacteria. Clin Chest Med 23(3):623–632, vii
Hadjiliadis D, Adlakha A, Prakash UB (1999) Rapidly growing mycobacterial lung infection in association with esophageal disorders. Mayo Clin Proc 74(1):45–51
Olivier KN, Weber DJ, Wallace RJ Jr, Faiz AR, Lee JH, Zhang Y, Brown-Elliot BA, Handler A, Wilson RW, Schechter MS, Edwards LJ, Chakraborti S, Knowles MR, Nontuberculous Mycobacteria in Cystic Fibrosis Study Group (2003) Nontuberculous mycobacteria. I: multicenter prevalence study in cystic fibrosis. Am J Respir Crit Care Med 167(6):828–834
Pomerantz M, Weber DJ, Wallace RJ Jr, Faiz AR, Lee JH, Zhang Y, Brown-Elliot BA, Handler A, Wilson RW, Schechter MS, Edwards LJ, Chakraborti S, Knowles MR, Nontuberculous Mycobacteria in Cystic Fibrosis Study Group (1991) Surgical management of resistant mycobacterial tuberculosis and other mycobacterial pulmonary infections. Ann Thorac Surg 52(5):1108–1111 discussion 1112
Shiraishi Y, Nakajima Y, Katsuragi N, Kurai M, Takahashi N (2004) Pneumonectomy for nontuberculous mycobacterial infections. Ann Thorac Surg 78(2):399–403
Jacobson K, Garcia R, Libshitz H, Whimbey E, Rolston K, Abi-Said D, Raad I (1998) Clinical and radiological features of pulmonary disease caused by rapidly growing mycobacteria in cancer patients. Eur J Clin Microbiol Infect Dis 17(9):615–621
Nash KA, Zhang Y, Brown-Elliott BA, Wallace RJ Jr (2005) Molecular basis of intrinsic macrolide resistance in clinical isolates of Mycobacterium fortuitum. J Antimicrob Chemother 55(2):170–177
Nash KA, Brown-Elliott BA, Wallace RJ Jr (2009) A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother 53(4):1367–1376
Nomura K, Ogawa M, Miyamoto H, Muratani T, Taniguchi H (2004) Antibiotic susceptibility of glutaraldehyde-tolerant Mycobacterium chelonae from bronchoscope washing machines. Am J Infect Control 32(4):185–188
Hsieh HC, Lu PL, Chen TC, Chang K, Chen YH (2008) Mycobacterium chelonae empyema in an immunocompetent patient. J Med Microbiol 57(Pt 5):664–667
Hazelton TR, Newell JD Jr, Cook JL, Huitt GA, Lynch DA (2000) CT findings in 14 patients with Mycobacterium chelonae pulmonary infection. AJR Am J Roentgenol 175(2):413–416
Wallace RJ Jr, Brown BA, Onyi GO (1992) Skin, soft tissue, and bone infections due to Mycobacterium chelonae chelonae: importance of prior corticosteroid therapy, frequency of disseminated infections, and resistance to oral antimicrobials other than clarithromycin. J Infect Dis 166(2):405–412
Brown-Elliott BA, Wallace RJ Jr (2002) Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev 15(4):716–746
Fowler SJ, French J, Screaton NJ, Foweraker J, Condliffe A, Haworth CS, Exley AR, Bilton D (2006) Nontuberculous mycobacteria in bronchiectasis: prevalence and patient characteristics. Eur Respir J 28(6):1204–1210
Wickremasinghe M, Ozerovitch LJ, Davies G, Wodehouse T, Chadwick MV, Abdallah S, Shah P, Wilson R (2005) Non-tuberculous mycobacteria in patients with bronchiectasis. Thorax 60(12):1045–1051
Esther CR Jr, Henry MM, Molina PL, Leigh MW (2005) Nontuberculous mycobacterial infection in young children with cystic fibrosis. Pediatr Pulmonol 40(1):39–44
Pierre-Audigier C, Ferroni A, Sermet-Gaudelus I, Le Bourgeois M, Offredo C, Vu-Thien H, Fauroux B, Mariani P, Munck A, Bingen E, Guillemot D, Quesne G, Vincent V, Berche P, Gaillard JL (2005) Age-related prevalence and distribution of nontuberculous mycobacterial species among patients with cystic fibrosis. J Clin Microbiol 43(7):3467–3470
Mussaffi H, Rivlin J, Shalit I, Ephros M, Blau H (2005) Nontuberculous mycobacteria in cystic fibrosis associated with allergic bronchopulmonary aspergillosis and steroid therapy. Eur Respir J 25(2):324–328
Iseman MD, Buschman DL, Ackerson LM (1991) Pectus excavatum and scoliosis. Thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. Am Rev Respir Dis 144(4):914–916
Henry MT, Rivlin J, Shalit I, Ephros M, Blau H (2004) Nontuberculous mycobacteria in non-HIV patients: epidemiology, treatment and response. Eur Respir J 23(5):741–746
McGrath EE, Bardsley P (2009) An association between Mycobacterium malmoense and coal workers’ pneumoconiosis. Lung 187(1):51–54
Thomson RM, Armstrong JG, Looke DF (2007) Gastroesophageal reflux disease, acid suppression, and Mycobacterium avium complex pulmonary disease. Chest 131(4):1166–1172
Koh WJ, Lee JH, Kwon YS, Lee KS, Suh GY, Chung MP, Kim H, Kwon OJ (2007) Prevalence of gastroesophageal reflux disease in patients with nontuberculous mycobacterial lung disease. Chest 131(6):1825–1830
Horsburgh CR Jr (1991) Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med 324(19):1332–1338
Jones D, Havlir DV (2002) Nontuberculous mycobacteria in the HIV infected patient. Clin Chest Med 23(3):665–674
Hocqueloux L, Lesprit P, Herrmann JL, de La Blanchardiere A, Zagdanski AM, Decazes JM, Modai J (1998) Pulmonary Mycobacterium avium complex disease without dissemination in HIV-infected patients. Chest 113(2):542–548
Marras TK, Morris A, Gonzalez LC, Daley CL (2004) Mortality prediction in pulmonary Mycobacterium kansasii infection and human immunodeficiency virus. Am J Respir Crit Care Med 170(7):793–798
Marras TK, Daley CK (2004) A systematic review of the clinical significance of pulmonary Mycobacterium kansasii isolates in HIV infection. J Acquir Immune Defic Syndr 36(4):883–889
Shelburne SA III, Hamill RJ (2003) The immune reconstitution inflammatory syndrome. AIDS Rev 5(2):67–79
Lawn SD, Bekker LG, Miller RF (2005) Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis 5(6):361–373
Phillips P, Bonner S, Gataric N, Bai T, Wilcox P, Hogg R, O’Shaughnessy M, Montaner J (2005) Nontuberculous mycobacterial immune reconstitution syndrome in HIV-infected patients: spectrum of disease and long-term follow-up. Clin Infect Dis 41(10):1483–1497
Shelburne SA III, Hamill RJ, Rodriguez-Barradas MC, Greenberg SB, Atmar RL, Musher DW, Gathe JC Jr, Visnegarwala F, Trautner BW (2002) Immune reconstitution inflammatory syndrome: emergence of a unique syndrome during highly active antiretroviral therapy. Medicine (Baltimore) 81(3):213–227
Malouf MA, Glanville AR (1999) The spectrum of mycobacterial infection after lung transplantation. Am J Respir Crit Care Med 160(5 Pt 1):1611–1616
Queipo JA, Broseta E, Santos M, Sánchez-Plumed J, Budía A, Jiménez-Cruz F (2003) Mycobacterial infection in a series of 1261 renal transplant recipients. Clin Microbiol Infect 9(6):518–525
Sexton P, Harrison AC (2008) Susceptibility to nontuberculous mycobacterial lung disease. Eur Respir J 31(6):1322–1333
Jacobson KL, Teira R, Libshitz HI, Raad I, Rolston KV, Terrand J, Whimbey E (2000) Mycobacterium kansasii infections in patients with cancer. Clin Infect Dis 30(6):965–969
Thaker H, Neilly IJ, Saunders PG, Magee JG, Snow MH, Ong EL (2001) Remember mycobacterial disease in hairy cell leukaemia (HCL). J Infect 42(3):213–214
Finucane K, Ambrey P, Narayan S, Archer CB, Dayan C (2003) Insulin injection abscesses caused by Mycobacterium chelonae. Diabetes Care 26(8):2483–2484
Jackson PG, Keen H, Noble CJ, Simmons NA (1980) Injection abscesses in a diabetic due to Mycobacterium chelonei var abscessus. Br Med J 281(6248):1105–1106
Kelly SE (1987) Multiple injection abscesses in a diabetic caused by Mycobacterium chelonei. Clin Exp Dermatol 12(1):48–49
Schadlow M, Laochumroonvorapong P, Burack L, Wu H, Sinha AA (2003) Nodules on the arm of a diabetic patient. Arch Dermatol 139(1):93–98
Nagaia T, Akiyama M, Mita Y, Tomizawa T, Dobashi K, Mori M (2000) Mycobacterium avium complex pleuritis accompanied by diabetes mellitus. Diabetes Res Clin Pract 48(2):99–104
Arend SM, Cerdá de Palou E, de Haas P, Janssen R, Hoeve MA, Verhard EM, Ottenhoff TH, van Soolingen D, van Dissel JT (2004) Pneumonia caused by Mycobacterium kansasii in a series of patients without recognised immune defect. Clin Microbiol Infect 10(8):738–748
Robillon JF, Sadoul JL, Guerin P, Iafrate-Lacoste C, Talbodec A, Santini J, Canivet B, Freychet P (1994) Mycobacterium avium intracellulare suppurative thyroiditis in a patient with Hashimoto’s thyroiditis. J Endocrinol Invest 17(2):133–134
Southern PM Jr (2004) Tenosynovitis caused by Mycobacterium kansasii associated with a dog bite. Am J Med Sci 327(5):258–261
Maimon N, Brunton J, Chan AK, Marras TK (2007) Fatal pulmonary Mycobacterium xenopi in a patient with rheumatoid arthritis receiving etanercept. Thorax 62(8):739–740
Thomas JE, Taoka CR, Gibbs BT, Fraser SL (2006) Fatal pulmonary Mycobacterium abscessus infection in a patient using etanercept. Hawaii Med J 65(1):12–15
van Ingen J, Boeree M, Janssen M, Ullmann E, de Lange W, de Haas P, Dekhuijzen R, van Soolingen D (2007) Pulmonary Mycobacterium szulgai infection and treatment in a patient receiving anti-tumor necrosis factor therapy. Nat Clin Pract Rheumatol 3(7):414–419
van de Vosse E, Hoeve MA, Ottenhoff TH (2004) Human genetics of intracellular infectious diseases: molecular and cellular immunity against mycobacteria and salmonellae. Lancet Infect Dis 4(12):739–749
Doffinger R, Smahi A, Bessia C, Geissmann F, Feinberg J, Durandy A, Bodemer C, Kenwrick S, Dupuis-Girod S, Blanche S, Wood P, Rabia SH, Headon DJ, Overbeek PA, Le Deist F, Holland SM, Belani K, Kumararatne DS, Fischer A, Shapiro R, Conley ME, Reimund E, Kalhoff H, Abinun M, Munnich A, Israël A, Courtois G, Casanova JL (2001) X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat Genet 27(3):277–285
Ohga S, Ikeuchi K, Kadoya R, Okada K, Miyazaki C, Suita S, Ueda K (1997) Intrapulmonary Mycobacterium avium infection as the first manifestation of chronic granulomatous disease. J Infect 34(2):147–150
Takahashi M, Ishizaka A, Nakamura H, Kobayashi K, Nakamura M, Namiki M, Sekita T, Okajima S (2000) Specific HLA in pulmonary MAC infection in a Japanese population. Am J Respir Crit Care Med 162(1):316–318
Koh WJ, Kwon OJ, Kim EJ, Lee KS, Ki CS, Kim JW (2005) NRAMP1 gene polymorphism and susceptibility to nontuberculous mycobacterial lung diseases. Chest 128(1):94–101
Ehrmantraut ME, Hilligoss DM, Chernick M, Steagall WK, Glasgow CG, Anderson VL (2003) Pulmonary nontuberculous mycobacterial infections are highly associated with mutations in CFTR. Am J Respir Crit Care Med 167:A708
Ryu YJ, Kim EJ, Lee SH, Kim SY, Suh GY, Chung MP, Kim H, Kwon OJ, Koh WJ (2007) Impaired expression of Toll-like receptor 2 in nontuberculous mycobacterial lung disease. Eur Respir J 30(4):736–742
Huang JH, Oefner PJ, Adi V, Ratnam K, Ruoss SJ, Trako E, Kao PN (1998) Analyses of the NRAMP1 and IFN-gammaR1 genes in women with Mycobacterium avium-intracellulare pulmonary disease. Am J Respir Crit Care Med 157(2):377–381
Woods GL (2002) The mycobacteriology laboratory and new diagnostic techniques. Infect Dis Clin North Am 16(1):127–144
Tenover FC, Crawford JT, Huebner RE, Geiter LJ, Horsburgh CR Jr, Good RC (1993) The resurgence of tuberculosis: is your laboratory ready? J Clin Microbiol 31(4):767–770
Glassroth J (2008) Pulmonary disease due to nontuberculous mycobacteria. Chest 133(1):243–251
Wallace RJ Jr, Dunbar D, Brown BA, Onyi G, Dunlap R, Ahn CH, Murphy DT (1994) Rifampin-resistant Mycobacterium kansasii. Clin Infect Dis 18(5):736–743
Valero G, Peters J, Jorgensen JH, Graybill JR (1995) Clinical isolates of Mycobacterium simiae in San Antonio, Texas. An 11-yr review. Am J Respir Crit Care Med 152(51):1555–1557
Dailloux M, Abalain ML, Laurain C, Lebrun L, Loos-Ayav C, Lozniewski A, Maugein J, French Mycobacteria Study Group (2006) Respiratory infections associated with nontuberculous mycobacteria in non-HIV patients. Eur Respir J 28(6):1211–1215
van Ingen J, Boeree MJ, de Lange WC, Dekhuijzen PN, van Soolingen D (2007) Impact of new American Thoracic Society diagnostic criteria on management of nontuberculous mycobacterial infection. Am J Respir Crit Care Med 176(4):418, author reply 419
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McGrath, E.E., Blades, Z., McCabe, J. et al. Nontuberculous Mycobacteria and the Lung: From Suspicion to Treatment. Lung 188, 269–282 (2010). https://doi.org/10.1007/s00408-010-9240-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-010-9240-9