Abstract
Background
Obstructive sleep apnea has been linked with metabolic syndrome characterized by dyslipidemia, dyscoagulation, hypertension, and diabetes mellitus type 2 and their cardiovascular consequences. This study was designed to determine the effects of 8 weeks of therapy with continuous positive airway pressure (CPAP) on insulin resistance, glucose, and lipid profile, and the relationship between leptin and insulin-resistance parameters in patients with moderate-to-severe obstructive sleep apnea.
Methods
In 44 patients, serum cholesterol, triglycerides, high-density lipoprotein, low-density lipoprotein, very low-density lipoprotein, leptin, and insulin parameters were measured at baseline and after 8 weeks of CPAP. Insulin resistance index was based on the homeostasis model assessment (HOMA-IR) method. Insulin sensitivity (HOMA-S) and insulin secretion capacity (HOMA-β) also were calculated. Thirteen patients were excluded from statistical analyses due to noncompliant CPAP usage (<4 h night−1).
Results
In 31 patients who used CPAP for ≥4 h night−1, CPAP therapy reduced total cholesterol (P < 0.05), low-density lipoprotein (P < 0.05), and leptin (P < 0.05). Circulating leptin levels showed significant correlation with both HOMA-S and HOMA-IR at baseline and follow-up (P = 0.03 for all). In addition, there was no correlation between HOMA-IR and the severity of sleep apnea, which was shown by apnea-hypopnea index.
Conclusions
In patients with moderate-to-severe obstructive sleep apnea, compliant CPAP usage may improve insulin secretion capacity, reduce leptin, total cholesterol, and low-density lipoprotein levels. Leptin showed significant relationship with insulin resistance, and this relationship remained after 8 weeks of CPAP therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea syndrome (OSAS) is a major public health problem because of its high prevalence besides its cardiovascular and metabolic consequences. The prevalence within the general population is frequently given as 2–4% of adult men and 1–2% of adult women [1, 2]. Although obstructive sleep apnea has been mostly treated as a “local abnormality” of the upper airways, accumulating evidence underlines its systemic aspects. A number of recent studies suggested the possibility that sleep apnea is a manifestation of the metabolic syndrome, i.e., dyslipidemia, dyscoagulation, hypertension and diabetes mellitus type 2, and their cardiovascular consequences [3, 4]. Furthermore, it has become clearer that the role of obesity in the genesis of sleep apnea is through its metabolic activity as well as its anatomic/mechanical impact. Indeed, OSAS shows a strong correlation with obesity and obesity-related variables, such as body mass index (BMI) [5], neck circumference [6], and visceral fat deposition [7]. Especially, visceral obesity, which is among the characteristic features of OSAS, is associated with insulin resistance. Insulin resistance, as indicated by an impaired biological response to insulin and eventually a reduction of insulin-mediated glucose utilization [8], is considered to be a key component of metabolic and cardiovascular disorders [9, 10]. Because obesity is recognized as an important risk factor with chance of reversibility for OSAS and is thought to affect the respiratory control system, control of body weight is clinically important in the management of the disease.
Leptin, the obese (ob) gene product, is a versatile 16 kDa peptide hormone secreted by adipocytes [11]. Leptin induces a complex response, including control of body weight and energy expenditure after interaction with specific receptors located in hypothalamus and in peripheral tissues [12]. Previous studies showed that circulating leptin levels are positively correlated with the severity of the disease in patients with OSAS independent of age and BMI [13, 14]. Furthermore, both short-term (3–4 days) and long-term (3 months) CPAP treatment significantly decreased leptin levels in patients with OSAS [15, 16]. Higher leptin concentrations in patients with OSAS may contribute to disease pathophysiology in several ways. It has been demonstrated in animal studies that chronic hyperleptinemia could promote blood pressure elevation [17] and platelet aggregation, together with arterial thrombosis [18]. These observations raise the possibility that leptin may have a role in development of hypertension and cardiovascular disease in patients with OSAS. The relationship between leptin and insulin seems to be more complex. Although a direct effect of insulin on ob mRNA could not be demonstrated in isolated rat adipocytes [19], insulin provoked a dose-dependent increase in leptin in cultured human fat cells [20]. Prolonged exposure to insulin increases plasma leptin concentrations both in vivo and in vivo, implying a role for insulin in chronic leptin regulation [12]. However, no post-prandial increase in serum leptin was observed, and 24-h profiles of circulating leptin were not correlated with insulin levels. It remains to be clarified whether elevated leptin is a cofactor in the induction of insulin resistance or vice versa in OSAS.
The purpose of this study was to examine whether serum leptin levels is associated with insulin resistance in the presence of sleep apnea and whether the nasal CPAP treatment has an impact on insulin resistance and circulating leptin levels in patients with OSAS. We hypothesized that reduction in circulating leptin levels and insulin resistance under CPAP treatment may explain the interaction between the two parameters when BMI is similar before and after treatment.
Methods
Study Population and Initial Assessments
The study population consisted of 44 (27 men) patients with OSAS who were examined by using digital polysomnography. All patients were free from diabetes mellitus type 2 and other known metabolic disorders, respiratory infection, heart diseases, and other respiratory disorders at the time of polysomnography. None of the patients were taking antihyperlipidemic, antihypertensive, or other drug treatments. They were asked to complete the Epworth Sleepiness Scale and a questionnaire about sleep symptoms and medical history. Diagnosis of OSAS was established on the basis of clinical and polysomnographic criteria. The average number of episodes of apnea and hypopnea per hour of sleep was calculated and expressed as apnea-hypopnea index (AHI). In addition to clinical symptoms, AHI > 15 was used as inclusion criterion. Liver function tests, renal function tests, and thyroid function tests were performed to determine comorbid disorders. Circulating total cholesterol, triglyceride, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) measurements also were performed by enzymatic methods using commercial kits as standard evaluations.
Overnight polysomnography (Somnologica, Flaga, Iceland) was performed between 11 p.m. and 7 a.m. Polysomnography consisted of simultaneous recordings of two channels EEG (C3A2 and C4A1), left and right electro-oculography, and chin electromyography from surface leads for sleep stating. In addition, air flow from nasal cannula, thoracic and abdominal strain gauges for respiratory effort, tracheal microphone for snoring, pulse oximetry for oxyhemoglobin level, and sensor for body position during sleep were used. Sleep staging and respiratory event scoring were performed manually according to standard criteria [21]. Obstructive apnea was defined as a cessation of airflow for at least 10 seconds and obstructive hypopnea as a decrease of the airflow signal amplitude by at least 50% accompanied by oxyhemoglobin desaturation of at least 3% or by an arousal.
CPAP Treatment
Automatic titration of the CPAP pressure (AutoSet spirit, ResMed Corp., California, USA) was performed during a subsequent night in the sleep laboratory under polysomnographic control and monitorization by a trained sleep laboratory technician. For each patient, an average CPAP pressure at which most of the apneas, hypopneas (AHI < 10 events h−1) and snoring were abolished in all body positions was determined. After the CPAP titration night, patients who were willing to accept the treatment were advised to use CPAP every night at the effective fixed pressure mode and to keep their diet and physical activity constant until metabolic studies were repeated at the end of 8 weeks. After the treatment period, all patients returned for clinical assessment and biochemical analyses. Compliance was measured electronically using a smartcard embedded in the CPAP machine. Data of 13 participants were excluded from statistical analysis because they show poor compliance to CPAP treatment.
Serum Leptin Measurements
Blood samples for leptin measurements were collected into ehtylendiamine tetra acetic acid (EDTA)-coated polypropylene tubes kept on ice, at 07:30 hour after an overnight fast. Tubes were centrifuged immediately at 1800 × g for 20 min at 0°C, and the clear plasma supernatant was then stored until leptin levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Human Leptin kit Code: RD191001100, Biovendor Laboratory Medicine, Czech Republic). All plasma samples were analyzed in duplicate and in the same batch.
Insulin Resistance
Insulin resistance was calculated by homeostasis assessment model (HOMA) as described previously in a representative Turkish adult group [22]. The HOMA is a mathematical model that allows values for insulin sensitivity and β-cell function (expressed as a percentage of normal) to be obtained if simultaneous fasting plasma glucose and fasting insulin concentrations are known [23]. According to that model, insulin resistance is estimated by the following formula: [fasting serum glucose (mmol/l) × fasting serum insulin (μU/ml)]/22.5. Insulin resistance in white southern Europeans has been defined as a HOMA score >3.99 [24]. Because insulin secretion is pulsatile and the optimal sample should be the mean of three results at 5-min intervals, we collected 0-, 5-, and 10-min samples for calculation. Fasting insulin was determined with electrochemiluminiscence immunoassay kits (Elecsys) on Roche Elecsys 1010/2010 (Roche Diagnostics GmbH, Germany), and plasma glucose was measured by glucose oxidase method on a Beckman autoanalyzer.
Statistical Analysis
The results are expressed as mean ± standard deviation (SD). BMI, circulating lipid parameters, insulin resistance parameters, and leptin levels were compared between conditions before and after CPAP using Wilcoxon test. Comparisons before and after CPAP included the data of 31 participants. The relationship between circulating leptin levels and insulin resistance parameters was tested by using Spearman correlation statistics. P > 0.05 was accepted as statistically significant.
Results
General baseline characteristics of study group are given in Table 1. There is no statistical differentiation between CPAP compliant and CPAP noncompliant patient’s characteristics (n:13). The mean age, BMI, Epworth score, and AHI of noncompliant patients were 52.9 ± 12.3, 32.6 ± 4.1, 12.3 ± 6.1, and 41.3 ± 21.2, respectively. Sixteen patients (36%) had moderate sleep apnea (15 < AHI < 30), whereas 28 (63%) patients had severe OSAS (AHI > 30). Mean CPAP pressure established by automatic titration was 7.8 ± 2.6 cmH2O. Comparisons of before and after CPAP therapy parameters are shown in Table 2. There was no statistically significant change in BMI after 8-week CPAP therapy. Subjective sleepiness score, assessed by Epworth Sleepiness Scale, was significantly reduced, which may show the effectiveness of CPAP therapy. Considering the blood lipid profile, cholesterol and low density lipoprotein levels were significantly reduced after CPAP treatment. Change in insulin resistance parameters are given in Table 3. Only statistically significant change was observed in HOMA-β levels after CPAP therapy. HOMA-β is a factor that shows insulin secretion capacity. Thus, these results may suggest that 8-week CPAP treatment increased insulin secretion capacity in OSAS patients. Circulating glucose and insulin levels showed no significant alterations.
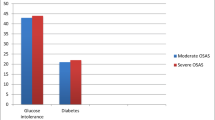
Figure 1A and B shows, in the whole study group, circulating leptin levels correlated with insulin resistance (HOMA-IR) at pretreatment evaluation (r = 0.41, P = 0.03) as well as at posttreatment evaluation (r = 0.45, P = 0.03), respectively. In addition, leptin levels were correlated with HOMA-S (Table 4). Finally, we were unable to find a relationship between the severity of OSAS and insulin resistance (Fig. 2).
Discussion
This study demonstrated that 8-week CPAP therapy reduced leptin, total cholesterol, and LDL cholesterol levels and increased insulin secretion capacity in patients with moderate-to-severe OSAS. In addition, circulating leptin levels were significantly correlated with insulin resistance and insulin sensitivity. These relationships remained after 8-week CPAP therapy. Previous studies showed that insulin sensitivity assessed by hyperinsulinemic clamp technique was negatively correlated with leptin levels. Eventually, serum leptin levels are higher in insulin-resistant men [25]. We have confirmed these observations in a subgroup of OSAS population. CPAP therapy lead to 16% increase for insulin secretion capacity (HOMA-β), which is comparable with previous findings that reported a 38% decrease for insulin resistance (HOMA-IR) after 8-weeks of CPAP [26] and a 24% increase for insulin sensitivity after 3 months of CPAP therapy [27]. In a recent study, Comondore et al. demonstrated similar results for improvement in insulin resistance and insulin levels by short-term CPAP therapy in minimally symptomatic patients with OSAS [28]. Finding improved insulin sensitivity without a concomitant change in body weight suggests that OSAS has an independent effect on insulin resistance. In this study, there was no significant change in BMI by CPAP therapy. It is well-established that leptin is essential to the normal regulation of body weight and energy expenditure. Levels of circulating leptin correlate with indexes of adiposity, including body fat mass, BMI, and percentage of body fat [29]. OSAS patients have elevated leptin concentrations. The higher leptin levels may contribute to preferential fat deposition (i.e., subcutaneous vs. visceral), which may predispose patients to develop OSAS. However, elevated leptin concentrations in patients with OSAS decrease by CPAP independently of changes in body weight [30], suggesting that other mechanisms (such as insulin sensitivity) apart from fat mass could be interrelated with increased leptin levels. It is known that leptin may regulate insulin release as part of an adipoinsular feedback [31]. On the other hand, insulin resistance has been shown to increase leptin secretion independent of body fat mass [32]. In our study, circulating leptin level was significantly correlated with insulin resistance before and after CPAP treatment.
Dyslipidemia, an established independent risk factor for coronary heart disease and atherosclerosis, is common in patients with OSAS. Blood lipid profiles in OSAS have not been widely studied. Buechner et al. [33] studied the effects of therapy on dyslipidemia in OSAS and found in 95 patients that total cholesterol and LDL cholesterol levels decreased significantly in patients treated for 6 months effectively with CPAP, BiPAP, and mandibular advancement device, whereas triglyceride, HDL cholesterol, and lipoprotein (a) levels remained unchanged. Comondore et al. demonstrated similar results in their parameters [28]. In a recent study, Dorkova et al. demonstrated that elevated total cholesterol, triglycerides, and LDL cholesterol decreased effectively after 8 weeks of CPAP therapy in patients with OSAS [34]. Our results confirm these previous findings: total cholesterol and LDL-cholesterol levels significantly decreased by the same duration of CPAP treatment in our patient group. The mechanism of total and LDL cholesterol lowering effect of CPAP may be speculative at the moment. One possible mechanism may be via nocturnal intermittent hypoxia resulting from repetitive apneas. Hypoxia may affect LDL receptor density or hepatic lipoprotein metabolism. Furthermore, hypoxia induces stimulation of sympathetic activity, which affects serum lipid composition by well-known metabolic effects of catecholamines, including gluconeogenesis, peripheral insulin resistance, and increased lipolysis [33]. Another mechanism may involve insulin resistance, which may increase total cholesterol and LDL cholesterol by decreasing the catabolism of LDL, by down-regulation of LDL receptors [33]. Finally, previous studies demonstrated that visceral fat significantly correlated with indexes of sleep apnea, such as apnea hypopnea index and minimum hemoglobin oxygen saturation [7]. LDL correlates with visceral fat mass and correction of sleep apnea by CPAP and may affect lipid profile (i.e., increased HDL cholesterol and decreased LDL cholesterol) by reducing visceral fat accumulation [15].
Several limitations of this study deserve comment. Insulin sensitivity was approximated by using HOMA-IR instead of the euglycemic clamp technique. Euglycemic clamp method is the “gold standard” for the evaluation of insulin sensitivity. However, this method is invasive and labor intensive. On the other hand, it is demonstrated that insulin sensitivity indices derived from the homeostatic model are strongly correlated to values obtained by the clamp method [35]. Second, there was no control group in study. Third, a relatively small number of patients with moderate-to-severe OSAS makes it difficult to extrapolate these results to a whole spectrum of sleep-disordered breathing.
Conclusions
Our study demonstrated a significant increase in the insulin secretion capacity and decrease in circulating leptin, total cholesterol, and LDL cholesterol by 8 weeks of CPAP treatment. Overall, these results contribute to better delineation of the relationship between glucose homeostasis parameters and leptin in the presence of obstructive sleep apnea and the protective effects of CPAP treatment against the development of metabolic syndrome in this patient group.
References
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328:1230–1235. doi:10.1056/NEJM199304293281704
Olson LG, King MT, Hensley MJ, Saunders NA (1995) A community study of snoring and sleep-disordered breathing: prevalence. Am J Respir Crit Care Med 152:711–716
Vgontzas AN, Bixler EO, Chrousos GP (2003) Metabolic disturbances in obesity versus sleep apnoea: the importance of visceral obesity and insulin resistance. J Intern Med 254:32–44. doi:10.1046/j.1365-2796.2003.01177.x
Vgontzas AN, Bixler EO, Chrousos GP (2005) Sleep apnea is a manifestation of the metabolic syndrome. Sleep Med Rev 9:211–224. doi:10.1016/j.smrv.2005.01.006
Ünal M, Öztürk L (2005) The effect of body mass index on the severity of obstructive sleep apnea. In: Ferrera LA (ed) Body mass index and health. Nova Science Publishers, New York, pp 81–96
Dancey DR, Hanly PJ, Soong C, Lee B, Shepard J, Hoffstein V (2003) Gender differences in sleep apnea: the role of neck circumference. Chest 123:1544–1550. doi:10.1378/chest.123.5.1544
Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, Kales A, Chrousos GP (2000) Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab 85:1151–1158. doi:10.1210/jc.85.3.1151
American Diabetes Association (1998) Consensus development conference on insulin resistance. Diabetes Care 21:1–5
Reaven GM (1988) Role of insulin resistance in human disease. Banting Lecture 1988. Diabetes 37:1595–1607. doi:10.2337/diabetes.37.12.1595
Despres JP, Lamarche B, Mauriege P, Cantin B, Dagenais GR, Moorjani S, Lupien PJ (1996) Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med 334:952–957. doi:10.1056/NEJM199604113341504
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432. doi:10.1038/372425a0
Frühbeck G, Jebb SA, Prentice AM (1998) Leptin: physiology and pathophysiology. Clin Physiol 18:399–419. doi:10.1046/j.1365-2281.1998.00129.x
Öztürk L, Ünal M, Tamer L, Çelikoğlu F (2003) The association of the severity of obstructive sleep apnea with plasma leptin levels. Arch Otolaryngol Head Neck Surg 129:538–540. doi:10.1001/archotol.129.5.538
Kapsimalis F, Varouchakis G, Manousaki A et al (2008) Association of sleep apnea severity and obesity with insulin resistance, C-reactive protein, and leptin levels in male patients with obstructive sleep apnea. Lung 186:209–217. doi:10.1007/s00408-008-9082-x
Chin K, Shimizu K, Nakamura T, Narai N, Masuzaki H, Ogawa Y, Mishima M, Nakamura T, Nakao K, Ohi M (1999) Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation 100:706–712
Saarelainen S, Lahtela J, Kallonen E (1997) Effect of nasal CPAP treatment on insulin sensitivity and plasma leptin. J Sleep Res 6:146–147
Aizawa-Abe M, Ogawa Y, Masuzaki H, Ebihara K, Satoh N, Iwai H, Matsuoka N, Hayashi T, Hosoda K, Inoue G, Yoshimasa Y, Nakao K (2000) Pathophysiological role of leptin in obesity-related hypertension. J Clin Invest 105:1243–1252. doi:10.1172/JCI8341
Konstantinides S, Schafer K, Koschnick S, Loskutoff DJ (2001) Leptin-dependent platelet aggregation and arterial thrombosis suggests a mechanism for atherothrombotic disease in obesity. J Clin Invest 108:1533–1540
Murakami T, Iida M, Shima K (1995) Dexamethasone regulates obese expression in isolated rat adipocytes. Biochem Biophys Res Commun 214:1260–1267. doi:10.1006/bbrc.1995.2422
Wabitsch M, Jensen PB, Blum WF et al (1996) Insulin and cortisol promote leptin production in cultured human fat cells. Diabetes 45:1435–1438. doi:10.2337/diabetes.45.10.1435
Rechtschaffen A, Kales AA (1968) A manual of standardized terminology, techniques and scoring for sleep stages of human subjects. National Institutes of Health publication No.204. Government Printing Office, Washington, DC
Gokcel A, Baltali M, Tarim E, Bagis T, Gumurdulu Y, Karakose H, Yalcin F, Akbaba M, Guvener N (2003) Detection of insulin resistance in Turkish adults: a hospital-based study. Diabetes Obes Metab 5:126–130. doi:10.1046/j.1463-1326.2003.00253.x
Wallace TM, Matthews DR (2002) The assessment of insulin resistance in man. Diabet Med 19:527–534. doi:10.1046/j.1464-5491.2002.00745.x
Ascaso JF, Romero P, Real JT, Lorente RI, Martinez-Valls J, Carmena R (2003) Abdominal obesity, insulin resistance and metabolic syndrome in a southern European population. Eur J Intern Med 14:101–106. doi:10.1016/S0953-6205(03)00022-0
Segal KR, Landt M, Klein S (1996) Relationship between insulin sensitivity and plasma leptin concentrations in lean and obese males. Diabetes 45:988–991. doi:10.2337/diabetes.45.7.988
Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R (2008) Effects of CPAP on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest 134:686–692. doi:10.1378/chest.08-0556
Harsch IA, Schahin SP, Radespiel-Troger M et al (2004) Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 169:156–162. doi:10.1164/rccm.200302-206OC
Comondore VR, Cheema R, Fox J et al (2008) The impact of CPAP on cardiovascular biomarkers in minimally symptomatic patients with obstructive sleep apnea: a pilot feasibility randomized crossover trial. Lung 186:209–217. doi:10.1007/s00408-008-9082-x
Ostlund RE, Yang JW, Klein S et al (1996) Relation between plasma leptin concentration and body fat, gender, diet, age, and metabolic covariates. J Clin Endocrinol Metab 81:3909–3913. doi:10.1210/jc.81.11.3909
Harsch IA, Konturek PC, Koebnick C, Kuehlein PP, Fuchs FS, Schahin SP, Wiest GH, Hahn EG, Lohmann T, Ficker JH (2003) Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment. Eur Respir J 22:251–257. doi:10.1183/09031936.03.00010103
Kieffer TJ, Heller RS, Habener JF et al (1996) Leptin receptors expressed on pancreatic beta-cells. Biochem Biophys Res Commun 224:522–527. doi:10.1006/bbrc.1996.1059
Kolaczynski JW, Nyce MR, Considine TV et al (1996) Acute and chronic effects of insulin on leptin production in humans: studies in vivo and in vitro. Diabetes 45:699–701. doi:10.2337/diabetes.45.5.699
Buechner NJ, Zidek W, Esser M, Haske M, Sanner BM (2001) Obstructive sleep apnea syndrome. Effects of therapy on dyslipidemia. Somnologie 5:97–102. doi:10.1046/j.1439-054X.2001.01159.x
Mazzone T, Foster D, Chait A (1984) In vivo stimulation of low-density lipoprotein degradation by insulin. Diabetes 33:333–338. doi:10.2337/diabetes.33.4.333
Wallace TM, Levy JC, Matthews DR (2004) Use and abuse of HOMA modeling. Diabetes Care 21:568–576
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Çuhadaroğlu, Ç., Utkusavaş, A., Öztürk, L. et al. Effects of Nasal CPAP Treatment on Insulin Resistance, Lipid Profile, and Plasma Leptin in Sleep Apnea. Lung 187, 75–81 (2009). https://doi.org/10.1007/s00408-008-9131-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-008-9131-5