Abstract
β2-Adrenergic agonists are known to improve muscle strength because of anabolic properties. The purpose of this study was to determine if long-term administration of a long-acting β2-adrenergic agonist to subjects with tetraplegia is associated with improvement in pulmonary function parameters and maximal static inspiratory and expiratory mouth pressures (MIP and MEP, respectively), measures of respiratory muscle strength. The study was a randomized, prospective, double-blind, placebo-controlled, crossover trial and conducted at the James J. Peters Veterans Affairs Medical Center. Thirteen subjects who had complete or incomplete tetraplegia for more than one year participated in the study. Eleven subjects completed the study. All were clinically stable outpatients without any history of asthma or use of inhaled bronchodilators. Following baseline measurements, patients were randomized to receive salmeterol or placebo from identically marked Diskus containers for 4 weeks. Following a 4-week washout period, the subjects were randomized to receive the alternate preparation for 4 weeks. Pulmonary function parameters and static mouth pressure were measured during baseline and during the fourth week of the two study periods. During the 4-week period of salmeterol administration, forced vital capacity, forced expiratory volume in 1 s, peak expiratory flow, MIP, and MEP improved significantly compared with placebo and baseline. Expiratory reserve volume increased significantly compared to baseline. Increases in MIP and MEP during salmeterol administration suggest improvement in respiratory muscle strength. However, this cannot be stated with certainty because MIP and MEP are dependent on volume parameters at which they are measured. Regardless of the mechanism, improvement in static mouth pressures indicates that salmeterol should benefit these individuals by improving cough effectiveness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In addition to providing bronchodilation, β2-adrenergic agents have been shown to have anabolic properties. Specifically related to spinal cord injury (SCI), in a double-blind, placebo-controlled, crossover study, Signorile et al. [25] found that administration of oral metaproterenol (80 mg/day) for 4 weeks was associated with an increase in arm muscle strength and muscle size. In addition, a placebo-controlled study of three individuals with SCI (two with tetraplegia and one with paraplegia) demonstrated that total work output during functional electrical stimulation cycling increased during salbutamol treatment (64%) compared with that during placebo treatment (27%) [20].
A previous study revealed that approximately 40% of subjects with tetraplegia demonstrated significant improvement in forced expiratory volume in 1 s (FEV1 > 12 % and 200 ml) following inhalation of metaproterenol sulfate, a short-acting β2-adrenergic agonist [27]. Additional findings that lower baseline FEV1 values correlated with heightened responsiveness to methacholine suggested that bronchodilatory responsiveness to metaproterenol sulfate was due to reduced baseline airway caliber [12]. Confirmation came from body plethysmography studies that revealed that mean baseline specific airway conductance (sGaw) values (1/cmH2O·s) were significantly lower among subjects with tetraplegia (0.16 ± 0.05) compared with subjects with paraplegia (0.26 ± 0.05) and able-bodied controls (0.27 ± 0.05) [24]. Importantly, following administration of metaproterenol sulfate by aerosol, subjects with tetraplegia experienced significantly greater improvement in sGaw (134 ± 25%) than those with paraplegia (17 ± 13%) [23]. Collectively, these studies revealed that subjects with tetraplegia were bronchoconstricted at rest and that administration of a short-acting β2-adrenergic agent induced significant bronchodilation. Baseline bronchoconstriction in tetraplegia has been attributed to unopposed vagal activity due to interruption of sympathetic innervation of the lung and/or reduced circulating epinephrine levels [27].
The purpose of this study was to determine if changes occur in maximum expiratory pressure (MEP) and maximum inspiratory pressure (MIP), markers of expiratory and inspiratory respiratory muscle strength, respectively, among subjects with tetraplegia following administration of a long-acting β2-adrenergic agent. A second goal was to determine if the agent provides sustained bronchodilation in these subjects.
Materials and Methods
Subjects
Thirteen clinically stable male subjects with chronic complete or incomplete cervical SCI (C4-C7 not requiring mechanical ventilation) for more than one year participated in the study. Subjects were selected who reported no history of pulmonary disease, atopy, or asthma, and none reported recent active pulmonary infections or hospitalization for acute medical illnesses. None were receiving inhaled bronchodilators. Study participants were recruited from among outpatients who were followed by the Spinal Cord Injury Service at the James J. Peters Veterans Affairs Medical Center. The study was approved by the institutional review board of the James J. Peters Veterans Affairs Medical Center, and informed consent was obtained from subjects before the investigation.
Study Design
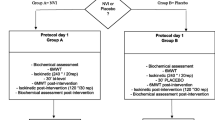
The study was randomized, prospective, double-blind, placebo-controlled with crossover. Following measurement of baseline parameters, subjects were randomized to receive salmeterol or placebo for 4 weeks (phase 1). Following a 4-week washout period, subjects received the alternate preparation for 4 weeks (phase 2). Subjects inhaled salmeterol (50 μg) or inactive powder from identically labeled Diskus devices of identical color twice daily during phase 1 and phase 2. Pulmonary function parameters were assessed during the baseline period and at the end of the fourth week of phase 1 and the fourth week of phase 2. Measurements in the pulmonary function laboratory were performed approximately 15 h after the last administration of salmeterol or placebo.
Measurements
Spirometric and lung volume parameters were obtained while subjects were seated in a variable-pressure, constant-volume, whole-body plethysmograph (Vmax/6200 Body Plethysmograph, VIASYS Healthcare, Yorba Linda, CA). Spirometry was performed according to American Thoracic Society standards [1]. As previously validated among subjects with tetraplegia, reproducible expiratory efforts were deemed acceptable in some individuals despite back-extrapolated volumes that were in excess of the standard limits and/or forced expiratory times of < 6 s [2]. Lung volume and spirometric parameters were expressed as absolute values and percent predicted based upon prediction equations of Crapo et al. [5] and standards by Morris et al. [19]. Maximal inspiratory and expiratory static mouth pressures (MIP and MEP, respectively) were measured using a Vmax 229 (VIASYS Healthcare, Yorba Linda, CA) system. Both were measured using a flange-style mouthpiece. Three maneuvers were performed at or near residual volume for determination of MIP and at or near or total lung capacity for determination of MEP. The highest value for each maneuver was used in data analysis.
Statistical Analyses
Descriptive statistics were performed on the continuous demographic variables and reported as mean ± standard deviation (SD). Frequency distributions were determined from the categorical descriptive variables and reported as number and percent of occurrence. A repeated-measures analysis of variance (ANOVA) was used to compare the outcome variables during baseline, phase 1, and phase 2. Percent change from baseline was calculated for the active/placebo (phases 1 and 2) for MIP and MEP. A one-way ANOVA was performed to determine MIP and MEP significant differences from zero for percent change during phases 1 and 2.
Results
Eleven subjects completed the study. Two dropped out because of transportation difficulties. Both had been randomized to receive salmeterol during phase 1. Demographics are shown in Table 1. During phase 1 seven subjects received placebo and four received salmeterol. Spirometric, lung volume, MIP, and MEP parameters are shown in Table 2. For the 11 subjects, regardless of whether salmeterol was administered during phase 1 or phase 2, salmeterol was associated with a significant increase in forced vital capacity (FVC), FEV1, peak expiratory flow (PEF), MIP, and MEP compared with placebo and baseline. Expiratory reserve volume increased significantly during salmeterol administration compared to baseline. Percent changes from baseline in MIP and MEP during placebo or salmeterol administration are shown in Figure 1A. A significant increase for both parameters occurred during administration of salmeterol. MEP also increased significantly during the placebo phase. Percent changes in MIP and MEP as related to order of administration are shown in Figure 1B. Although MIP and MEP rose by approximately 20% in the four subjects given salmeterol during phase 1, the values did not reach statistical significance compared with baseline. Both MIP and MEP rose significantly compared with baseline for the seven subjects given salmeterol during phase 2. Of note, although not of statistical significance, MIP and MEP values were still elevated at the end of phase 2 for the four subjects given salmeterol during phase 1 (approximately 8 weeks after the last administration of salmeterol).
Discussion
We found that subjects with tetraplegia without a history of asthma experienced significant improvement in spirometric parameters following inhalation of a long-acting β2-adrenergic agent during a 4-week study period. The findings supplement previous observations that administration of a short-acting β2-agonist improved spirometric parameters in tetraplegia patients [27]. Improvement in PEF, similar to FEV1, measures relative changes in bronchi and reflects airway bronchial responsiveness and lability. Salmeterol has greater affinity for β2-adrenoceptors than short-acting agents because of lipophilic properties, which contributes to prolonged activity exceeding 12 h [13]. Salmeterol improves pulmonary function, reduces dyspnea, and improves quality of life for patients with asthma and chronic obstructive pulmonary disease [14, 21]. In this study, it was not possible to determine if chronic administration of salmeterol improved respiratory symptoms because of the small number of subjects. Improvement in respiratory complaints would be anticipated because it was previously shown that the majority of subjects with tetraplegia demonstrate airway hyperreactivity in response to methacholine, histamine, and ultrasonically nebulized distilled water comparable to that found among patients with mild asthma [9, 11, 26] and that essentially all are bronchoconstricted at rest [24]. Also, the majority of subjects with tetraplegia are aware of breathlessness at rest or on exertion [28].
Improvement in MIP and MEP with salmeterol introduces the possibility that these parameters increased because of improvement in respiratory muscle strength of innervated but atrophic and deconditioned muscles due to associated systemic effects of the drug. MIP and MEP are considered global measures of inspiratory and expiratory muscle strength [10]. Following inhalation of salmeterol, peak plasma concentrations occur within 5-15 min [18] as a result of direct absorption from the airways and the gastrointestinal tract [3]. However, in this study it cannot be concluded with certainty that salmeterol improved respiratory muscle strength because static mouth pressures are affected by the lung volume at which they are measured. Although in this study total lung capacity and residual volume, at which MEP and MIP were measured, did not change significantly, it is possible that small changes in these parameters associated with bronchodilation could have influenced the findings because FVC increased significantly. Interpretation of static mouth pressure findings is also confounded by findings that MEP values increased significantly during the placebo phase, possibly as a result of a “learning effect.” Of note, although not of statistical significance, MIP and MEP values had not returned to baseline approximately 8 weeks after the last administration of salmeterol. These findings could represent a “learning effect” or sustained improvement in respiratory muscle strength due to the effects of salmeterol.
Of interest, Signorile et al. [25] determined in a double-blind, placebo-controlled, crossover study of subjects with tetraplegia given an oral short-acting β2-adrenergic agent (metaproterenol) that forearm muscle strength and size increased significantly during the time of active drug administration. Also, a placebo-controlled study of three individuals with SCI (two with tetraplegia and one with paraplegia) demonstrated that total work output during functional electrical stimulation cycling increased during salbutamol treatment (64%) compared with that during placebo treatment (27%) [20]. Among able-bodied individuals it was shown that oral salbutamol improved quadricep strength, hamstring strength, and MIP [17], and, given along with resistance exercise, it augmented strength gains [4]. In a small pilot study, administration of oral albuterol to individuals with facioscapulohumeral muscular dystrophy was associated with an increase in lean body mass and muscle strength [15]. Support for improvement in expiratory muscle strength in the current study comes from the additional finding that expiratory reserve volume (ERV) increased during the time of salmeterol administration compared with that at baseline. Improvement in ERV and reduction in RV associated with pectoralis muscle training among patients with tetraplegia has been attributed to improvement in expiratory muscle strength [7].
In summary, regardless of whether static mouth pressures increased in the current study because of changes in lung volumes or because of improvement in respiratory muscle strength, the findings reveal that subjects with tetraplegia are able to generate higher expiratory pressures in association with administration of salmeterol. Elevated expiratory pressures should improve cough effectiveness, particularly because contraction of the pectoralis major causes dynamic airway compression during expiratory efforts in a substantial proportion of subjects with tetraplegia [8]. Defective cough, rather than inadequate ventilation, causes atelectasis and mucus retention, two of the more important causes of morbidity among subjects with spinal cord injury [6, 16, 22].
References
American Thoracic Society (1995) Standardization of spirometry: 1994 update. Am Rev Respir Dis 152:1107–1136
Ashba J, Garshick E, Tun CG, et al. (1993) Spirometry: acceptability and reproducibility in spinal cord injured subjects. J Am Paraplegia Soc 16:197–203
Bennett JA, Harrison TW, Tattersfield AE (1999) The contribution of the swallowed fraction of an inhaled dose of salmeterol to its systemic effects. Eur Respir J 13:445–448
Caruso JF, Signorile JF, Perry AC, et al. (1995) The effects of albuterol and isokinetic exercise on the quadriceps muscle group. Med Sci Sports Exerc 27:1471–1476
Crapo RO, Morris AH, Clayton PD, et al. (1982) Lung volumes in healthy nonsmoking adults. Bull Eur Physiopathol Respir 18:419–425
DeVivo MJ, Black KJ, Stover SL (1993) Causes of death during the first 12 years after spinal cord injury. Arch Phys Med Rehabil 74:248–254
Estenne M, Knoop C, Vanvaerenbergh J, Heilporn A, De Troyer A (1989) The effect of pectoralis muscle training in tetraplegic patients. Am Rev Respir Dis 139:1218–1222
Estenne M, Van Muylem A, Gorini M, et al. (1994) Evidence of dynamic airway compression during cough in tetraplegic patients. Am J Respir Crit Care Med 150:1081–1085
Fein ED, Grimm DR, Lesser M, et al. (1998) The effects of ipratropium bromide on histamine-induced bronchoconstriction in subjects with cervical spinal cord injury. J Asthma 35:49–55
Gounden P (1997) Static respiratory pressures in patients with post-traumatic tetraplegia. Spinal Cord 35:43–47
Grimm DR, Arias E, Lesser M, et al. (1999) Airway hyperresponsiveness to ultrasonically nebulized distilled water in subjects with tetraplegia. J Appl Physiol 86:1165–1169
Grimm DR, Chandy D, Almenoff PL, Schilero G, Lesser M (2000) Airway hyperreactivity in subjects with tetraplegia is associated with reduced baseline airway caliber. Chest 118:1397–1404
Johnson M, Butchers PR, Coleman RA, et al. (1993) The pharmacology of salmeterol. Life Sci 52:2131–2143
Jones PW, Bosh TK (1997) Quality of life changes in COPD patients treated with salmeterol. Am J Respir Crit Care Med 155:1283–1289
Kissel JT, McDermott MP, Natarajan R, et al. (1998) Pilot trial of albuterol in facioscapulohumeral muscular dystrophy. Neurology 50:1402–1406
Lemons VR, Wagner FC (1994) Respiratory complications after cervical spinal cord injury. Spine 19:2315–2320
Martineau L, Horan MA, Rothwell NJ, Little RA (1992) Salbutamol, a β2-adrenoceptor agonist, increases skeletal muscle strength in young men. Clin Sci 83:615–621
Meyer JM, Wenzel CL, Kradjan WA (1993) Salmeterol: A novel, long-acting beta2-agonist. Ann Pharmacother 27:1478–1487
Morris JF, Koski A, Johnson LC (1971) Spirometric standards for healthy nonsmoking adults. Am Rev Respir Dis 103:57–67
Murphy RJL, Hartkopp A, Gardiner PF, Kjaer M, Beliveau L (1999) Salbutamol effect in spinal cord injured individuals undergoing functional electrical stimulation training. Arch Phys Med Rehabil 80:1264–1267
Ramirez-Venegas A, Ward J, Lentine T, Mahler DA (1997) Salmeterol reduces dyspnea and improves lung function in patients with COPD. Chest 112:336–340
Reines HD, Harris RC (1987) Pulmonary complications of acute spinal cord injuries. Neurosurgery 21:193–196
Schilero GJ, Grimm D, Spungen A, Lenner R, Lesser M (2004) Bronchodilator responses to metaproterenol sulfate among subjects with spinal cord injury. J Rehabil Res Dev 41:59–64
Schilero GJ, Grimm DR, Bauman WA, Lenner R, Lesser M (2005) Assessment of airway caliber and bronchodilator responsiveness in subjects with spinal cord injury. Chest 127:149–155
Signorile JF, Banovac K, Gomez M, et al. (1995) Increased muscle strength in paralyzed patients with spinal cord injury: Effect of a beta-2 adrenergic agonist. Arch Phys Med Rehabil 76:55–58
Singas E, Lesser M, Spungen AM, et al. (1996) Airway hyperresponsiveness to methacholine in subjects with spinal cord injury. Chest 110:911–915
Spungen AM, Dicpinigaitis PV, Almenoff PL, Bauman WA (1993) Pulmonary obstruction in individuals with cervical spinal cord lesions unmasked by bronchodilator administration. Paraplegia 31:404–407
Spungen AM, Grimm DR, Lesser M, et al. (1997) Self-reported prevalence of pulmonary symptoms in subjects with spinal cord injury. Spinal Cord 35:652–657
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grimm, D.R., Schilero, G.J., Spungen, A.M. et al. Salmeterol Improves Pulmonary Function in Persons with Tetraplegia. Lung 184, 335–339 (2006). https://doi.org/10.1007/s00408-006-0011-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-006-0011-6