Abstract
Mitogen-activated protein kinases (MAPKs) belong to the group of serine/threonine kinases that are rapidly activated in response to growth factor stimulation. In adult mammalian cells, the MAPK family includes extracellular signal-regulated kinases 1 and 2 (ERK1 and ERK2 or p44mapk and p42mapk), which translocate to the nucleus and integrate signals from second messengers leading to cellular proliferation or differentiation. However, the specific role of MAPKs in neonatal pulmonary vascular smooth muscle is not well understood. Expression of p44mapk and p42mapk in primary cultured pulmonary vascular smooth muscle cells from neonatal (1–2 day old) rats was identified by Western immunoblot analysis. Treatment with 10 nM endothelin-1 (ET-1), a potent vasoconstrictor with vascular mitogenic properties, induced phosphorylation of both p44mapk and p42mapk , but treatment with the exogenous nitric oxide (NO) donor sodium nitroprusside inhibited both p44mapk and p42mapk phosphorylation by ET-1. The specific cGMP-dependent protein kinase (PKG) inhibitor KT5823, the nonspecific nitric oxide synthase (NOS) inhibitor L-NAME, and the specific NOS 1 blocker NPLA all significantly enhanced both p44mapk and p42mapk phosphorylation by ET-1. Collectively, these data demonstrate the expression and phosphorylation of specific MAPKs in rat neonatal pulmonary vascular smooth muscle and suggests that the NO signaling pathway modulates MAPK activation by ET-1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mitogen-activated protein kinases (MAPKs) are important messengers in signal transduction pathways that are stimulated by many different types of cell surface receptors. MAPKs are a family of 40–46-kDa cytosolic serine/threonine kinases that participate in the transduction of mitogenic and differentiation-promoting signals to the cell nucleus. In mammalian cells the MAPK family includes the specific MAPKs, extracellular signal-regulated kinases 1 and 2 (ERK1 and ERK2, or p44mapk and p42mapk, respectively), which require tyrosine and threonine phosphorylation for maximal activation [4]. Activated MAPKs translate to the nucleus for regulation of transcription factors for cellular growth and differentiation [13, 35, 38].
MAPKs have recently been identified in contractile smooth muscle. Childs et al. [9] reported the presence of active MAPK in rat aortic smooth muscle, and Khalil and Morgan [20] have shown that MAPK is activated and translocates from the cytosol to the membrane upon pharmacologic stimulation in ferret aortic smooth muscle. Marrero et al. [27] reported that ERK1 mediated cellular responses to angiotensin II, and studies have shown that G-protein–coupled agonists such as lysophosphatidic acid and thrombin activate MAPK via a pertussin toxin–sensitive mechanism [17, 28]. Numerous receptors for vasoactive substances are coupled to G proteins including thromboxane, prostaglandins, leukotrienes, and platelet-activating factor [15, 31, 40].
Endothelin-1 (ET-1) is a 21-amino-acid peptide originally isolated from the supernatants of cultured porcine aortic endothelial cells [47]. ET-1 has been found in the lung and cultured pulmonary endothelial cells [30], and pulmonary blood vessels possess ET-1 receptors [24]. In addition, ET-1 is a vasoactive and mitogenic substance [2, 6], and studies suggest that ET-1 activates MAPKs in vascular smooth muscle [11, 46, 49]. The vasodilator nitric oxide (NO) is important in the transition of the pulmonary circulation from fetal to postnatal life. NO is primarily produced in the pulmonary endothelium by upregulation of endothelial nitric oxide synthase (NOS) in fetal life [36], and it activates soluble guanylate cyclase, increasing synthesis of guanosine 3′, 5′-cyclic monophosphate (cGMP) leading to vasorelaxation of the pulmonary vasculature [5]. It is thought that attenuated production of NO and, therefore, cGMP may contribute to the pathophysiology of a variety of forms of neonatal pulmonary vascular disease states.
Recent evidence suggests a relationship between ET-1 and NO in the vasculature. ET-1 has been reported to cause release of NO and subsequently cGMP in the isolated rat aorta [42], which in turn may modulate ET-1-induced vascular contraction. In the fetal lung, it is documented that ET-1 causes pulmonary vasodilation through a NO-dependent mechanism, which is most abundant at term [16, 41]. Although ET-1-dependent pathways have been implicated in the activation of MAPK [22, 29], little is known about the effect of NO on ET-1-induced MAPK signaling in pulmonary vascular smooth muscle.
In light of these previous investigations, the present study was done to determine the effect of NO on ET-1-induced MAPK activation in rat neonatal pulmonary vascular smooth muscle cells (NPVSM). Specifically, we determined in NPVSM: (1) p44mapk and p42mapk expression, (2) if ET-1 activates p44mapk and p42mapk, (3) if NO modulates ET-1-induced MAPK activation, (4) the effect of NOS inhibitors on ET-1 activation of MAPK, and (5) the effect of a specific inhibitor of cGMP-dependent protein kinase (PKG) on activation of MAPK by ET-1. The rat NPVSM model was chosen because evidence is emerging that these signal transduction pathways have specific neonatal vascular cellular functions including mitogenesis and contractility [3], phenomena which may be accelerated in disease states such as persistent pulmonary hypertension of the newborn (PPHN).
Materials and Methods
NPVSM Isolation
Lung lobes were excised from 1–2-day-old neonate rats and placed into ice cold physiological saline solution (PSS-I) consisting of NaCl 137 mM, KCl 5.4 mM, Hepes 10 mM, KH2PO4 0.4 mM, NaHCO3 4.2 mM, NaH2PO4 0.4 mM, and CaCl2 0.05 mM. The pulmonary blood vessels were dissected under a stereomicroscope, the endothelium was removed, and the adventitia was carefully teased away. The vascular tissue was then dissociated enzymatically at 35°C for 1 in an incubation solution of PSS-I containing the following: collagenase type II 1 mg/ml (Sigma), elastase 0.2 mg/ml (Worthington), trypsin inhibitor 0.5 mg/ml, bovine serum albumin (BSA) 2.0 mg/ml (Sigma), and DNAase type I 0.1 mg/ml (Sigma). After incubation, 5.0 ml of PSS-I containing 2.0 mg/ml BSA was added to the cell solution. The tissue was then triturated gently which allowed individual smooth muscle cells to fall away from the larger pieces of undigested tissue with minimal damage to the cells. These cells typically exhibit morphology characteristics of vascular smooth muscle cells (see NPVSM identification procedure below). Isolated cells were seeded in 60-mm culture dishes at a density of 9 × 104 cells/cm2 and grown to 75% confluency (6–7 days) to obtain a primary culture (passage 0) for the cell culture experiments.
NPVSM Identification
The positive identification of NPVSM cells was done using α-actin monoclonal antibody, which is widely used to identify smooth muscle cells [8, 10, 19]. NPVSM cells isolated from neonatal pulmonary vessels were placed on 13-mm coverslips coated with fibronectin and allowed to grow to approximately 75% confluency. After confluency, the coverslips were washed in phosphate-buffered saline (PBS). The PBS was removed and the NPVSM cells were fixed for 10 min in methanol at −20°C. The cells were then washed 3 × in PBS, and FITC-conjugated α-actin monoclonal antibody (Sigma) was added to the cells and incubated for 45 min in a humidified chamber (37°C). The coverslips were then washed 3 × in PBS, mounted on a microscope slide with Biomedia Gel Mount, and visualized using an IMT2-RFL microscope with epifluoresence. Cells that stained yellow (α-actin monoclonal antibody) and exhibited the characteristic “hill and valley” pattern of growth were identified as smooth muscle in origin as has been shown in other studies [8, 10, 19].
MAPK Signaling in NPVSM
For the MAPK expression experiments, time course treatments were done with (1) 10 nM ET-1 alone (n = 4), (2) pretreatment with the exogenous NO donor sodium nitroprusside (10 μM) for 15 min before ET-1 (n = 4), (3) pretreatment with 100 μM L-NAME (nonspecific NOS inhibitor) for 30 min before ET-1 (n = 4), (4) pretreatment with 1 μM NPLA (specific NOS 1 inhibitor) for 30 min before ET-1 (n = 4), and (5) pretreatment with 1 μM KT5823 (specific PKG antagonist) for 30 min before ET-1 (n = 4). For the Western blots, after the time course treatments the cells were washed in PBS and homogenate buffer containing 0.29 M sucrose, 10 mM Tris-HCL (pH 7.4), 1 mM EDTA, 1 mM EGTA, and 20 μg/ml each of leupeptin, soybean trypsin inhibitor, aprotinin, and phenylmethylsulfonyl fluoride. The cells were harvested and triturated five times with a 1-ml syringe and 22 gauge needle and prepared for Western blot analysis as previously described [26, 27, 43]. After the proteins were transferred to a nitrocellulose membrane, they were blotted with phospho-specific MAPK antibodies (New England Biolabs, Inc.) at 1:1000 dilution. These antibodies recognize the catalytically activated forms (phosphorylated on tyrosine residue 204) of p44mapk and p42mapk (ERK1 and ERK2, respectively). The pMAPKases were visualized using a horseradish peroxidase conjugated to goat anti-mouse at 1:2000 dilution and an enhanced chemiluminescence kit. Blots were loaded onto Fuji radiographic film, and autoradiographs were then quantified by densitometry using imaging software (NTH Image 1.61).
Drugs
ET-1 was purchased from Peptides International, and all other drugs were purchased from Calbiochem.
Statistical Analysis
All values are expressed as means ± SE. Significance was determined using an analysis of variance for within group and between group comparisons. If a significant F ratio was found, then specific statistical comparisons were made using Bonferroni–Dunn post hoc test. Statistical significance was accepted when p < 0.05.
Results
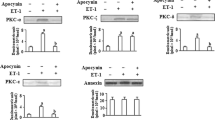
Total p44mapk and p42mapk protein expression for each treatment group is shown (Figs. 1–5), and the effect of 10 nM ET-1 on p44mapk and p42mapk phosphorylation in NPVSM is shown in Figure 1. ET-1 induced phosphorylation of both p44mapk and p42mapk from 5 to 30 min with a peak response observed at 10 min (Fig. 1). Treatment with the exogenous NO donor sodium nitroprusside (10 μM) significantly inhibited ET-1-induced p44mapk and p42mapk phosphorylation at 5, 10, and 30 min. (Fig. 2; p < 0.05) compared with ET-1 alone (Fig. 1), indicating that NO production attenuates ET-1-induced MAPK activation. To determine if decreasing the production of NO via NOS inhibition would modulate the effect of ET-1 on expression of p44mapk and p42mapk, experiments were done using the nonspecific NOS inhibitor L-NAME and the specific NOS 1 inhibitor NPLA to treat NPVSM before ET-1 exposure. As seen in Figure 3, 100 μM L-NAME significantly increased (p < 0.05) MAPK phosphorylation by ET-1 compared with ET-1 treatment alone at 1, 5, 10, and 30 min (Fig. 1). In addition, this same phenomenon was observed in the presence of 1 μM NPLA, even at 60 min (Fig. 4; p < 0.05), indicating that NO production via NOS 1 modulates ET-1-induced MAPK phosphorylation in NPVSM. Since NO activates PKG via cGMP, experiments were also done with the specific PKG inhibitor KT5823 to determine if PKG modulates the response to ET-1. Figure 5 shows that PKG blockade with 1 μM KT5823 significantly enhances p44mapk and/or p42mapk phosphorylation by ET-1 compared with ET-1 treatment alone (Fig. 1) at 1, 5, 10, 30, and 60 min (p < 0.05), indicating that PKG activation by NO also has an effect on MAPK phosphorylation by ET-1. Collectively, these data indicate that p44mapk and p42mapk phosphorylation by ET-1 in NPVSM is modulated by NO production and subsequent PKG signaling.
Effect of endothelin-1 (ET-1) on tyrosine phosphorylation of p44mapk and p42mapk. NPVSM were incubated in serum-free medium for 24 h and then treated with 10 nM ET-1 for the times indicated. Cells were lysed, and lysates were immunoblotted with phosphotyrosine-specific and nonphosphospecific anti-p44mapk and anti-p42mapk antibodies. Shown are representative densitometric analysis and immunoblots (inset) of four immunoblots probed with the phosphotyrosine-specific antibody (mean ± SE). P-Tyr, phosphotyrosine. *Significantly different from zero, p < 0.05.
Effect of sodium nitroprusside (SNP) and ET-1 on tyrosine phosphorylation of p44mapk and p42mapk. NPVSM were incubated in serum-free medium for 24 h and then pretreated with 10 μM SNP for 15 min before treatment with 10 nM ET-1 for the times indicated. Cells were lysed, and lysates were immunoblotted with phosphotyrosine-specific and nonphosphospecific anti-p44mapk and anti-p42mapk antibodies. Shown are representative densitometric analysis and immunoblots (inset) of four immunoblots probed with the phosphotyrosine-specific antibody (mean ± SE). P-Tyr, phosphotyrosine. +Significantly different from ET-1 alone, p < 0.05.
Effect of L-NAME and ET-1 on tyrosine phosphorylation of p44mapk and p42mapk. NPVSM were incubated in serum-free medium for 24 h and then pretreated with 100 μM L-NAME for 30 min before treatment with 10 nM ET-1 for the times indicated. Cells were lysed, and lysates were immunoblotted with phosphotyrosine-specific and nonphosphospecific anti-p44mapk and anti-p42mapk antibodies. Shown are representative densitometric analysis and immunoblots (inset) of four immunoblots probed with the phosphotyrosine-specific antibody (mean ± SE). P-Tyr, phosphotyrosine. *Significantly different from zero, p < 0.05. +Significantly different from ET-1 alone, p < 0.05.
Effect of NPLA and ET-1 on tyrosine phosphorylation of p44mapk and p42mapk. NPVSM were incubated in serum-free medium for 24 h and then pretreated with 1 μM NPLA for 30 min before treatment with 10 nM ET-1 for the times indicated. Cells were lysed, and lysates were immunoblotted with phosphotyrosine-specific and nonphosphospecific anti-p44mapk and anti-p42mapk antibodies. Shown are representative densitometric analysis and immunoblots (inset) of four immunoblots probed with the phosphotyrosine-specific antibody (mean ± SE). P-Tyr, phosphotyrosine. *Significantly different from zero, p < 0.05. +Significantly different from ET-1 alone, p < 0.05.
Effect of KT5823 and ET-1 on tyrosine phosphorylation of p44mapk and p42mapk. NPVSM were incubated in serum-free medium for 24 h and then pretreated with 1 μM KT5823 for 30 min before treatment with 10 nM ET-1 for the times indicated. Cells were lysed, and lysates were immunoblotted with phosphotyrosine-specific and nonphosphospecific and anti-p44mapk and anti-p42mapk antibodies. Shown are representative densitometric analysis and immunoblots (inset) of four immunoblots probed with the phosphotyrosine-specific antibody (mean ± SE). P-Tyr, phosphotyrosine. *Significantly different from zero, p < 0.05. +Significantly different from ET-1 alone, p < 0.05.
Discussion
The results of this study demonstrate: (1) the expression and phosphorylation of MAPKs in rat neonatal pulmonary vascular smooth muscle (NPVSM) by ET-1, (2) NO-mediated inhibition of ET-1-induced MAPK phosphorylation, and (3) that NOS inhibition and PKG inhibition enhances the effect of ET-1 on both p44mapk and p42mapk phosphorylation in NPVSM. Mitogen-activated protein kinases (MAPKs) are important messengers in signal transduction pathways that are stimulated by many different types of cell surface receptors. MAPKs are a family of 40–46-kDa cytosolic serine/threonine kinases that participate in the transduction of mitogenic and differentiation-promoting signals to the cell nucleus. In mammalian cells the MAPK family includes the specific MAPKs, extracellular signal-regulated kinases 1 and 2 (ERK1 and ERK2, or p44mapk and p42mapk, respectively), which require tyrosine and threonine phosphorylation for maximal activation [4]. Specifically, the activated MAPKs translate to the nucleus for regulation of transcription factors for cellular growth and differentiation [13, 35, 38].
In this study ET-1 induced significant phosphorylation of both p44mapk and p42mapk in NPVSM. These results agree with previous studies that reported that ET-1 activates MAPKs and stimulates DNA synthesis in vascular smooth muscle via ETA receptor mediation [11, 14, 44, 46, 48, 49]. ET-1 activates ERK1/2 in rabbit carotid artery vascular smooth muscle cells to induce mitogenesis [18] and elicit contraction in coronary smooth muscle [7] and activate MAPK in pulmonary artery smooth muscle and human coronary artery smooth muscle [46, 49]. In addition, Kubo et al. [23] observed that the endothelin precursor big endothelin-1 produced an increase in MAP kinase activity in vascular smooth muscle cells, and Malarkey et al. [25] reported that ET-1 activates MAP kinase in tracheal smooth muscle cells.
Results from this study also suggest that NO production inhibits ET-1-induced MAPK phosphorylation because the exogenous NO donor sodium nitroprusside inhibited phosphorylation of both p44mapk and p42mapk at all ET-1 treatment time points. In contrast, NOS inhibition amplified the NPVSM phosphorylation response to ET-1. Specifically, ET-1, in the presence of the nonspecific NOS inhibitor L-NAME, or the specific NOS 1 blocker NPLA induced greater p44mapk and p42mapk phosphorylation compared with ET-1 alone.
The physiological significance of the relationship between ET-1 and NO in neonatal vascular smooth muscle is not well understood, but may involve a balance of vasomotor tone in the neonate. ET-1 has been reported to induce release of NO that leads to an increase in cGMP tissue content, which modulates vasodilation in adult human pulmonary blood vessels [33]. The endothelin receptor subtype B (ETB), which is most abundant at term, mediates paradoxic vasodilation through a NO-dependent mechanism [16, 41], and studies suggest that NO regulates ETA receptors in vitro through a cGMP-dependent mechanism via activation of the cAMP-dependent protein kinase [34].
The vasodilator nitric oxide (NO) is important in the transition of pulmonary circulation from fetal to postnatal life. Primarily produced in the pulmonary endothelium by upregulation of endothelial nitric oxide synthase in fetal life [36], NO activates soluble guanylate cyclase, increasing synthesis of guanosine 3′, 5′-cyclic monophosphate (cGMP) leading to vasorelaxation of the pulmonary vasculature [5]. Physiologically, it is possible that NOSs are developmentally regulated in the fetus and contribute to the distribution of blood flow [45] and to marked lung growth and vessel mitogenesis [12, 32]. In contrast, it has also been reported that NO donors inhibit neonatal pulmonary artery smooth muscle cell growth [1] and adult systemic vascular smooth muscle proliferation [21] via an increase in cGMP and p44mapk and p42mapk phosphorylation.
It is thought that attenuated production of NO and, therefore, of cGMP may contribute to the pathophysiology of a variety of forms of neonatal pulmonary vascular disease states. Shaul et al. [37] showed that endothelial NOS gene expression is decreased in fetal lambs with pulmonary hypertension, and Thomae et al. [39] speculated that NO may alter vascular smooth muscle growth and pulmonary vascular remodeling in conditions complicated by pulmonary hypertension and treated with inhaled NO.
In summary, the results of this study revealed the expression of both p44mapk and p42mapk in primary cultured pulmonary vascular smooth muscle cells from neonatal (1–2 day old) rats identified by Western immunoblot analysis. Treatment with ET-1, a potent vasoconstrictor with vascular mitogenic properties, induced phosphorylation in both p44mapk and p42mapk. The NO donor sodium nitroprusside inhibited the MAPK phosphorylatory response to ET-1, and the specific PKG inhibitor KT5823, the nonspecific NOS inhibitor L-NAME, and the specific NOS 1 blocker NPLA all significantly enhanced phosphorylation of both p44mapk and p42mapk by ET-1. Collectively, these data demonstrate the expression and phosphorylation of MAPKs in rat neonatal pulmonary vascular smooth muscle and suggest that NO modulates MAPK activation by ET-1. Future studies need to address the specific physiological conditions under which NO regulates pulmonary vascular smooth muscle mitogenesis.
References
Ambalavanan N, Mariani G, Bulger A, et al. (1999) Role of nitric oxide in regulating neonatal porcine pulmonary artery smooth muscle cell proliferation. Biol Neonate 76:291–300
Barman SA, Pauly JR (1995) Mechanism of action of endothelin-1 in the canine pulmonary circulation. J Appl Physiol 79:2014–2020
Berra E, Diaz–Meco MT, Dominguez I, et al (1993) Protein kinase C ζ isoform is critical for mitogenic signal transduction. Cell 74:555–563
Bhalla US, Ram PT, lyengar R (2002) MAP kinase phosphatase as a locus of flexibility in a mitogen-activated protein kinase signaling network. Science 297:1018–1023
Bloch KD, Filippov G, Sanchez LS, et al (1997) Pulmonary soluble guanylate cyclase, a nitric oxide receptor, is increased during the perinatal period. Am J Physiol 272:L400–406
Bobik A, Grooms A, Millar JA, et al (1990) Growth factor activity of endothelin on vascular smooth muscle. Am J Physiol 258:C408–C415
Cain AE, Tanner DM, Khalil RA (2002) Endothelin-1 induced enhancement of coronary smooth muscle contraction via MAPK-dependent and MAPK-independent [Ca2+] (i) sensitization pathways. Hypertension 39:543–549
Carlin S, Yang KXF, Donnelly R, et al (1999) Protein kinase C isoforms in human airway smooth muscle cells: activation of PKC-ζ during proliferation. Am J Physiol 276:L506–L512
Childs TJ, Watson WH, Sanghera JS, et al (1992) Phosphorylation of smooth muscle caldesmon by mitogen-activated protein (MAP) kinase and expression of MAP kinase in differentiated smooth muscle cells. J Biol Chem 267:22853–22859
Damron DS, Nadim HS, Hong SJ, et al (1998) Intracellular translocation of PKC isoforms in canine pulmonary artery smooth muscle cells by ANG II. Am J Physiol 274:L278–288
Fei J, Viedt C, Soto U, et al (2000) Endothelin-1 and smooth muscle cells. Induction of jun amino-terminal kinase through an oxygen radical-sensitive mechanism. Arterioscler Thromb Vasc Biol 20:1244–1249
Garg UC, Hassid A (1989) Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest 83:1774–1777
Gille H, Sharrocks AD, Shaw PE (1992) Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature 358:414–417
Hafizi S, Alien SP, Goodwin AT, et al (1999) Endothelin-1 stimulates proliferation of human coronary smooth muscle cells via the ETA receptor and is co-mitogenic with growth factors. Atherosclerosis 146:351–359
Hwang SB (1988) Identification of a second putative receptor of platelet-activating factor from human polymorphonuclear leukocytes. J Biol Chem 263:3225–3233
Ivy D, LeCras T, Parker T, et al (2000) Developmental changes in endothelin expression and activity in ovine fetal lung. Am J Physiol 278:L785–793
Kahan C, Seuwen K, Meloche S, et al (1992) Co-ordinate biphasic activation of p44mapk-mitogen activation protein kinase and S6 kinase by growth factors in hamster fibroblasts. J Biol Chem 267:13369–13375
Kawanabe Y, Hashimoto N, Masaki T et al (2002) Extracellular Ca2+ influx and endothelin-1 induced intracellular mitogenic cascades in rabbit internal carotid artery vascular smooth muscle cells. J Cardiovasc Pharmacol 40:307–314
Kelleher MD, Abe MK, Chao TO, et al (1995) Role of MAP kinase activation in bovine tracheal smooth muscle mitogenesis. Am J Physiol 268:L894–L901
Khalil RA, Morgan KG (1993) PKC-mediated redistribution of mitogen-activated protein kinase during smooth muscle cell activation. Am J Physiol 265:C404–411
Kibbe MR, Li J, Nie S, et al (2000) Inducible nitric oxide (iNOS) expression upregulates p21 and inhibits vascular smooth muscle cell proliferation through p42/44 mitogen-activated protein kinase activation and independent of p53 and cyclic guanosine monophosphate. J Vasc Surg 31:1214–1228
Kribben A, Wieder ED, Li X, et al (1993) AVP-induced activation of MAP kinase in vascular smooth muscle cells is mediated through protein kinase C. Am J Physiol 265:C939–945
Kubo T, Ibusuki T, et al (2001) Mitogen-activated protein kinase activity regulation role of angiotensin and endothelin systems in vascular smooth muscle cells. Eur J Pharmacol 41:27–34
Lippton HL, Hauth TA, Cohen GA, et al (1993) Functional evidence for different endothelin receptors in the lung. J Appl Physiol 75:38–48
Malarkey K, Belham CM, Paul A, et al (1995) The regulation of tyrosine kinase signaling pathways by growth factor and G-protein-coupled receptors. Biochem J 309:361–375
Marrero MB, Schieffer B, Ma H, et al (1996) Ang II-induced tyrosine phosphorylation stimulates phospholipase C-I and Cl channels in mesangial cells. Am J Physiol 270:C1834–1842
Marrero MB, Schieffer B, Li B, et al (1997) Role of janus kinase/signal transducer and activator of transcription and mitogen-activated protein kinase cascades in angiotensin II and platelet-derived growth factor-induced vascular smooth muscle cell proliferation. J Biol Chem 272:24684–24690
McLees A, Graham A, Malarkey K, et al (1995) Regulation of lysophosphatidic acid-stimulated tyrosine phosphorylation of pp42 mitogen-activated protein kinase C and pertussis toxin sensitive mechanisms in EAhy926 cells. Biochem J 307:743–748
Molloy CJ, Taylor DS, Weber H (1993) Angiotensin II stimulation of protein tyrosine phosphorylation and protein kinase activation in rat aortic smooth muscle cells. J Biol Chem 263:7338–7345
Naruse M, Naruse K, Kurimoto F, et al (1989) Radioimmunoasay for endothelin and immunoreactive endothelin in culture medium of bovine endothelial cells. Biochem Biophys Res Commun 160:662–668
Olson NC, Kruse–Elliot KT, Whorton AR, et al (1993) Pertussis toxin attenuates platelet-activating factor-induced pulmonary hemodynamic alterations in pigs. Am J Physiol 264:L213–221
Parker TA, Le Cras TD, Kinsella J, et al (2000) Developmental changes in endothelial nitric oxide synthase expression and activity in ovine fetal lung. Am J Physiol 278:L202–208
Pussard G, Gascard JP, Gorenne I, et al (1995) Endothelin-1 modulates cyclic GMP production and relaxation in human pulmonary vessels. J Pharmacol Exp Ther 274:969–975
Redmond EM, Cahill PA, Hodges R, et al (1996) Regulation of endothelin receptors by nitric oxide in cultured rat vascular smooth muscle cells. J Cell Physiol 166:469–479
Robinson MJ, Cobb M.H (1997) Mitogen-activated protein kinase pathways. Curr Opin Cell Biol 9:180–186
Shaul PW (1997) Ontogeny of nitric oxide in the pulmonary vasculature. Semin Perinatol 21:381–392
Shaul PW, Yuhanna IS, German Z, et al (1997) Pulmonary endothelial NO synthase gene expression is decreased in fetal lambs with pulmonary hypertension. Am J Physiol 272:L1005–1012
Su B, Karin M (1996) Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol 8:402–411
Thomae KR, Nakayama DK, Billiar TR, et al (1995) The effect of nitric oxide on fetal pulmonary artery smooth muscle growth. J Surg Res 59:337–343
Thommes KB, Hoppe J, Vetter H, et al (1996) The synergistic effect of PDGF-AA and IGF-1 on VSMC proliferation might be explained by the differential activation of their intracellular signaling pathways. Exp Cell Res 226:59–66
Tod ML, Cassin S (1992) Endothelin-1–induced pulmonary arterial dilation is reduced by N-nitro-L-arginine in fetal lambs. J Appl Physiol 72:1730–1734
Toupouzis S, Huggins JP, Pelton JT, et al (1991) Modulation by endothelium of the responses induced by endothelin-1 and by some of its analogues in rat isolated aorta. Br J Pharmacol 102:545–549
Wang X, Shaw S, Amiri F, et al (2002) Inhibition of the JAK/STAT signaling pathway prevents the high glucose-induced increase in TGF-β and fibronectin synthesis in mesangial cells. Diabetes 51:3505–3509
Wang Y, Rose PM, Webb ML, et al (1994) Endothelins stimulate mitogen-activated protein kinase through either ETA or ETB. Am J Physiol 278:C1130–1135
Weiner CP, Thompson LP (1997) Nitric oxide and pregnancy. Semin Perinatol 21:367–380
Yamboliev IA, Hruby A, Gerthoffer WT (1998) Endothelin-1 activates MAP kinases and c-Jun in pulmonary artery smooth muscle. Pulmon Pharmacol Ther 11:205–208
Yanagisawa M, Kurihara H, Kimura S, et al (1988) A novel potent vasoconstrictor peptide produced from endothelial cells. Nature Lond 332:411–415
Yang Z, Krasnici N, Luscher TF (1999) Endothelin-1 potentiates human smooth muscle cell growth to PDGF: effects of ETA and ETB receptor blockade. Circulation 100:5–8
Yoshizumi M, Kim S, Kagami S, et al (1998) Effect of endothelin-1 (1-31) on extracellular signal-regulated kinase and proliferation of human coronary artery smooth muscle cells. Br J Pharmacol 125:1019–1027
Acknowledgments
The author gratefully acknowledge the technical assistance of Louise Meadows and Heather Redd. This work was supported by a Research Grant award (6-FY01-289) from the March of Dimes Birth Defect Foundation and National Heart, Lung, and Blood Institute Grant HL-68026.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barman, S.A. Effect of Nitric Oxide on Mitogen-Activated Protein Kinases in Neonatal Pulmonary Vascular Smooth Muscle. Lung 183, 325–335 (2005). https://doi.org/10.1007/s00408-005-2545-4
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00408-005-2545-4