Abstract
To our knowledge no data have been reported on the contribution to acute increase in dyspnea by the respiratory muscles in obese nonsmoking subjects. To better focus on this topic, we studied seven obese subjects and an age-matched normal control group, assessing baseline pulmonary function, breathing pattern, esophageal pressure (Pes), and gastric (Pga) and transdiaphragmatic (Pdi) pressures. Pes was also recorded during a sniff maneuver (Pessn). During a hypercapnic rebreathing test we recorded inspiratory swing in Pes (Pessw), expiratory changes in Pga, and inspiratory swings in Pdi (Pdisw). Change in inspiratory capacity was considered the mirror image of end-expiratory lung volume (EELV). Dyspnea was assessed by a modified Borg scale. Under control conditions, patients exhibited a reduced expiratory reserve volume and intrinsic positive end-expiratory pressure (PEEPi). At the end of hypercapnic stimulation, compared with controls our obese subjects exhibited greater respiratory frequency (Rf), shorter expiratory time, greater Pessw, and lower Pdisw. Increases in EELV and PEEPi were found in the obese subjects but not in controls. Changes in Borg correlated with changes in PETCO2, VE, Pessw (%Pessn), and Pdisw to a greater extent in patients than in controls. Stepwise regression analysis indicated the amount of variability in Borg that was predicted by both Pdisw (r2 = 0.31, p < 0.0004), and Pessw (%Pessn) (r2 = 0.09, p < 0.005) in controls, and by Pessw (%Pessn) (r2 = 0.40, p <0.00001) in obese subjects. We conclude that the rib cage muscles contributed to dyspnea to a greater extent in this subset of obese subjects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Current hypotheses on the origin of dyspnea emphasize the importance of respiratory muscle effort, which reflects the increased central motor command [10, 17, 19, 20]. Respiratory discomfort gets worse with increasing respiratory loads [18], dynamic hyperinflation [24], inspiratory threshold load [7], and respiratory muscle weakness [14]. Dynamic hyperinflation alters the geometrical configuration of the inspiratory muscles, putting them in a less favorable part of the length–tension curve away from its optimal length [9]. In these circumstances increased motor command to the muscles and reduced muscle strength are the important mechanisms for dyspnea [17, 18].
Studies in healthy humans have shown that the imposition of expiratory flow loads shifts the perception of difficulty in breathing from a balanced contribution of the respiratory muscle groups to a prevalent contribution of the rib cage inspiratory muscles and the abdominal expiratory muscles [6, 16, 32, 33]. Based on reports on expiratory flow limitation (EFL) and inspiratory threshold load (ITL) in spontaneously breathing obese subjects [25], we hypothesize that in this subset of patients EFL and ITL overtax the respiratory rib cage muscles such that the sensation of respiratory difficulty would preferentially refer to an increased drive to the respiratory muscles other than the diaphragm.
As yet no data have been reported on the contribution to acute changes in dyspnea by the respiratory muscles in obese patients. The present study was carried out to verify the above hypothesis in seven obese nonsmoking subjects who in a previous sleep study had exhibited ITL while breathing spontaneously. Carbon dioxide rebreathing was used as a standard stimulus to increase ventilation in this study [8].
Patients and Methods
Subjects
Seven subjects (4 males), with body mass index (BMI) ≥ 36.11 kg/m2, were referred to the Section of Respiratory Diseases at the Department of Internal Medicine at the University of Florence. Inclusion criteria were life-long nonsmoker and absence of cardiac disorders. In accordance with the criteria established by the American Thoracic Society [1], there was no evidence that any patient suffered from chronic obstructive pulmonary disease or bronchial asthma. A group of 14 normal subjects matched for gender and age (20–65 years) was studied as a control. They were either members of our institution or medical students. All were free of cardiopulmonary disorders. Their demographic characteristics (BMI-22–26 kg/m2) and lung function were strictly normal (range as percentage of the predicted value): total lung capacity (TLC)-98%–110%; vital capacity (VC)-98%–108%; forced expiratory volume in 1 s (FEV1) 93%–110%.
Measurements
Routine spirometry was obtained with a water-sealed spirometer (Pulmonet Godard). Functional residual capacity was measured by helium dilution technique as previously described [28]. The normal values for lung volumes are those proposed by European Respiratory Society (ERS) [26]. During both inspiratory and expiratory efforts against a closed airway, maximal inspiratory (MIP) and expiratory pressures (MEP) were measured as previously described [15].
After baseline routine testing, the ventilatory pattern was evaluated during room-air breathing. From the spirogram we derived inspiratory time (TI), expiratory time (TE), total time of the and respiratory cycle (TTOT), inspiratory tidal volume (VT), and mean inspiratory flow (VT/TI). Respiratory frequency (Rf = 1/TTOT × 60) and minute ventilation (VE = VT × Rf) were also calculated. Expired CO2 (PETCO2) was sampled continuously at the mouth by an infrared CO2 meter (Datex Normocap, Helsinki, Finland). The values for dead space and resistance of the system up to a flow of 4 L/s were 201 ml and 0.94 cm H2O/(L/s), respectively. In the apparatus we used, the inspiratory line was separated from the expiratory one by a one-way valve (Hans-Rudolph) connected to a Fleisch type 3 pneumotachograph (Beckman Instruments, Shiller Park, IL). The flow signal was integrated into volume.
In all patients and in seven controls esophageal pressure (Pes) was measured with an esophageal latex balloon (length-10 cm; air volume-0.5 ml) introduced via the nose. A marker was placed on the polyethylene tubing 40 cm from the balloon tip [21]. The catheter was connected to a differential pressure transducer (Validyne, Northridge, CA). Transpulmonary pressure (PL) was obtained as the difference between mouth pressure and Pes.
In controls and in six of the seven patients, gastric pressure (Pga) was measured with a similar balloon catheter system connected to a second differential transducer. This balloon was positioned in the stomach with the tip 65–70 cm from the nostrils and contained 1 ml of air. Pressure and flow signals were recorded onto an IBM-compatible personal computer by a RTI 800 A/D card (an eight-channel analog/digital board at 100-Hz sampling rate).
The highest (most negative in sign) Pes was evaluated at functional respiratory capacity (FRC) during a maximal sniff maneuver (Pesmaxsn) [11], which was repeated until three measurements with less than 5% variability were recorded. The highest value of Pesmaxsn was used for subsequent analysis.
Volume and time components of the respiratory cycle and pressures were averaged in each patient over 30 consecutive breaths.
Hypercapnic Hyperoxic Rebreathing Tests
A hypercapnic hyperoxic test was performed following the procedure recommended by Read [27]. Rebreathing was terminated when the PETCO2 reached 72–74 mmHg. Changes in volume and time components of breathing pattern and pressures were continuously recorded. Inspiratory capacity (1C) was measured every 30–40 s. In each subject the rebreathing test was repeated twice on the same day with an interval of 60 min between each test. Ventilatory and pressure response slopes were averaged for each subject. Details of the procedures have been described elsewhere [30].
Protocol
The subjects were tested on two separate days. On the first day they were acquainted with the laboratory equipment, trained to breathe quietly into a mouthpiece, and trained to perform maximum inspiratory and expiratory and transdiaphragmatic pressure maneuvers. On the second day, after a 5-min period of rest, the subject was seated comfortably in an armchair and started to breathe into a pneumotachograph; when stable PETCO2 values were obtained, the pattern of breathing and Pes and Pga swings during two periods of quiet breathing over 20 min were recorded. After that, the hypercapnic rebreathing test was carried out.
Dyspnea
Under control conditions and every 30 s during the rebreathing test, subjects were asked to quantify the sensation of breathlessness by pointing to a score on a large Borg scale from 0 (none) to 10 (maximal) [5]. Specifically, the subjects were requested to quantify the intensity of breathlessness by relating it to their common experience. The scale was a continuous vertical linear display with ten verbal descriptors of the extent of the symptom which correspond to those of the 10-point Borg category scale. The subjects were instructed to indicate how dyspneic they felt with reference to the category descriptors.
Data Analysis
To the extent that total lung capacity does not change appreciably during an increase in ventilation [35], the changes in IC were thought to accurately reflect the changes in dynamic end-expiratory lung volume (EELV). We calculated end-inspiratory lung volume (EILV) by adding VT to EELV.
Pes was used as an index of pleural pressure and Pga as that of abdominal pressure. From the pressure signals we measured the following: Pes in all patients and Pga in five patients at end-inspiration (PesEI and PgaEI, respectively) and end-expiration (PesEE and PgaEE, respectively) at zero flow points. ΔPga was the maximal variation in gastric pressure during expiration. Pessw was measured from beginning to end inspiration at zero flow points and was expressed both as an absolute value (cm H2O) and as a percentage of Pessn. Pessw (%Pessn) represents the force required for breathing relative to the maximal inspiratory force available and is henceforth referred to as inspiratory muscle effort. The difference in esophageal pressure between end-expiration and the initiation of inspiratory flow was considered as an expression of dynamic positive end-expiratory alveolar pressure (PEEPi) [13].
The transdiaphragmatic pressure (Pdi) was obtained by subtracting Pes from Pga. Pdi at end-expiration during quiet breathing was assumed to be zero. The difference between PgaEI and PesEI was PdiEI. Pdisw was measured from beginning to end-inspiration and was expressed as an absolute value (cm H2O).
One concern with PEEPi being an expression of preinspiratory muscle activity (decrease in Pes) is the concomitance of expiratory abdominal muscle contraction and then relaxation [23, 35]. Because of this we subtracted the decrease in Pga from the concomitant change in Pes before the beginning of inspiratory flow (Fig. 1) [2].
Record of flow, esophageal pressure (Pes), gastric pressure (Pga), and transdiaphragmatic pressure (Pdi) at two levels of end-tidal carbon dioxide tension (PETCO2) in one representative obese subject (patient 2 in Table 1). The arrows indicate the onset of the inspiratory effort and the vertical line corresponds to the onset of the inspiratory flow. Intrinsic positive end-expiratory alveolar pressure (PEEPi) was calculated as negative deflection in Pes (bars, a) that preceded the start of the inspiratory flow. As seen in the figure, a positive preinspiratory Pes was present before the beginning of rebreathing and it increased along with inspiratory swing in Pes (Pessw) over the test.
Statistics
A parametric statistical procedure was used to test differences and a t test for paired samples. Changes during hypercapnic stimulus were evaluated by two-way analysis of variance (ANOVA). Individual regression analysis was performed by Pearson’s correlation coefficient. The relationships between the Borg score as a dependent variable and other independent variables were analyzed by applying the general linear model to multiple variables: We put into the model the variable that in the single model presented significant Wald χ2 values [31]. After checking the statistical significance of Wald χ2 values, we used multiple regression analysis for pooled data with stepwise regression to assess how much of the variability in the Borg score as dependent variable was predicted by other variables as independent. We retained the data of this analysis when the variables entered in the final model were the same as those obtained in general model analysis. The level of significance was settled at p < 0.05. All statistical procedures were carried out by Intercooled Stata 6.0 for Windows (Stata Corporation, College Station, TX). Data are presented as mean ± SE.
Results
Spirometry
FRC (patients 4, 5, 7) and expiratory reserve volume (ERV) (patients 1 and 4–7) were either mildly or moderately reduced (< mean predicted value −1.65 RSD), respectively, in obese patients; VC and IC, FEV1, MIP and MEP, and PaCO2 were normal (within mean predicted value ± 1.65 RSD ), with Pa O2 being mildly reduced in all patients but two (patients 1 and 3) (Table 1).
Breathing Pattern, Ventilation, and Operational Lung Volumes
Both controls and patients exhibited significant increases in VT (p < 0.000008 and p < 0.00001, respectively), VT/Ti (p < 0.000001 and p < 0.00001, respectively), and EILV (p < 0.00001 for both) at the end of hypercapnic stimulation. Unlike controls, patients exhibited an increase in Rf (from 16 ± 1 cycles/min to 22 ± 1 cycles/min, p < 0.00001), shorter TE (from 2 ±0.17 s to 1.36 ± 0.18 s, p < 0.001), and increases in EELV (from 2.57 ± 0.23 L to 3.45 ± 0.42 L, p < 0.00001) (Fig. 2).
Schematic representation of the change in breathing pattern from quiet breathing to end of hypercapnic stimulation for controls (left panel) and patients (right panel). The ascendent limb is the mean inspiratory flow (VT/TI); the descendent limb is the mean expiratory flow; the total time is the sum of inspiratory time and expiratory time. (•) quiet breathing, (○) end-rebreathing. Note that the start of inspiration is at a high lung volume (increase in EELV) in patients.
Slopes and intercepts of the relationship between changes in VE and changes in PETCO2 were similar in the two groups (1.6 ± 1 and 1.75 ±4 L/mmHg; −68 ±53 and −77 ± 23 L, for patients and controls, respectively).
Mechanics
Both controls and patients exhibited increases in Pdisw (p < 0.00001 and p < 0.0006) and in Pessw (p < 0.00001 and p < 0.0001, respectively); however, increase in Pdisw was lower (p < 0.04) and increase in Pessw was greater (p < 0.02) in patients.
ΔPga significantly increased, even if remarkably, in only 3 controls (1.35 ± 0.2 cm H2O to 15.0 ± 5.7 cm H2O), and PEEPi increased (p < 0.00001) over the hypercapnic rebreathing in all patients but one (patient 3) (Table 3 and Fig. 3). Also, changes in Pessw (%Pessn) related significantly to both EELV and EILV during hypercapnic rebreathing in patients (p < 0.03–0.01).
Patients’ increase in respiratory pressures over quiet breathing (QB) during hypercapnic stimulation. Values are mean and SE. (•) Pdisw, (▲) Peep dyn, (▼) Pessw (%Pessn), (♦) ΔPga. Pdisw: inspiratory swing in transdiaphragmatic pressure; PEEPi: intrinsic positive end — expiratory pressure; Pessw (%Pessn): inspiratory swing in esophageal pressure (Pessw) as % of Pes sniff (Pessn); ΔPga: maximal variation in gastric pressure during expiration.
Dyspnea
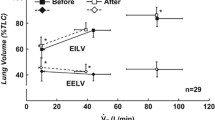
Changes in the Borg Score were consistently related to changes in PETCO2, VE, Pessw (%Pessn), and Pdisw (p < 0.05–0.005) to a greater extent in patients than in controls (Table 4); changes in ΔPga or PEEPi were not correlated with changes in the Borg score in either group. Significant relationships between operational lung volumes (EELV and EILV) and the Borg score were also found in patients (Fig. 5).
In multivariate analysis, Borg score was significantly related (Wald χ2 = 60.39, p <0.0001) to Pdisw (z = 3.4, p < 0.001) and Pessw (%Pessn) (z = 2.83, p < 0.005) in controls, and to Pessw (%Pessn) (z = 3.49, p < 0.0001) in patients (Wald χ2 = 157, p < 0.00001).
Stepwise regression analysis indicated the amount of variability in the Borg score (r2 = 0.40) predicted by Pdisw (r2 = 0.31, p < 0.0004) with an additional 9% variability predicted by Pessw (%Pessn) (r2 = 0.09, p < 0.005) in controls. Pessw (%Pessn) (r2 = 0.40, p < 0.0001) was the sole independent predictor of the variability in the Borg score in obese subjects.
Discussion
We have shown that dynamic hyperinflation and inspiratory threshold load (PEEPi) progressively increased during hypercapnic stimulation in our subset of obese subjects. In the control group Pdisw and Pessw (%Pessn) were the principal correlates to the Borg score in controls, while Pessw (%Pessn) contributed to a greater extent to the Borg score in patients.
Critique of Methods
Direct and simple precise information on activation of the respiratory muscles would have been obtained by electromyography (EMG) of the respiratory muscles. However, the use of pressure signals to assess respiratory muscle activity has been shown to be as good as EMG [33]. With regard to the small number of patients, 12 patients were recruited but the complexity of the methodology, protocol, and mostly the patients’ ability to provide reproducible maneuvers limited the number of participants in the study.
Discussion of the Results
Expiratory flow limitation and intrinsic positive end-expiratory pressure (PEEPi) have been found recently in quietly breathing obese subjects [25]. These findings are consistent with the contention that breathing at low lung volume decreases the expiratory flow reserve and may lead to airway flow limitation [3]. In this regard, expiratory reserve volume was significantly smaller than predicted in the study by Pankow et al. [25] as in most patients in the present research. We expanded the data of Pankow et al. in that PEEPi, which was measured in all but one patient while seated, increased with increasing ventilation. Because of the flow limitation at rest or the rapid increase in ITS at the beginning of the CO2 rebreathing, patients tended to dynamically hyperinflate such that functional residual capacity remained above the relaxation volume of the respiratory system. At end-expiration the positive pressure was counterbalanced by the inspiratory muscle action before inspiratory flow started. Another potential reason for the increase in PEEP is a short expiratory time. Its shortening with hypercapnia allows less time for exhaling pulmonary volume and does not enable the pressure in alveoli to equalize the downstream pressure. However, the reduction in expiratory time was mild, while the time constant was likely to be short because of the reported increase in chest wall elastance in obese subjects [22]. For these reasons we believe it unlikely that the reduced expiratory time contributed importantly to PEEPi.
In line with our previous studies in controls and in patients with multisystem diseases [29], the rib cage muscles were involved mostly in the perception of dyspnea in patients. These data are consistent with the observation that the increase in dynamic hyperinflation enhances the inspiratory rib cage muscle contribution to inflate the respiratory system, relative to that of the diaphragm [7]. In addition, the increase in mean inspiratory flow (Fig. 2), which reduces maximal force-generating capacity [33], contributed to the increased Pessw (%Pessn).
The reasons why inspiratory activity of the diaphragm does not appear to be independently involved in dyspnea in obese patients are likely to be complex. There is evidence that in healthy subjects breathing against an expiratory load the inspiratory activity of the rib cage muscles is more involved in the perception of inspiratory effort than that of the diaphragm [6, 16, 33]. Bradley et al. [6] found a strong correlation between the score effort and Pes-to-maximal Pes ratio in both fresh and fatigued diaphragmatic conditions. Ward et al. [34] found that the sense of effort that accompanied diaphragmatic fatigue correlated with a rising inspiratory activity of the rib cage muscles but not with an increased diaphragmatic activation. They postulated that the sensory changes might be a result of an increased perception of central inspiratory motor output preferentially directed to the rib cage muscles. More recently, Kaiser et al. [16] found that the pressure swings across the diaphragm do not explain much of the variance in perception of respiratory difficulty in healthy subjects during flow-limited cycling exercise. In turn, an increased drive to the respiratory muscles other than the diaphragm, or receptors within these muscles, would appear to be the prime candidate for generating the sensation of respiratory effort in conditions of expiratory flow limitation [16].
An increase in expiratory gastric pressure swings reflects the expiratory recruitment and activity of the abdominal muscles, aimed at improving both length and preinspiratory configuration of the diaphragm during dynamic hyperinflation [16, 35]. In line with studies in healthy humans [12], we found that gastric pressure swings inconsistently increased in both groups and did not correlate with dyspnea in the conditions of the present study.
Unlike the study by Kaiser et al. [16], we found that end-tidal carbon dioxide tension (PETCO2) did not independently contribute to the variability in the Borg score. However, it is difficult to compare studies where increase in CO2 is the main input for ventilation (VE) with other studies where many sources of increase in VE vary over the test. Had we maintained constant VE and pressure production or prohibited their increase, we could have evaluated the increasing effect of CO2 as an independent contributor to dyspnea; the alteration in blood CO2 tension sensed by the chemoreceptors may contribute per se to breathing sensation [4]. Clague et al. [8] recently found, however, that during CO2 rebreathing, the inspiratory effort sensation was mainly related to the inspiratory rib cage tension–time index, with arterial CO2 tension having only a small independent effect on sensation.
In conclusion, inspiratory effort and dynamic hyperinflation with attendant PEEPi increase during stimulated breathing in patients. Compared with controls, the rib cage muscles mostly contribute to dyspnea in our subset of obese subjects.
References
American Thoracic Society (1987) Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis 136:7225–7244
Appendini L, Patessio A, Zanaboni S, et al (1994) Physiologic effects of positive end-expiratory pressure and mask pressure support during exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 149:1069–1076
Babb TG (1999) Mechanical ventilatory constraints in aging, lung disease, ad obesity: perspectives and brief review. Med Sci Sports Exerc 31 (1 Suppl):S12–22
Banzett RB, Lansing RW, Brown R, et al (1990) ‘Air hunger’ from increased PCO2 persists after complete neuromuscular blocks in humans. Respir Physiol 81:1–17
Borg GA (1982) Psychophisycal bases of perceived exertion. Med Sci Sports Exerc 14:377–381
Bradley TD, Chartrand DA, Fitting JW, et al (1986) The relation of inspiratory effort sensation to fatiguing patterns of the diaphragm. Am Rev Respir Dis 134:1119–1124
Chen RC, Yan S (1999) Perceived respiratory difficulty during inspiratory threshold load and hyperinflationary loadings. Am J Respir Crit Care Med 159:720–727
Clague JE, Carter J, Coakley J, et al (1994) Respiratory effort perception at rest and during carbon dioxide rebreathing in patients with dystrophia myotonica. Thorax 49:240–244
Eldridge FL, Vaughn KZ (1977) Relationship of thoracic volume and airway occlusion pressure: muscular effects. J Appl Physiol 43:312–321
Gandevia SC (1988) Neural mechanisms underlying the sensation of breathlessness: kinaesthetic parallels between respiratory and limb muscles. Aust N Z J Med 18:83–91
Gorini M, Misuri G, Corrado A, et al (1996) Breathing pattern and carbon dioxide retention in severe chronic obstructive pulmonary disease. Thorax 51:677–683
Grimby G, Goldman M, Mead J (1976) Respiratory muscle action inferred from rib cage and abdominal V-P partitioning. J Appl Physiol 41:739–751
Haluszka J, Chartrand DA, Grassino A, et al (1990) Intrinsic PEEP and arterial PCO2 in stable patients whit chronic obstructive lung disease. Am Rev Respir Dis 141:1194–1197
Hamilton AL, Killian KJ, Summers E, et al (1995) Muscle strength, symptom intensity and exercise capacity in patients with cardiorespiratory disorders. Am J Respir Crit Care Med 152:2021–31
Iandelli I, Gorini M, Misuri G, et al (2001) Assessing inspiratory muscle strength in patients with neurological and neuromuscular diseases. Comparative evaluation of two noninvasive techniques. Chest 119:1108–1113
Kayser B, Sliwinski P, Yan S, et al (1997) Respiratory effort sensation during exercise with induced expiratory-flow limitation in healthy humans. J Appl Physiol 83:936–947
Killian KJ, Gandevia SC, Summers E, et al (1984) Effect of increased lung volume on perception of breathlessness, effort and tension. J Appl Physiol 57:686–691
Killian KJ, Campbell EJM (1995) Dyspnea. In: Roussos C (eds) The Thorax, part B. Applied Physiology. Marcel Dekker, New York, pp 1709–1747
Leblanc P, Summers E, Inman MD, et al (1988) Inspiratory muscles during exercise. A problem of supply and demand. J Appl Physiol 64:2482–2489
McCloskey DI (1981) Corollary discharges: motor commands and perception. In: Brookhart JM, Mountcastle VB (eds). Handbook of Physiology, The Nervous System, Section I, vol 2, part 2. American Physiological Society, Bethesda MD, pp 1415–1447
Milic Emili J, Mead J, Turner JM, et al (1964) Improved technique for estimating pleural pressure from esophageal balloons. J Appl Physiol 19:207–211
Nairnark A, Cherniak RM (1960) Compliance of the respiratory system and its components in health and obesity. J Appl Physiol 15:377–382
Ninane V, Yernault YC, De Troyer A (1993) Intrinsic PEEP in patients with chronic obstructive pulmonary disease. Role of expiratory muscles. Am Rev Respir Dis 148:1037–1042
O’Donnell DE (1994) Breathlessness in patients with chronic airflow limitation. Chest 106:904–912
Pankow W, Podszus T, Gutheil T, et al (1998) Expiratory flow limitation and intrinsic positive end-expiratory pressure in obesity. J Appl Physiol 85:1236–1243
Quanjer PH, Tammeling GJ, Cotes JE, et al (1993) Lung volumes and forced ventilatory flows. Standardization of lung function tests. Eur Respir J 6(Suppl 16): 5–40
Read DJC (1967) A clinical method for assessing the ventilatory response to carbon dioxide. Aust Ann Med 16:20–32
Scano G, Garcia Herreros P, Stendardi D, et al (1980) Cardiopulmonary adaptation to exercise in coal miners. Arch Environ Health 35:360–366
Scano G, Seghieri G, Mancini M, et al (1999) Dyspnoea, peripheral airway involvement and respiratory muscle effort in patients with Type I diabetes mellitus under good metabolic control. Clin Sci 96:499–506
Scano G, Filippelli M, Romagnoli I, et al (2000) Hypoxic and hypercapnic breathlessness in patients with type I diabetes mellitus. Chest 117:960–967
Stata Reference Manual (1999) Release 6, College Station, TX, Stata Corp., pp 338–359
Suzuki S, Suzuki J, Ishii T, et al. (1992) Relationship of respiratory effort sensation to expiratory muscle fatigue during expiratory threshold loading. Am Rev Respir Dis 145:461–466
Ward ME, Corbeil C, Gibbons W, et al (1988) Optimization of respiratory muscle relaxation during mechanical ventilation. Anesthesiology 69:29–35
Ward ME, Eidelman DG, Stubbing DG, et al (1988) Respiratory sensation and pattern of respiratory muscle activation during diaphragm fatigue. J Appl Physiol 65:2181–2189
Yan S, Kayser B (1997) Differential inspiratory muscle pressure contributions to breathing during dynamic hyperinflation. Am J Respir Crit Care Med 156:497–503
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants of the Università degli Studi di Firenze.
Rights and permissions
About this article
Cite this article
Lotti, P., Gigliotti, F., Tesi, F. et al. Respiratory Muscles and Dyspnea in Obese Nonsmoking Subjects. Lung 183, 311–323 (2005). https://doi.org/10.1007/s00408-005-2544-5
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00408-005-2544-5