Abstract
The purpose of this study was to review our experience with patients who had a definitive diagnosis of follicular bronchiolitis (FB), and to describe in detail the clinical and pathological findings, looking for common clinical aspects that may help to identify this entity. Ours is a community 750 bed teaching hospital that acts as a tertiary referral center for several subspecialties, including thoracic surgery. Six patients with a morphological diagnosis of FB, defined by the presence of coalescent germinal centers adjacent to airways, were included. Lung biopsy was obtained by thoracotomy in all patients (2 women and 4 men, mean age 53 years). In one patient FB was associated with advanced AIDS, and in another with prolonged exposure to polyethylene-flock. In 4 patients no condition previously associated with FB was found. Five patients had a history of repeated respiratory infections, 3 patients complained of dyspnea and none had peripheral blood eosinophilia. After a mean follow-up of 25 months, 2 patients responded well to steroid therapy; 3 patients suffered symptomatic exacerbations that required an increase in the steroid dose and 1 patient was not treated with steroids. The most important contribution of this series is the description of a subset of patients with FB who were not associated with other processes. These patients present relatively homogeneous clinical and pathological pictures that do not differ greatly from secondary forms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Follicular bronchiolitis (FB) is a well-defined pathological condition consisting of abundant hyperplastic lymphoid follicles with reactive germinal centers distributed along the bronchioles [1, 2]. Most cases are associated with collagen vascular disease (especially rheumatoid arthritis (RA) and Sjögren’s syndrome), immunodeficiency, or appearing in an heterogeneous group of patients with frequent peripheral blood eosinophilia [1, 2, 3]. Recently, FB has been considered the characteristic lesion of those patients with nylon flock-associated interstitial lung disease [4].
We review our experience and describe the clinical and pathological characteristics of these patients, looking for common clinical aspects that may help to identity this rare entity.
Materials and Methods
We retrospectively reviewed the pathology reports of patients diagnosed with FB during a 4-year period (March 1998 to February 2001). After being revised by two independent observers, patients with a morphological diagnosis of FB, defined by the presence of coalescent germinal centers adjacent to airways, were included. Only patients in whom this finding was the predominant or the only abnormality histologically seen were accepted.
Clinical, radiological, and functional data from these patients at the time of diagnosis and during the follow-up were collected and analyzed. Specific questions were asked in an effort to determine the existence of familial histories of pulmonary disease, ciliary abnormalities, and the possibility of acquired or congenital immunodeficiency states and occupational exposure.
Unfixed specimens were received in the Surgical Pathology Department immediately following excision, and fixed in 10% buffered formalin for light microscopy. Three micrometer sections of paraffin-embedded tissues were stained with hemotoxylin and eosin (H&E), orceine van Gieson stain, and Masson trichrome for pathological examination.
In addition to the presence of diffuse lymphoid hyperplasia, pulmonary parenchyma was evaluated for interstitial cellular infiltrates, areas of lipid and organized pneumonia, parenchymal scarring, acute inflammation, and intrabronchiolar suppurative exudates.
The immunohistochemistry study was performed using the estreptavidine biotin peroxidase method (LSAB-kit universal, Dako, Barcelona, Spain). CD20/L26, CD79a/1B117, CNA.42 for follicular dendritic cells, and the bcl-2 (Concepta, Barcelona, Spain) were used as specific markers. For detecting Epstein-Barr virus (EBV), an in situ hybridization (PNA-EBER, Dako, Barcelona, Spain) was used.
ANA tests were performed with a commercially available kit that used an indirect immunofluorescent antibody method with a human epithelial (HEP-2) cell line as substrate (ANA Hep-2 IF test system, Meridan Diagnostics Europe SRL, Milano, Italy). Immunofluorescence was considered positive if observed at titers of ≥1:40. The rheumatoid factor test was performed by nefelometry using the Immunochemistry System Image together with the Calibrator 5 Plus (Beckman Coulter, Inc, Fullerton, California, USA).
Results
Six patients, 2 women and 4 men, mean age of 53 years (range 32–73), were diagnosed as having FB. Three additional patients were excluded: one because of specific pathological lesions of aspergillosis, 1 because of predominant lesions of organized pneumonia, and 1 because of adjacent bronchiectasis. Biopsy was obtained by thoracotomy in all 6 patients. Lung biopsy specimens were obtained from at least 2 lobes in 5 cases. Diagnostic open lung biopsy (OLB), because of undiagnosed diffuse lung disease, was the initial aim in 4 patients; in the 2 remaining patients, lung tissue specimens were obtained by a thoracotomy initially planned with a tumor-removal objective. In 2 patients, a disease known to be associated with FB was identified: 1 had advanced AIDS and the other had a prolonged exposure to polyethylene-flock. This latter case has been previously reported [5]. In 4 patients, an extensive diagnostic workup revealed no condition previously associated with FB. All but 1 patient had a history of repeated respiratory infections; 3 patients complained of dyspnea but none had peripheral blood eosinophilia (Table 1). Mean follow-up was 25 months (3–48 months).
Two patients responded well to steroid therapy and had a relapse-free course after steroids were withdrawn although 1 of them died of a massive abdominal hemorrhage 1 year after diagnosis. Three patients suffered symptomatic exacerbations that required an increase in the steroid dose. One patient, currently complaining of mild dyspnea, was not treated with steroids. Pulmonary function tests showed a mild restrictive disorder in most patients, with a more marked decrease in diffusing capacity. These changes were stable, recurrent, or progressive during the follow-up (Table 2). High resolution CT scan showed nodules in all patients ranging in size from 1 to 3 mm with a predominant centrilobular distribution (Table 3).
In addition to characteristic features of FB, other incidental pathological lesions were found (Table 4). In the immunohistochemistry, follicular lymphoid cells were predominantly positive for B markers (CD20 and CD79a), with a prominent population of dendritic follicular cells. When using bcl-2, mantle and marginal zone lymphoid cells were positive whereas germinal centers were negative. Staining for EBV in all patients resulted in a positive lymphoid component in the AIDS patient. A brief description of each patient is reported below.
Patient 1
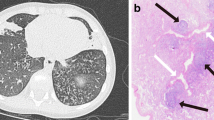
A 73-year-old man, ex-smoker of 20 pack-years, was referred for surgery because of a 1 cm right upper lobe (RUL) nodule. During the previous 2 years he had complained of a cough, recurrent yellow sputum, occasional hemoptysis, and more recently, dyspnea on effort. By thoracotomy, 4 nodules showing FB, 2 sited in the RUL, and 1 each in the middle lobe and the right lower lobe (RLL), were identified and removed (Fig. 1).
In the following days, the patient presented with fever, cough, and purulent sputum. A high resolution CT scan showed nodular infiltrates in the left upper lobe (LUL) and the right lower lobe (RLL) and widespread peripheral micronodules. Prednisone 45 mg/day was added to antibiotic therapy and later tapered in the next 5 months. Cough and mild breathlessness persisted until death, 1 year after diagnosis, caused by disruption of an abdominal aneurysm.
Patient 2
A never-smoker 61-year-old man was admitted to the hospital because of a 3-month history of recurrent bouts of fever, productive cough, and a 6 kg weight loss. Chest X-ray showed bilateral patchy alveolar infiltrates. A fiberbronchoscopy disclosed purulent secretions with a positive culture for Haemophilus influenzae, and a transbronchial biopsy showed mild interstitial fibrosis and pneumocytes hyperplasia. An open lung biopsy (OLB) showed follicular bronchiolis (FB) (Fig. 2).
Symptoms subsided and pulmonary infiltrates cleared after 15 days of treatment with prednisone 1 mg/kg/day. Steroid therapy was tapered for the next 9 months to suppression. Two years later, symptoms and alveolar infiltrates relapsed and again showed a good response to steroid therapy. He has been free of symptoms during the last 15 months of follow-up.
Patient 3
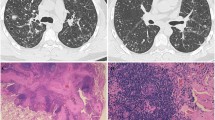
A 32-year-old woman, smoker of 10 pack/years, was admitted to the hospital because of low-grade fever, pleuritic chest pain, and an infiltrate in the middle lobe. She had a 10-year history of cough and sputum production, and recurrent episodes of pneumonia during the last 4 years. On admission, chest X-ray showed infiltrates in the middle lobe and lingula. Pulmonary function tests showed a severe restrictive dysfunction. Bronchoscopy showed acute inflammation and purulent secretions. Transbronchial biopsy was nondiagnostic. OLB showed FB (Fig. 3).
Prednisone 1 mg/kg/day, later tapered along the next 6 months to suppression, was begun with symptomatic and functional improvement and clearing of the pulmonary infiltrates.
Patient 4
A 61-year-old man, smoker of 120 pack/years was submitted to surgery for OLB because of invalidating dyspnea and bilateral pulmonary infiltrates. He had a 12-year history of productive cough with frequent exacerbations and progressive dyspnea on effort. At admission, a chest X-ray showed a bilateral interstitial infiltrate and a calcified granuloma in the RUL. A pulmonary function study disclosed an obstructive defect with a negative bronchodilator response. PaO2 was 7.2 kPa. An OLB of the left upper lobe showed follicular bronchiolitis (Fig. 4).
Prednisone 1 mg/kg/day and oxygen therapy were started. Prednisone was tapered along the next 30 months but the patient required dose increments because of frequent symptomatic exacerbations. Plain chest radiographs have remained unchanged. Ambulant oxygen therapy has been maintained during that period.
Patient 5
A never-smoker 54-year-old woman, previously reported in detail [5], presented with a 12-month history of progressive pulmonary impairment after 7 years of occupational exposure to rotary-cut polyethylene. An OLB revealed FB. Prednisone 1 mg/kg/day was administered for 4 months without apparent functional improvement. One year later, the patient remained on inhaled steroid and beta-agonists, requiring short courses of steroids for exacerbations of respiratory problems.
Patient 6
A never-smoker 35-year-old man was admitted to our hospital to remove a para-vertebral mass. He had a history of mild asthma that abated when he was 16 years old. Six years before admission, he presented with a herpes zoster infection and recently he had been diagnosed with AIDS. At the time of admission his only symptom was a left side chest pain. When thoracotomy was performed he had been on treatment for 2 months with zidovudine, lamivudine, and lopinavir/ritonavir highly active antiretroviral therapy (HAART). Chest X-ray and a vertebral CT scan showed a left para-vertebral mass without evidence of pulmonary anomalies. At thoracotomy, micronodules in lingula were detected and removed and follicular bronchiolitis resulted. A para-vertebral leiomyoma was removed. A high resolution CT scan performed a few days after thoracotomy disclosed diffuse pulmonary interstitial micronodules. Three months later, he complained of mild dyspnea and presented a mild restrictive functional defect.
Discussion
Follicular bronchiolitis is a well-defined pathological entity consisting of abundant lymphoid follicles limited to the peribronchiolar area. As occurs with other bronchiolitis, its presence may lack any clinical significance (FB pattern) or its manifestations may be confounded with those of the causal or associated disease.
The cause of FB remains unknown. It has been suggested that this syndrome may result from a hypersensitivity phenomenon to an unidentified antigen or it may be related to situations of prostrated infection [1]. When considered as a separate syndrome, FB has been associated with collagen vascular diseases, predominantly rheumatoid arthritis, or immunodeficiency states.
Probably the most important contribution of this series is the description of a subset of 4 patients with FB not associated with other processes, since this entity has not been previously reported in detail in a group of patients. Moreover, some cases of FB considered in the literature [6, 7] to be idiopathic presented peripheral eosinophilia and consequently could be included in a heterogeneous group proposed by Yousem et al. [1] in which eosinophilia was the only common characteristic of an associated hypersensitivity disorder. The patients herein who were considered idiopathic have neither a known associated disorder nor peripheral eosinophilia.
The relationship between bronchiectasis and FB is generally admitted. The presence of peribronchiolar hyperplastic follicles distal to local or focal obstructive bronchiectasis is considered a usual and secondary phenomenon [1]. However, in the case of diffuse bronchiectasis, a secondary role of the peribronchiolar lymphoid proliferation is difficult to ascertain. In fact, the two conditions more frequently associated with the syndrome of FB, rheumatoid arthritis, and immunodeficiency are recognized among the causes of this type of bronchial dilatation [8, 9]. The presence of diffuse bronchiectasis could not be demonstrated in any of the 4 cases herein which are considered to be idiopathic. Although in one, the CT scan disclosed focal bronchiectasis in the left lower lobe, the specimens showing FB were obtained from the 3 lobes of the opposite lung.
All patients in this series had negative rheumatoid factor. Two of those considered idiopathic had positive antinuclear antibody but at a low titer (1/80) that has a relatively low specificity for rheumatoid arthritis [10]. The possibility that these patients will develop RA in the future cannot be ruled out but seems remote given the absence of rheumatic manifestations at diagnosis and during the follow-up, that at the moment of this report has been extended for a mean period of 25 months.
Interestingly, these patients presented a relatively homogeneous clinical, radiological, and functional picture that does not differ greatly from that reported in patients with a definitive evidence of collagen vascular disease or other secondary forms [1, 2, 3]. Recurrent respiratory infections and progressive shortness of breath were the major presenting symptoms that, together with persistent pulmonary infiltrates unresponsive to antibiotics, accounted for a diagnostic surgical intervention, in some cases to exclude neoplasm.
As in previous patients studied by CT [11, 12], in all patients herein described with idiopathic or secondary forms, nodules ranging in size from 1 to 3 mm with a predominant centrilobular distribution were the most common finding. Although a cystic form secondary to bronchiolar obstruction has been described (reported in 1 patient) it seems to be an exceptional finding [13]. Pulmonary function tests revealed restrictive disease in the majority of nonsmoker patients, in whom the diffusing capacity appears as the most sensitive test. As in other series that report the evolution [1], in spite of steroid therapy most patients of the present series have evidence of stable, recurrent, or progressive pulmonary disease.
The only patient with a medium titer (1/160) serum-positive ANA was the one with AIDS, a situation known to be related to the elevation of this marker. It is known that viruses and bacteria are likely to induce a production of auto-antibodies without there necessarily being an associated pathology or clinical condition. The possibility that FB in this patient was a consequence of the antiretroviral treatment and so representing an immune restoration disease cannot be ruled out, because when the disorder was diagnosed he was in antiretroviral therapy. HAART-induced immune recovery produces not only an increase in memory and naïve T cells but an enhancement of lymphoproliferative responses with an increase in interleukin 2 receptor expression (CD25+) [14, 15]. Gilquin et al. [16] raised the concern that thymic-mediated redevelopment of the T-cell repertoire during HAART might be abnormal, thereby allowing for the proliferation of autoreactive T cells.
The FB secondary to inhalation of nylon flock appears as the first example of this pathological condition with a known definitive causal agent [4]. Interestingly, the FB in the woman described here was considered idiopathic for a long period of time, reflecting the under-recognition of the risks of the exposure to polyethylene dust.
In conclusion, in our experience most patients with a definitive pathological diagnosis of this rare disorder lack a known cause and do not present any associated disorder. The clinical characteristics, course, and outcome do not differ greatly with those of the secondary forms.
References
SA Yousem TV Colby CB Carrington (1985) ArticleTitleFollicular bronchiolitis. Hum Pathol 16 700–706 Occurrence Handle1:STN:280:BiqB3s3oslU%3D Occurrence Handle4007845
H Hayakawa A Sato S Imokawa et al. (1996) ArticleTitleBronchiolar disease in rheumatoid arthritis. Am J Respir Crit Care Med 154 1531–1536 Occurrence Handle1:STN:280:ByiD28bnvFI%3D Occurrence Handle8912776
TI Fortoul F Cano-Valle E Oliva et al. (1985) ArticleTitleFollicular bronchiolitis in association with connective tissue diseases. Lung 163 305–314 Occurrence Handle1:STN:280:BimD28vit1U%3D Occurrence Handle3934469
WL Eschenbacher K Kreiss MD Lougheed et al. (1999) ArticleTitleNylon flock-associated interstitial lung disease. Am J Respir Crit Care Med 159 2003–2008 Occurrence Handle1:STN:280:DyaK1M3otVKltg%3D%3D Occurrence Handle10351952
E Barroso MD Ibañez FI Aranda et al. (2003) ArticleTitlePolyethylene flock-associated interstitial lung disease in a Spanish woman. Eur Respir J . .
JI Mayordomo J Díaz JL Aranda et al. (1992) ArticleTitleBronquiolitis folicular no asociada a enfermedades sistémicas. Med Clín (Bar) 98 438 Occurrence Handle1:STN:280:By2B3snhslM%3D
I Alfageme Michavila D Martínez Parra C Escalante Aguilar et al. (1994) ArticleTitleBronquiolitis folicular: una causa infrecuente de neumopatia interstitial. Arch Bronconeumol 30 407–409 Occurrence Handle1:STN:280:ByqD2sbhtVw%3D Occurrence Handle7987550
MJ McMahon DR Swinson S Shettar et al. (1993) ArticleTitleBronchiectasis and rheumatoid arthritis: a clinical study. Ann Rheum Dis 52 776–779 Occurrence Handle1:STN:280:ByuD2s7ltVU%3D Occurrence Handle8250608
AF Barker (2002) ArticleTitleBronchiectasis. N Engl J Med 346 1383–1393 Occurrence Handle10.1056/NEJMra012519 Occurrence Handle11986413
EM Tan TE Feltkamp JS Smolen et al. (1997) ArticleTitleRange of antinuclear antibodies in “healthy” individuals. Arthritis Rheum 40 1601–1611 Occurrence Handle1:STN:280:ByiH28vhvFc%3D Occurrence Handle9324014
NL Müller RR Miller (1995) ArticleTitleDiseases of the bronchioles: CT and histopathologic findings. Radiology 196 3–12 Occurrence Handle1:STN:280:ByqA3cvgsVU%3D Occurrence Handle7784583
SJ Howling DM Hansell AU Wells et al. (1999) ArticleTitleFollicular bronchiolitis: Thin-section CT and histologic findings. Radiology 212 637–642 Occurrence Handle1:STN:280:DyaK1MvgslKlsQ%3D%3D Occurrence Handle10478225
InstitutionalAuthorNameMassachusetts General Hospital (2001) ArticleTitleCase records of the Massachusetts General Hospital (Case 17-2001) N Engl J Med 344 1701–1708
T Wendland H Furrer PL Vernazza et al. (1999) ArticleTitleHAART in HIV-infected patients: restoration antigen-specific CD4 T-cell responses in vitro is correlated with CD4 memory T-cell reconstitution, whereas improvement in delayed type hypersensitivity is related to a decrease in viraemia. AIDS 13 1857–1862 Occurrence Handle10.1097/00002030-199910010-00007 Occurrence Handle1:CAS:528:DyaK1MXmvVWgtrs%3D Occurrence Handle10513643
JM Naccache M Antoine M Wislez et al. (1999) ArticleTitleSarcoid-like pulmonary disorder in human immunodeficiency virus-infected patients receiving antiretroviral therapy. Am J Respir Crit Care Med 159 2009–2013 Occurrence Handle1:STN:280:DyaK1M3otVKltw%3D%3D Occurrence Handle10351953
J Gilquin JP Viard V Jubault et al. (1998) ArticleTitleDelayed occurrence of Graves’ disease after immune restoration with HAART. Lancet 352 1907–1908 Occurrence Handle1:STN:280:DyaK1M%2FnvFCquw%3D%3D Occurrence Handle9863795
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romero, S., Barroso, E., Gil, J. et al. Follicular Bronchiolitis: Clinical and Pathologic Findings in Six Patients . Lung 181, 309–319 (2003). https://doi.org/10.1007/s00408-003-1031-0
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00408-003-1031-0