Abstract
Emotional dysregulation (ED) is being increasingly recognized as a core feature of attention-deficit/hyperactivity disorder (ADHD), but the pathophysiological underpinnings remain unclear. In this study, we provide meaningful electrophysiological evidence of ED in adult patients with ADHD (n = 39) compared to healthy controls (n = 40) by exploring the electrophysiological correlates of the emotion regulation strategies reappraisal, distraction, and expressive suppression. Event-related potentials (ERPs) were recorded during passive viewing of neutral and negative images, as well as during emotion regulation. The patients with ADHD exhibited increased frontal late positive potential (LPP) amplitudes during passive viewing of the aversive images and during emotion regulation. Compared with the healthy controls, a subgroup of medication-naïve patients with ADHD (n = 25) also exhibited larger centroparietal LPP amplitudes and provided more negative ratings of the aversive and neutral images. Both the frontal and centroparietal LPP amplitudes were associated with ADHD symptom severity. However, no significant deficit in LPP modulation during emotion regulation was found. These findings strongly support the clinical observation of increased emotional responsivity toward negative stimuli and difficulty during the implementation of emotion regulation strategies and thus encourage the implementation of emotion regulation modules in the treatment of adult patients with ADHD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a childhood-onset neurodevelopmental condition that is associated with impairment in the domains of attention, activity, and impulse control [1]. With a persistence rate of approximately 60% [2, 3] and a prevalence of 2.5% [4], ADHD remains a significant cause of social and occupational impairment in adulthood. Although the main body of ADHD research has focused on inattentiveness and hyperactivity/impulsivity, more recent efforts to delineate the underlying deficits have increasingly targeted emotional dysregulation (ED) [5, 6]. Shaw et al. have reported prevalence rates of ED ranging from 34 to 70% in adults with ADHD [6]. Importantly, ED has been found to independently contribute to the prediction of impairment in various domains, such as social and occupational functioning and marital satisfaction [7, 8]. Despite the ample evidence of ED as a common and disabling symptom cluster of ADHD, few studies have addressed the underlying pathophysiological mechanisms [9].
Beyond ADHD, Fernandez et al. [10] have recently proposed emotion regulation as a key transdiagnostic factor within the Research Domain Criteria framework [11]. According to the extended process model of emotion regulation [12, 13], ED may occur at any of the following stages: identifying emotions that need regulating, selecting an emotion regulation strategy, implementing the selected strategy, and monitoring the implemented strategy over time. Thus, the study of the time course of emotion regulation and the study of the strategies that target different stages of the emotion regulation process may provide further insight into the deficits that lead to ED in adults with ADHD.
In the past decade, the event-related potential (ERP) technique has been successfully implemented in studies of emotion regulation. Particularly, the late positive potential (LPP), a centroparietally maximal, emotion-sensitive component that begins approximately 300 ms after stimulus presentation and lasts for several seconds, has been studied extensively in this context [14]. Reductions in the LPP amplitudes elicited by aversive stimuli following the implementation of emotion regulation strategies, such as reappraisal, distraction, and expressive suppression, have been repeatedly demonstrated in healthy subjects [15,16,17,18,19,20]. Furthermore, the LPP has been found to be a suitable measure of ED, as indicated by the diminished or abnormal LPP modulation during the use of emotion regulation strategies, in various clinical populations [21,22,23,24,25]. The frontal aspect of the LPP (i.e., frontal LPP) has been studied in terms of the cognitive effort associated with the implementation of emotion regulation strategies [17, 26].
The only ERP study of reappraisal effects in ADHD was conducted with pediatric patients, and the results indicate reduced LPP modulation during reappraisal in patients with ADHD [27]. However, there were no reappraisal effects in either group, making these results difficult to interpret. Other studies of the neural and psychophysiological ED correlates in patients with ADHD have mostly used indirect ED measures, utilizing facial stimuli or incorporating emotional distractors into well-established executive function paradigms [28,29,30,31]. Even fewer studies have investigated the correlates of ED in adult ADHD populations, and the results are ambiguous. While some evidence indicates increased initial processing of negative facial stimuli, as reflected by the elevated P1 and N170 amplitudes [32], sustained processing of emotional stimuli, as reflected by the LPP amplitude, was found to be reduced [33] or similar to that of the healthy controls [34].
These findings map onto the most influential theoretical models of ADHD, which suggest a prominent role of emotional dysfunctions in ADHD psychopathology, albeit within different frameworks. For example, Barkley has postulated that ED is but another manifestation of a general deficit in executive functioning [35]. Correspondingly, Petrovic and Castellanos [36] have suggested a continuum of top-down dysregulation extending from mostly non-emotional (i.e., “cold”) executive functions to emotional (i.e., “hot”) executive functions. Within this framework, they have located emotional ADHD presentations on the intermediate levels of this continuum. A different hypothesis states that ED in patients with ADHD results more directly from abnormally strong emotional responses rather than from executive function impairment [37, 38].
In this study, we chose a direct approach to further explore ED mechanisms in adult patients with ADHD. To this end, we recorded ERPs during a modified version of the emotion regulation task from Thiruchselvam et al. [20]. The participants viewed neural and negative images from the International Affective Picture System (IAPS; [39]) and were instructed to downregulate their emotions using the emotion regulation strategies reappraisal, distraction, and expressive suppression. Using strategies that affect different emotion regulation stages [40] and are associated with activation in different neural networks [41], we aimed to achieve a better understanding of the specific processes affected in ADHD. Cognitive reappraisal, i.e., reinterpreting the meaning of emotional stimuli, is arguably the most extensively studied emotion regulation strategy in terms of electrophysiology and functional imaging [14, 41]. Since reappraisal requires elaborate processing of the stimulus, it affects the emotion generation process relatively late (i.e., 1500 ms post-stimulus [20]). Distraction, i.e., diverting attention away from the emotional stimulus, has been shown to attenuate the LPP prior to reappraisal [18, 20], thus suggesting an early disruption of emotional processing [40]. Findings concerning the effect of expressive suppression on the LPP are more heterogeneous, with some evidence of a regulative effect of expressive suppression [18, 42], but also contrary findings [43].
Based on previous findings, we expected the patients with ADHD to exhibit heightened emotional reactivity, as indicated by overall larger LPP amplitudes and more negative/arousing ratings of the IAPS images. Further, we hypothesized that, compared with the healthy controls, the patients with ADHD would show impaired emotion regulation reflected by an attenuated centroparietal LPP and valence/arousal modulation during emotion regulation versus passive viewing of aversive pictures. Finally, given the executive deficit in ADHD [44, 45], we expected increased cognitive effort in the ADHD subjects, as indicated by heightened frontal LPP amplitudes. We formulated no specific hypotheses regarding the three emotion regulation strategies, given the explorative nature of this question.
Methods
Participants
Forty-four adults with a diagnosis of ADHD according to the DSM-5 [1] and 45 healthy controls (HC) matched for age, gender, and years of education participated in the study. We recruited the patients with ADHD from the Outpatient Clinic of the Department of Psychiatry and the Outpatient Clinic of the Department of Psychology at the University of Münster. The controls were recruited through advertisements in local newspapers. All participants were pre-screened with the WHO’s Adult ADHD Self-Report Scale (ASRS; [46]). The ADHD diagnoses in childhood and adulthood were verified by a trained clinical psychologist with the structured Diagnostic Interview for ADHD in adults (DIVA 2.0; [47]). Twenty-one patients with ADHD met the criteria for the combined ADHD subtype, and 18 patients met the criteria for the predominantly inattentive subtype. Of the patients with ADHD, ten subjects were taking stimulant medication, and four were taking stimulants in combination with antidepressants (two subjects were taking selective serotonin reuptake inhibitors; one subject was taking a selective serotonin-norepinephrine reuptake inhibitor, and one subject was taking a norepinephrine-dopamine reuptake inhibitor). For the sake of readability, we refer to the subjects as either medicated or unmedicated. However, note that all subjects discontinued their stimulant medication 24 h prior to testing. We used the 22-item ADHD self-report scale (ADHD-SR; [48]) and the Wender Utah Rating Scale (WURS-K German short version; [49]) to assess the current and lifetime severity of ADHD symptoms in the ADHD group and to identify any HCs with current or lifetime ADHD. The comorbid diagnoses were assessed with the Structured Clinical Interview for the DSM-IV [50]. The comorbid psychiatric disorders identified in the ADHD group included mild dysthymic disorder (n = 2), mild social phobia (n = 1), and a not otherwise specified eating disorder (n = 1). Additionally, we assessed depressive symptoms with the Beck Depression Inventory-II (BDI-II; [51]). The general intellectual ability of all participants was estimated with the vocabulary and matrix reasoning subtests from the German version of the Wechsler Adult Intelligence Scale (WAIS-IV; [52]). Attention was assessed using the Frankfurt Attention Inventory (FAIR-2; [53]. We also assessed habitual use of emotion regulation strategies using the Emotion Regulation Questionnaire (ERQ; [54]) and emotion regulation skills using a German self-report measure (SEK-27; [55]).
The exclusion criteria for both groups included bipolar disorder, psychotic disorder, obsessive–compulsive disorder, a severe major depressive episode within the past 5 years, substance abuse or dependence, borderline personality disorder (screened with the Borderline Symptom List BSL-23; [56]), neurological disorders, brain damage, and a serious head injury. In the ADHD group, one subject was excluded due to cannabis abuse, and another was excluded due to intellectual disability. In the control group, we excluded two more subjects due to above-threshold ADHD symptom scores and below-average performance on the FAIR-2. The data of three patients with ADHD and three controls were discarded due to excessive EEG artifacts (>25%). The final sample consisted of 39 patients with ADHD and 40 controls. The demographical and clinical characteristics of both groups are displayed in Table 1. The groups did not differ among the demographic variables or IQ scores. As expected, the patients with ADHD reported higher current and childhood ADHD symptoms (ADHD-SR, WURS-K) and elevated depression scores (BDI-II) compared with those of the HCs. Additionally, the patients with ADHD performed significantly worse on the FAIR-2. All participants had normal or corrected-to-normal vision, were fluent in German, and provided written informed consent. The study protocol was approved by the local Medical Ethics Committee according to the Declaration of Helsinki (1964). The participants received financial compensation at an hourly rate of € 10.
Apparatus and stimuli
We selected 120 IAPS images (96 negative, 24 neutral)Footnote 1 for the five experimental conditions (neutral view, negative view, reappraisal, distraction, suppression). The negative and the neutral image sets differed in the IAPS-derived ratings of valence (M = 2.56, SD = 0.46 for negative; M = 5.03, SD = 0.23 for neutral) and arousal (M = 5.87, SD = 0.67 for negative; M = 2.94, SD = 0.32 for neutral). The negative images were divided into four sets of 24 pictures, which were assigned to the four emotional conditions: Negative View, Reappraisal, Distraction, and Suppression [20]. The sets were matched for valence, arousal (all p values >0.9), the presence of human characteristics, and content (e.g., accidents and hurt animals). The assignment of the sets to the conditions was varied between the subjects. The stimuli were presented centrally on a color monitor using Inquisit 3 Software [57]. The viewing distance was held constant at 1.5 m, resulting in a visual angle of 6.5° × 9.1°. The testing was conducted in a sound-attenuated Faraday chamber.
Emotion regulation task
Upon arrival, the participants received a general description of the experiment and completed the informed consent form. Then, EEG/EOG electrodes were attached, and the participants received detailed instructions for the emotion regulation task. The participants were asked to either experience their natural emotional response to the negative and neutral pictures or to decrease their negative emotions using the emotion regulation strategies reappraisal, distraction, or suppression (cf. [15, 18]). In the reappraisal trials, the participants were instructed to mentally change the meaning of the depicted situation by either imagining a positive outcome, finding a less negative interpretation, or viewing the scene from a detached perspective. In the distraction condition, the participants were instructed to generate neutral thoughts unrelated to the picture (e.g., imagining a neutral environment, such as their desk or neighborhood, or imagining a complex geometric shape). In the suppression condition, the participants were instructed to hide any expression of negative feelings. Prior to testing, the participants were trained in the regulation strategies and received feedback from the experimenter. Each participant’s correct understanding of the instructions was then tested in five practice trials.
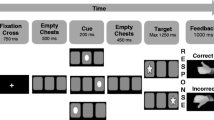
The trial sequence started with a white fixation cross on a black screen (2000 ms), followed by the instruction cue (view, reappraise, distract, or suppress; 2000 ms) and the picture (8000 ms). To facilitate the switching between the trial types, we color-coded the cue screen and the background of the following image according to the condition (cf. [20]). After picture offset, the participants rated their emotions and arousal on the 9-point Self-Assessment Manikin scales [58]. The participants were asked to attend to the pictures during the entire presentation time, without closing their eyes or averting their gaze. The participants were instructed to apply the emotion regulation strategies only after the picture onset and not in advance.
The experiment was divided into 6 blocks of 20 trials (4 negative view trials, 12 negative regulation trials and 4 neutral trials per block) with 15 s breaks between the blocks. We chose a blockwise presentation of the regulation conditions (reappraisal, distraction, and suppression) to prevent subjects from combining the strategies. The sequence of trials within each block was randomized for each participant, and the presentation order of the blocks was counterbalanced. The duration of the entire experiment was ca. 35 min. After the experiment, the participants rated the difficulty and successful implementation of distraction, reappraisal, and suppression, as well as those of the reduction of negative feelings and arousal with the regulation strategies on 5-point Likert scales.
EEG recording and analysis
The continuous electroencephalogram (EEG) was recorded with a SynAmps amplifier and SCAN 4.3 software (Neuroscan, Inc.) at 28 electrode sites (Fp1, Fp2, F7, F3, Fz, F4, F8, FC5, FC1, FC2, FC6, C3, Cz, C4, CP5, CP1, CP2, CP6, T7, T8, P7, P3, Pz, P4, P8, POz, O1, O2) and the mastoids using an electro-cap-system (Waveguard™). The Ag/AgCL electrodes were arranged according to the international extended 10–20 system. During recording, the electrode site Cz served as the online reference, and AFz served as the ground electrode. The electrooculogram (EOG) was monitored with bipolar tin cup electrodes positioned above and below the right eye (vertical EOG) and at the outer canthi of both eyes (horizontal EOG). The skin impedance was maintained below 5 kΩ. The EEG and EOG signals were amplified by a factor of 1000 and recorded at a sampling rate of 1000 Hz. The offline data analyses were performed using BrainVision Analyzer 2.1 software (Brain Products, Munich, Germany). First, the data were down-sampled to 250 Hz by spline interpolation and bandpass filtered from 0.05 to 30 Hz (24 dB/oct). After removal of the large muscle artifacts and extreme offsets following visual inspection, the data were epoched (−500 to 8000 ms) and baseline-corrected using the whole epoch as the baseline [59, 60]. The eye blinks and horizontal eye movements were then corrected using independent component analysis (ICA, cf. [61]). The data were re-referenced to the linked mastoids and epochs containing voltage steps of more than 50 µV between the sample points, and voltage differences of more than 200 µV within a 200 ms interval were automatically rejected. The residual artifacts were removed following visual inspection. The artifact-free data were segmented into epochs that began 200 ms before the onset of the pictures and lasted for 5000 ms. We used the 200 ms before the picture onset for baseline correction. The resulting waveforms were then averaged for each group and condition (neutral view, negative view, reappraisal, distraction, and suppression). The number of artifact-free trials (M = 23.24, SD = 0.89) did not differ significantly between the conditions or the groups (p = 0.171 and p = 0.218, respectively).
In accordance with previous research, the centroparietal LPP was evaluated as the average amplitude from 300 to 5000 ms collapsed over the four centroparietal electrodes Cz, CP1, CP2, and Pz (e.g., [62,63,64]). We also conducted an additional analysis of the centroparietal LPP in order to investigate the temporal dynamics of the emotion regulation strategies. To this end, we evaluated the centroparietal LPP in five consecutive time windows: 300–1000, 1000–2000, 2000–3000, 3000–4000, and 4000–5000 ms [19]. On the basis of previous studies (e.g., [17, 26]), the frontal LPP was evaluated as the mean amplitude at Fz between 800 and 1100 ms. The ERPs were low-pass filtered at 12 Hz (48 dB/oct) for visual presentation.
Statistical analyses
First, we analyzed the SAM ratings of valence and arousal as well as the centroparietal LPP amplitudes using mixed-model repeated measures analyses of variance (rmANOVAs) with the factors group (ADHD, HC) and emotion (negative view, neutral view) to determine the emotion effects. The emotion regulation effects (centroparietal LPP, SAM ratings) and the cognitive effort (frontal LPP) were evaluated with rmANOVAs, including the factors group (ADHD, HC) and regulation strategy (negative view, reappraisal, distraction, suppression). To investigate the temporal dynamics of the regulation effects, we included the factor time window (300–1000 ms, 1–2, 2–3, 3–4, 4–5 s) in the analysis. We used planned contrasts to compare the three regulation strategies to the negative viewing condition. For all other significant main effects and interactions, we performed post hoc tests with Bonferroni-Holm adjusted p values. Three HCs and five ADHD subjects who did not exhibit an electrophysiological response to the negative pictures (i.e., LPPs <0 µV) were excluded from the emotion regulation analyses. One subject with ADHD was excluded from the rating analyses due to a misunderstanding of the SAM scales. To account for medication effects, we conducted additional rmANOVAs including only subjects who were not using medication. The Greenhouse–Geisser correction was used when appropriate. We set the threshold for statistical significance at p < 0.01 for correlational analyses and at p < 0.05 for all other analyses.
Results
Ratings of valence and arousal
Emotion effects
The rmANOVA of valence ratings revealed a large effect of emotion on the valence ratings (F 1, 76 = 310.4, p < 0.001, η 2p = 0.803, Table 2) with lower ratings of the negative than the neutral images. Neither the group effect (F 1, 76 = 3.074, p = 0.084, η 2p = 0.039, d = 0.40) nor the group by emotion interaction reached significance (F 1, 76 = 1.537, p = 0.219, η 2p = 0.020). However, the reanalysis of the emotion effect including only the unmedicated ADHD subjects revealed a significant group effect (F 1, 62 = 5.723, p = 0.020, η 2p = 0.085, d = 0.61), indicating overall more negative ratings in the unmedicated ADHD group versus the control group. The rmANOVA of the arousal ratings revealed a significant effect of emotion (F 1, 76 = 206.1, p < 0.001, η 2p = 0.731). The negative pictures were rated as more arousing than the neutral ones. Neither the effect of group (F 1, 76 = 0.106, ns) nor the group by emotion interaction (F 1, 76 = 2.459, p = 0.121, η 2p = 0.031) was significant.
Regulation effects
We found a significant main effect of regulation strategy on the valence ratings (F 3, 228 = 59.882, p < 0.001, η 2p = 0.441, ε = 0.67). Planned contrasts confirmed more positive valence ratings in all three regulation strategies compared with those of the viewing condition (reappraisal: F 1, 76 = 90.80, p < 0.001, η 2p = 0.544, distraction: F 1, 76 = 63.54, p < 0.001, η 2p = 0.455, suppression: F 1, 76 = 6.43, p = 0.013, η 2p = 0.078). Neither the effect of group (F 1, 76 = 0.847, ns) nor the group by regulation strategy interaction (F 1, 76 = 1.280, p = 0.281, η 2p = 0.017) was significant. Concerning the arousal ratings, we found a significant group by regulation strategy interaction (F 3, 228 = 2.918, p = 0.047, η 2p = 0.037, ε = 0.81). Follow-up ANOVAs for each group revealed significant effects of regulation strategy in both groups (ADHD: F 3, 111 = 4.524, p = 0.012, η 2p = 0.109, ε = 0.72; HCs: F 3, 117 = 14.972, p < 0.001, η 2p = 0.277, ε = 0.79). Post hoc tests showed that the HCs rated the negative pictures as less arousing when using reappraisal and distraction than when using suppression (reappraisal: t 39 = 4.01, p = 0.001; distraction: t 39 = 3.68, p = 0.001). In the ADHD group, the arousal ratings did not vary among the strategies used (smallest p = 0.18).
Late positive potential (LPP)
Emotion effects
The rmANOVA on the LPP amplitudes revealed a significant main effect of emotion (F 1, 77 = 10.651, p = 0.002, η 2p = 0.122; see Table 2), indicating larger LPPs for the negative than for the neutral images. Neither the effect of group (F 1, 77 = 0.932, ns) nor the group by emotion interaction (F 1, 77 = 0.474, ns) was significant. The averaged waveforms per group and condition are presented in Fig. 1. The analysis of the emotion effect including only the unmedicated subjects yielded a trend for a significant main effect of group (F 1, 63 = 3.941, p = 0.051, η 2p = 0.059, d = 0.51), indicating larger LPP amplitudes in the unmedicated ADHD subjects than in the HCs. The group by emotion interaction remained non-significant (F 1, 63 = 0.086, ns).
Regulation effects
We found a significant effect of regulation strategy on the LPP amplitudes (F 3, 207 = 3.237, p = 0.023, η 2p = 0.045, Fig. 2). Compared to the viewing condition, planned contrasts confirmed reduced LPP amplitudes when the subjects used suppression (F 1, 69 = 7.645, p = 0.007, η 2p = 0.100) and, at a trend level, reappraisal (F 1, 69 = 3.957, p = 0.051, η 2p = 0.054), but not distraction (F 1, 69 = 0.244, ns). The group effect was significant at a trend level (F 1, 69 = 3.908, p = 0.052, η 2p = 0.054, d = 0.47), indicating larger LPP amplitudes in the ADHD group than in the HCs. The analysis of regulation effects including only the unmedicated subjects revealed a significant group effect (F 1, 57 = 8.095, p = 0.006, η 2p = 0.124, d = 0.77) with the same pattern of results (Fig. 3). The group by regulation strategy interaction was not significant (F 3, 207 = 0.285, ns).
Mean (and SE) centroparietal LPPs of the healthy controls, the patients with ADHD, and the sub-group of the unmedicated patients with ADHD for the neutral view, negative view, reappraisal, distraction, and suppression conditions averaged over CP1, CP2, Cz, and Pz. The error bars represent the standard errors
Concerning the cognitive effort, the rmANOVA on the frontal LPP amplitudes revealed significant main effects of group (F 1, 77 = 4.099, p = 0.046, η 2p = 0.51, d = 0.46) and regulation strategy (F 3, 231 = 3.095, p = 0.028, η 2p = 0.39) but no significant group by regulation strategy interaction (F 3, 231 = 0.317, ns). The subjects with ADHD exhibited larger frontal LPPs than the HCs (see Fig. 4). Post hoc tests revealed larger frontal LPPs during reappraisal than during suppression (t 78 = 2.879. p = 0.03, d = 0.29). We obtained similar results including only the unmedicated subjects in the analysis. There was a significant group effect (F 1, 63 = 7.083, p = 0.010, η 2p = 0.101, d = 0.69) indicating larger frontal LPPs in the unmedicated ADHD group than those in the control group. The group by regulation strategy interaction was not significant (F 3, 189 = 0.657, ns).
To account for possible timing effects, we conducted a rmANOVA of the LPP amplitudes including the factor time window. In addition to the significant main effect of regulation strategy (F 3, 207 = 3.331, p = 0.021, η 2p = 0.46) and a trend for a group effect (F 1, 69 = 3.817, p = 0.055 η 2p = 0.52), we also found a significant time window by regulation strategy interaction (F 12, 828 = 2.548, p = 0.023, η 2p = 0.36, ε = 0.462). To delineate the time courses of the regulation effects, we conducted pairwise comparisons of the LPPs in the view condition with the LPPs in each of the three regulation conditions for each time window. The results are presented in Table 3. We found that suppression attenuated the LPPs from the very beginning, while reappraisal attenuated the LPPs only in the last two time windows (3000–4000 and 4000–5000 ms). Interestingly, the effect of suppression was no longer significant in the last time window. There were no significant interactions with the factor group (smallest p = 0.715). The main effect of time on the LPP amplitudes was not significant (F 4, 276 = 2.979, p = 0.071, η 2p = 0.41, ε = 0.366).
Questionnaire data
After adjusting for multiple comparisons, we found that the patients with ADHD reported significantly more difficulty devising reappraisals than the HCs (U = 546.5, p = 0.036). All other comparisons (i.e., difficulty implementing distraction and suppression, reduction of negative feelings and arousal with the regulation strategies) were not significant (smallest p = 0.096). Concerning the habitual emotion regulation (ERQ), the patients with ADHD reported more frequent use of suppression (t 76 = 2.96, p = 0.008, d = 0.67; Table 1) and less frequent use of reappraisal than the HCs (t 76 = 2.60, p = 0.011, d = 0.59). In addition, the patients with ADHD reported significantly lower appraisals of their own emotion regulation skills (SEK-27) than those of the HCs (t 76 = 7.59, p < 0.001, d = 1.72). In the ADHD group, habitual reappraisal was associated with smaller centroparietal LPP amplitudes in the suppression condition (r = −0.44 p = 0.005). In the unmedicated subjects, habitual reappraisal was associated with smaller frontal LPPs during the viewing of the negative images (r = −0.52, p = 0.009) and smaller centroparietal LPPs during suppression (r = −0.54 p = 0.006).
Clinical correlates
In the ADHD group, the symptom severity (ADHD-SR full scale) was associated with larger frontal LPPs during the passive viewing of the negative pictures (r = 0.53, p = 0.001) and larger centroparietal LPPs during suppression (r = 0.46, p = 0.004). After excluding the medicated subjects, we found even stronger associations between ADHD symptom severity and the frontal LPPs during passive viewing (r = 0.63, p = 0.001) and a significant correlation between symptom severity and the centroparietal LPP amplitudes during reappraisal (r = 0.58, p = 0.003), which was not evident in the whole ADHD group. The correlations between the electrophysiological measures and the depression symptoms (BDI-II) were not significant (smallest p = 0.014).
Discussion
The study is the first to provide electrophysiological evidence of ED in adult patients with ADHD using a paradigm that directly targets emotion regulation. The results confirm our hypothesis of abnormally elevated emotional reactivity to negative stimuli in adult patients with ADHD, as indicated by the larger centroparietal LPP amplitudes and the more negative valence ratings of the aversive and neutral IAPS images. We found no significant deficit in the centroparietal LPP modulation or the self-reported valence and arousal ratings after the use of the emotion regulation strategies in the patients with ADHD. However, the processing of aversive stimuli as well as emotion regulation was associated with greater cognitive effort in patients with ADHD than in healthy controls, which was reflected by the larger frontal LPP amplitudes. Correspondingly, the patients with ADHD exhibited lower self-appraisals of emotion regulation skills, more difficulty generating reappraisals, and less habitual use of reappraisal but more use of suppression. Both the centroparietal and frontal LPPs were associated with ADHD symptom severity.
To date, the neural and physiological correlates of increased emotional reactivity have been mostly obtained from pediatric patients with ADHD [28,29,30,31]. Until now, the enhanced and sustained processing of emotional stimuli have not been replicated on an electrophysiological level in adults [35, 36]. Therefore, our findings provide valuable support of the persistence of emotional hyper-reactivity into adulthood and corroborate the behavioral findings of heightened emotional interference in adults with ADHD (e.g., [65, 66]). Enhanced emotional reactivity was particularly strong in the unmedicated ADHD subjects. This finding is consistent with the results from the randomized controlled trials showing positive stimulant effects on ED symptoms in adults with ADHD [67,68,69]. On a neural level, Posner and colleagues have also demonstrated attenuation of the atypical mPFC [28] and the amygdala [29] activations when the subjects were tested with methylphenidate. Importantly, in our sample, the beneficial stimulant effects appear to persist, despite discontinuation of the medication 24 h prior to testing.
The finding of the enhanced frontal LPPs (i.e., cognitive effort) during the processing of the aversive stimuli and emotion regulation is consistent with the concepts that link ED in patients with ADHD to executive dysfunctions [36, 70]. Frontal hyperactivation also provides a possible explanation for the intact self-reported emotion regulation and the unimpaired centroparietal LPP modulation in our ADHD subjects, which could be attributed to compensational mechanisms [71, 72]. Taken together, our findings support both the theoretical concepts of ED in patients with ADHD discussed above, that is, the abnormally strong emotional responses [38] and top-down emotion regulation difficulties [35, 36].
Within the framework suggested by Sheppes et al. [13], our results suggest difficulties in several stages of emotion regulation in adult ADHD. For example, the abnormally elevated emotional reactivity may lead to the selection of maladaptive regulation strategies in patients with ADHD. As shown by Shafir et al. [26] in healthy adults, high emotional intensity leads to the preference of regulatory tactics that prevent emotional processing (such as suppression) over tactics that require in-depth processing (such as reappraisal). This result is consistent with our finding of more frequent habitual suppression than reappraisal in the adults with ADHD. However, the choice of suppression over reappraisal could also stem from difficulties during the implementation phase [13]. The patients with ADHD may overrepresent regulation tactics, which offer immediate effects (such as suppression), over strategies that offer long-term benefits (such as reappraisal) due to difficulties in focusing on the long-term beneficial courses of action. Correspondingly, the suppression effects in the present study were limited to the early time windows of the LPP. The frontal hyper-activation during emotion regulation also indicates difficulties during the implementation stage. Finally, although the patients with ADHD exhibited increased cognitive effort over the relatively short presentation time in our experiment, the significantly lower self-appraisals of emotion regulation skills may lead to dysregulation during the monitoring stage in longer lasting, emotionally challenging everyday life situations. Patients with ADHD might stop regulation efforts prematurely due to low emotion regulation self-efficacy [73]. This hypothesis should be explored in future research using paradigms with higher ecological validity.
The potential limitations of the present findings must be considered. First, although the patients discontinued medication, the stimulant effects appear to have persisted. Previous findings of maintained medication effects in patients with ADHD after treatment discontinuation have been discussed in terms of lasting neurobiological changes [74] and prolonged alleviation of ADHD symptoms promoting the development of adaptive coping skills [75]. While we recruited a considerable number of medication-naïve subjects and were able to confirm the hypothesis of heightened emotional reactivity, the reduced sample size in the subanalyses could have led to insufficient power to discover aberrant LPP modulation in the unmedicated patients. Since heterogeneity is a major concern in ADHD research, future studies should consider exploring ED in larger medication-naïve samples and different subtypes of ADHD (e.g., [76]). Second, as typically observed in ADHD samples, there was a moderate increase in the depression symptoms in our ADHD group. However, comorbid depression is an unlikely explanation for our results, since the correlations between the electrophysiological measures and the BDI-II scores were not significant. Further, depression has been repeatedly found to be associated with blunted not increased LPP amplitudes [77,78,79]. We assume that the heightened BDI-II scores mostly stem from the overlap of the BDI-II items with the ADHD symptomatology [80] and symptom distress. Finally, we were not able to replicate the LPP attenuation during distraction. Inspection of the post-experiment questionnaires revealed that a considerable number of subjects from both groups used positive rather than neutral distraction, which might have led to enhanced LPPs [81].
The present study might also have some clinical implications which need further validation. In the light of emotional hyper-reactivity and difficulty with the implementation of the emotion regulation strategies, the regular overall time courses of emotion regulation and the intact centroparietal LPP modulation encourage the implementation of emotion regulation modules in the treatment of patients suffering from ADHD. Training patients to reinterpret aversive experiences might be particularly promising, given that the literature has linked reappraisal to psychological well-being (e.g., [82]) and the association of ADHD symptom severity with the enhanced LPPs during reappraisal. In addition to clinical training, neurofeedback, such as the fMRI-inspired electroencephalography method developed by Keynan et al. [83], might be another avenue for the treatment of emotional hyper-reactivity in patients with ADHD.
Notes
Codes of the IAPS images: negative [set 1]: 1050, 2276, 2717, 2456, 3500, 5961, 6200, 6250, 6312, 6560, 9163, 6510, 3230, 9570, 9300, 9321, 9400, 9423, 9424, 9560, 9610, 9901, 9911, 9922 [set 2]: 1052, 2141, 2710, 2799, 3530, 5971, 6210, 6243, 6315, 6540, 6825, 6830, 9007, 9183, 9301, 9322, 9419, 9425, 9427, 9561, 9620, 9903, 9905, 9941 [set 3]: 1033, 2301, 2753, 2900, 6571, 5972, 6230, 6231, 6360, 6550, 6831, 6370, 3220, 9185, 9302, 9325, 6520, 9428, 9429, 9571, 9611, 9900, 9910, 9925 [set 4]: 1040, 2455, 2750, 9332, 9414, 5973, 6260, 6263, 6530, 6350, 6838, 6213, 9043, 9187, 9320, 9326, 9421, 9426, 2683, 9140, 9600, 9902, 9904, 9623; neutral: 2038, 2102, 2190, 2377, 2393, 2397, 2411, 2440, 2493, 2516, 2745.1, 2880, 5510, 5740, 7002, 7003, 7009, 7026, 7052, 7059, 7090, 7493, 7950, 9210.
References
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. Author, Washington, DC
Biederman J, Petty CR, Clarke A et al (2011) Predictors of persistent ADHD: an 11-year follow-up study. J Psychiatr Res 45:150–155. doi:10.1016/j.jpsychires.2010.06.009
Sibley MH, Swanson JM, Arnold LE et al (2016) Defining ADHD symptom persistence in adulthood: optimizing sensitivity and specificity. J Child Psychol Psychiatry n/a-n/a. doi:10.1111/jcpp.12620
Simon V, Czobor P, Bálint S et al (2009) Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry J Ment Sci 194:204–211. doi:10.1192/bjp.bp.107.048827
Retz W, Stieglitz R-D, Corbisiero S et al (2012) Emotional dysregulation in adult ADHD: what is the empirical evidence? Expert Rev Neurother 12:1241–1251. doi:10.1586/ern.12.109
Shaw P, Stringaris A, Nigg J, Leibenluft E (2014) Emotion dysregulation in attention deficit hyperactivity disorder. Am J Psychiatry 171:276–293. doi:10.1176/appi.ajp.2013.13070966
Barkley RA, Murphy KR (2010) Impairment in occupational functioning and adult ADHD: the predictive utility of executive function (EF) ratings versus EF tests. Arch Clin Neuropsychol 25:157–173. doi:10.1093/arclin/acq014
Surman CBH, Biederman J, Spencer T et al (2013) Understanding deficient emotional self-regulation in adults with attention deficit hyperactivity disorder: a controlled study. Atten Deficit Hyperact Disord 5:273–281. doi:10.1007/s12402-012-0100-8
Herrmann MJ, Biehl SC, Jacob C, Deckert J (2010) Neurobiological and psychophysiological correlates of emotional dysregulation in ADHD patients. Atten Deficit Hyperact Disord 2:233–239. doi:10.1007/s12402-010-0047-6
Fernandez KC, Jazaieri H, Gross JJ (2016) Emotion regulation: a transdiagnostic perspective on a new RDoC domain. Cogn Ther Res 40:426–440. doi:10.1007/s10608-016-9772-2
Insel T, Cuthbert B, Garvey M et al (2010) Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 167:748–751. doi:10.1176/appi.ajp.2010.09091379
Gross JJ (1998) The emerging field of emotion regulation: an integrative review. Rev Gen Psychol 2:271–299
Sheppes G, Suri G, Gross JJ (2015) Emotion regulation and psychopathology. Annu Rev Clin Psychol 11:379–405. doi:10.1146/annurev-clinpsy-032814-112739
Hajcak G, MacNamara A, Olvet DM (2010) Event-related potentials, emotion, and emotion regulation: an integrative review. Dev Neuropsychol 35:129–155. doi:10.1080/87565640903526504
Hajcak G, Nieuwenhuis S (2006) Reappraisal modulates the electrocortical response to unpleasant pictures. Cogn Affect Behav Neurosci 6:291–297
Moran TP, Jendrusina AA, Moser JS (2013) The psychometric properties of the late positive potential during emotion processing and regulation. Brain Res 1516:66–75. doi:10.1016/j.brainres.2013.04.018
Moser JS, Hartwig R, Moran TP et al (2014) Neural markers of positive reappraisal and their associations with trait reappraisal and worry. J Abnorm Psychol 123:91–105. doi:10.1037/a0035817
Paul S, Simon D, Kniesche R et al (2013) Timing effects of antecedent- and response-focused emotion regulation strategies. Biol Psychol 94:136–142. doi:10.1016/j.biopsycho.2013.05.019
Schönfelder S, Kanske P, Heissler J, Wessa M (2014) Time course of emotion-related responding during distraction and reappraisal. Soc Cogn Affect Neurosci 9:1310–1319. doi:10.1093/scan/nst116
Thiruchselvam R, Blechert J, Sheppes G et al (2011) The temporal dynamics of emotion regulation: an EEG study of distraction and reappraisal. Biol Psychol 87:84–92. doi:10.1016/j.biopsycho.2011.02.009
Horan WP, Hajcak G, Wynn JK, Green MF (2013) Impaired emotion regulation in schizophrenia: evidence from event-related potentials. Psychol Med 43:2377–2391. doi:10.1017/S0033291713000019
Strauss GP, Kappenman ES, Culbreth AJ et al (2013) Emotion regulation abnormalities in schizophrenia: cognitive change strategies fail to decrease the neural response to unpleasant stimuli. Schizophr Bull 39:872–883. doi:10.1093/schbul/sbs186
Strauss GP, Kappenman ES, Culbreth AJ et al (2015) Emotion regulation abnormalities in schizophrenia: directed attention strategies fail to decrease the neurophysiological response to unpleasant stimuli. J Abnorm Psychol 124:288–301. doi:10.1037/abn0000017
Paul S, Simon D, Endrass T, Kathmann N (2015) Altered emotion regulation in obsessive-compulsive disorder as evidenced by the late positive potential. Psychol Med. doi:10.1017/S0033291715001610
Zhang B-W, Xu J, Chang Y et al (2016) Impaired cognitive reappraisal in panic disorder revealed by the late positive potential. NeuroReport 27:99–103. doi:10.1097/WNR.0000000000000504
Shafir R, Schwartz N, Blechert J, Sheppes G (2015) Emotional intensity influences pre-implementation and implementation of distraction and reappraisal. Soc Cogn Affect Neurosci 10:1329–1337. doi:10.1093/scan/nsv022
Van Cauwenberge V, El Kaddouri R, Hoppenbrouwers K, Wiersema JR (2017) To make a molehill out of a mountain: an ERP-study on cognitive reappraisal of negative pictures in children with and without ADHD. Clin Neurophysiol 128:529–537. doi:10.1016/j.clinph.2017.01.008
Posner J, Maia TV, Fair D et al (2011) The attenuation of dysfunctional emotional processing with stimulant medication: an fMRI study of adolescents with ADHD. Psychiatry Res 193:151–160. doi:10.1016/j.pscychresns.2011.02.005
Posner J, Nagel BJ, Maia TV et al (2011) Abnormal amygdalar activation and connectivity in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 50(828–837):e3. doi:10.1016/j.jaac.2011.05.010
Brotman MA, Rich BA, Guyer AE et al (2010) Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am J Psychiatry 167:61–69. doi:10.1176/appi.ajp.2009.09010043
López-Martín S, Albert J, Fernández-Jaén A, Carretié L (2013) Emotional distraction in boys with ADHD: neural and behavioral correlates. Brain Cogn 83:10–20. doi:10.1016/j.bandc.2013.06.004
Raz S, Dan O (2015) Altered event-related potentials in adults with ADHD during emotional faces processing. Clin Neurophysiol 126:514–523. doi:10.1016/j.clinph.2014.06.023
Köchel A, Leutgeb V, Schienle A (2012) Affective inhibitory control in adults with attention deficit hyperactivity disorder: abnormalities in electrocortical late positivity. Neurosci Lett 530:47–52. doi:10.1016/j.neulet.2012.09.053
Herrmann MJ, Schreppel T, Biehl SC et al (2009) Emotional deficits in adult ADHD patients: an ERP study. Soc Cogn Affect Neurosci 4:340–345. doi:10.1093/scan/nsp033
Barkley RA (2010) Deficient emotional self-regulation is a core component of ADHD. J ADHD Relat Disord 1:5–37
Petrovic P, Castellanos FX (2016) Top-down dysregulation—from ADHD to emotional instability. Front Behav Neurosci. doi:10.3389/fnbeh.2016.00070
Posner J, Kass E, Hulvershorn L (2014) Using stimulants to treat ADHD-related emotional lability. Curr Psychiatry Rep 16:478. doi:10.1007/s11920-014-0478-4
Sonuga-Barke EJS (2002) Psychological heterogeneity in AD/HD—a dual pathway model of behaviour and cognition. Behav Brain Res 130:29–36
Lang PJ, Bradley MM, Cuthbert BN (2008) International affective picture system (IAPS): affective ratings of pictures and instruction manual. Technical Report A-8. University of Florida, Gainesville
Gross JJ (1998) Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. J Pers Soc Psychol 74:224–237. doi:10.1037/0022-3514.74.1.224
Ochsner KN, Silvers JA, Buhle JT (2012) Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci 1251:E1–E24. doi:10.1111/j.1749-6632.2012.06751.x
Murata A, Moser JS, Kitayama S (2013) Culture shapes electrocortical responses during emotion suppression. Soc Cogn Affect Neurosci 8:595–601. doi:10.1093/scan/nss036
Gan S, Yang J, Chen X, Yang Y (2015) The electrocortical modulation effects of different emotion regulation strategies. Cogn Neurodyn 9:399–410. doi:10.1007/s11571-015-9339-z
Barkley RA (1997) Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 121:65–94. doi:10.1037/0033-2909.121.1.65
Willcutt EG, Doyle AE, Nigg JT et al (2005) Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry 57:1336–1346. doi:10.1016/j.biopsych.2005.02.006
Kessler RC, Adler L, Ames M et al (2005) The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med 35:245–256
Kooij JJS (2012) Adult ADHD. Diagnostic assessment and treatment, 3rd edn. Springer, London
Rösler M, Retz-Junginger P, Retz W, Stieglitz RD (2008) HASE—Homburger ADHS-Skalen für Erwachsene. Hogrefe, Göttingen
Retz-Junginger P, Retz W, Blocher D et al (2002) Wender Utah Rating Scale (WURS-k) Die deutsche Kurzform zur retrospektiven Erfassung des hyperkinetischen Syndroms bei Erwachsenen. Nervenarzt 73:830–838. doi:10.1007/s00115-001-1215-x
Wittchen HU, Zaudig M, Fydrich T (1997) Strukturiertes Klinisches Interview für DSM-IV. Hogrefe, Göttingen
Beck AT, Steer RA, Brown GK (1996) BDI-II, Beck depression inventory: manual. Psychological Corp.; Harcourt Brace, San Antonio, Boston
Wechsler D (2008) Wechsler adult intelligence scale fourth edition (WAIS-IV) Deutsche Version. Hg.; von F. Petermann. Pearson Assessment, Frankfurt/Main
Moosbrugger H, Oehlschlägel J (2011) Frankfurter Aufmerksamkeits-Inventar 2 (FAIR-2). Huber, Bern
Abler B, Kessler H (2009) Emotion Regulation Questionnaire—Eine deutschsprachige Fassung des ERQ von Gross und John. Diagnostica 55:144–152
Berking M, Znoj H (2008) Entwicklung und Validierung eines Fragebogens zur standardisierten Selbsteinschätzung emotionaler Kompetenzen (SEK-27). Z Für Psychiatr Psychol Psychother 56:141–153
Bohus M, Kleindienst N, Limberger MF et al (2009) The short version of the borderline symptom list (BSL-23): development and initial data on psychometric properties. Psychopathology 42:32–39
Millisecond Software (2010) Inquisit. Millisecond Software, Seattle
Lang PJ (1980) Behavioral treatment and bio-behavioral assessment: computer applications. In: Sidowski JB, Johnson JH, Williams TA (eds) Technol. Ment. Health Care Deliv. Syst. Ablex, Norwood, NJ, pp 119–137
Groppe DM, Makeig S, Kutas M (2009) Identifying reliable independent components via split-half comparisons. NeuroImage 45:1199–1211. doi:10.1016/j.neuroimage.2008.12.038
Zakeri Z, Assecondi S, Bagshaw AP, Arvanitis TN (2014) Influence of signal preprocessing on ICA-based EEG decomposition. In: XIII Mediterr. Conf. Med. Biol. Eng. Comput. 2013. Springer, Cham, pp 734–737
Jung T-P, Makeig S, Humphries C et al (2000) Removing electroencephalographic artifacts by blind source separation. Psychophysiology 37:163–178. doi:10.1017/S0048577200980259
Hajcak G, Dunning JP, Foti D (2009) Motivated and controlled attention to emotion: time-course of the late positive potential. Clin Neurophysiol 120:505–510. doi:10.1016/j.clinph.2008.11.028
MacNamara A, Hajcak G (2010) Distinct electrocortical and behavioral evidence for increased attention to threat in generalized anxiety disorder. Depress Anxiety 27:234–243. doi:10.1002/da.20679
Parvaz MA, Moeller SJ, Goldstein RZ, Proudfit GH (2015) Electrocortical evidence of increased post-reappraisal neural reactivity and its link to depressive symptoms. Soc Cogn Affect Neurosci 10:78–84. doi:10.1093/scan/nsu027
Marx I, Krause J, Berger C, Häßler F (2014) Dissociable patterns in the control of emotional interference in adults with attention-deficit/hyperactivity disorder (ADHD) and in adults with alcohol dependence. PLoS One 9:e107750. doi:10.1371/journal.pone.0107750
Marx I, Domes G, Havenstein C et al (2011) Enhanced emotional interference on working memory performance in adults with ADHD. World J Biol Psychiatry Off J World Fed Soc Biol Psychiatry 12(Suppl 1):70–75. doi:10.3109/15622975.2011.599213
Marchant BK, Reimherr FW, Halls C et al (2010) OROS methylphenidate in the treatment of adults with ADHD: a 6-month, open-label, follow-up study. Ann Clin Psychiatry Off J Am Acad Clin Psychiatr 22:196–204
Marchant BK, Reimherr FW, Robison RJ et al (2011) Methylphenidate transdermal system in ADHD adhd and impact on emotional and oppositional symptoms. J Atten Disord 15:295–304. doi:10.1177/1087054710365986
Rösler M, Retz W, Fischer R et al (2010) Twenty-four-week treatment with extended release methylphenidate improves emotional symptoms in adult ADHD. World J Biol Psychiatry 11:709–718. doi:10.3109/15622971003624197
Van Cauwenberge V, Sonuga-Barke EJS, Hoppenbrouwers K et al (2015) “Turning down the heat”: is poor performance of children with ADHD on tasks tapping “hot” emotional regulation caused by deficits in “cool” executive functions? Res Dev Disabil 47:199–207. doi:10.1016/j.ridd.2015.09.012
Francx W, Oldehinkel M, Oosterlaan J et al (2015) The executive control network and symptomatic improvement in attention-deficit/hyperactivity disorder. Cortex J Devoted Study Nerv Syst Behav 73:62–72. doi:10.1016/j.cortex.2015.08.012
Halperin JM, Schulz KP (2006) Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychol Bull 132:560–581. doi:10.1037/0033-2909.132.4.560
Bigman YE, Mauss IB, Gross JJ, Tamir M (2016) Yes I can: expected success promotes actual success in emotion regulation. Cogn Emot 30:1380–1387. doi:10.1080/02699931.2015.1067188
Buitelaar J, Asherson P, Soutullo C et al (2015) Differences in maintenance of response upon discontinuation across medication treatments in attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol 25:1611–1621. doi:10.1016/j.euroneuro.2015.06.003
Biederman J, Mick ES, Surman C et al (2010) A randomized, 3-phase, 34-week, double-blind, long-term efficacy study of osmotic-release oral system-methylphenidate in adults with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 30:549–553. doi:10.1097/JCP.0b013e3181ee84a7
Surman CBH, Biederman J, Spencer T et al (2011) Deficient emotional self-regulation and adult attention deficit hyperactivity disorder: a family risk analysis. Am J Psychiatry 168:617–623. doi:10.1176/appi.ajp.2010.10081172
Foti D, Olvet DM, Klein DN, Hajcak G (2010) Reduced electrocortical response to threatening faces in major depressive disorder. Depress Anxiety 27:813–820. doi:10.1002/da.20712
MacNamara A, Kotov R, Hajcak G (2015) Diagnostic and symptom-based predictors of emotional processing in generalized anxiety disorder and major depressive disorder: an event-related potential study. Cogn Ther Res 40:275–289. doi:10.1007/s10608-015-9717-1
Weinberg A, Perlman G, Kotov R, Hajcak G (2016) Depression and reduced neural response to emotional images: distinction from anxiety, and importance of symptom dimensions and age of onset. J Abnorm Psychol 125:26–39. doi:10.1037/abn0000118
Steer RA, Ranieri WF, Kumar G, Beck AT (2003) Beck depression inventory-II items associated with self-reported symptoms of ADHD in adult psychiatric outpatients. J Pers Assess 80:58–63. doi:10.1207/S15327752JPA8001_14
Schupp HT (2000) Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology 37:257–261
Gross JJ, John OP (2003) Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol 85:348–362. doi:10.1037/0022-3514.85.2.348
Keynan JN, Meir-Hasson Y, Gilam G et al (2016) Limbic activity modulation guided by functional magnetic resonance imaging-inspired electroencephalography improves implicit emotion regulation. Biol Psychiatry 80:490–496. doi:10.1016/j.biopsych.2015.12.024
Acknowledgements
The authors would like to thank Claudia Schmidt and David Jendryczko for their support during data acquisition.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (DFG, PE 1882/2-1).
Rights and permissions
About this article
Cite this article
Shushakova, A., Ohrmann, P. & Pedersen, A. Exploring deficient emotion regulation in adult ADHD: electrophysiological evidence. Eur Arch Psychiatry Clin Neurosci 268, 359–371 (2018). https://doi.org/10.1007/s00406-017-0826-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-017-0826-6