Abstract
Dopamine D2 receptors (DRD2) have been strongly implicated in reward processing of natural stimuli and drugs. Using the approach-avoidance task (AAT), we recently demonstrated that smokers show an increased approach-bias toward smoking-related cues but not toward naturally rewarding stimuli. Here, we examined the contribution of the DRD2 Taq1B polymorphism to smokers’ and non-smokers’ responsivity toward smoking versus naturally rewarding stimuli in the AAT. Smokers carrying the minor B1 allele of the DRD2 Taq1B polymorphism showed reduced approach behavior for food-related pictures compared to non-smokers with the same allele. In the group of smokers, a higher approach-bias toward smoking-related compared to food-related pictures was found in carriers of the B1 allele. This pattern was not evident in smokers homozygous for the B2 allele. In addition, smokers with the B1 allele reported fewer attempts to quit smoking relative to smokers homozygous for the B2 allele. This is the first study demonstrating that behavioral shifts in response to smoking relative to natural rewards in smokers are mediated by the DRD2 Taq1B polymorphism. Our results indicate a reduced natural-reward brain reactivity in smokers with a genetically determined decrease in dopaminergic activity (i.e., reduction of DRD2 availability). It remains to be determined whether this pattern might be related to a different outcome after psychological cessation interventions, i.e., AAT modification paradigms, in smokers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to dual-process models, addictive behaviors occur as a consequence of an imbalance between a slowly operating reflective instance and a fast, approach-oriented, or impulsive instance [1, 2]. The latter includes automatic approach-biases toward drug-related cues which represent important triggers for both the initiation of drug intake and the “urge” to continue chronic drug use. In recent years, new paradigms have been developed for both the assessment and modification of such drug-cue induced automatic approach tendencies in the context of different addictions. The approach-avoidance task (AAT) [3] has been used to measure existing approach-biases in heroin [4], cannabis [5], alcohol [6] and nicotine addiction [7]. Likewise, several training versions of the AAT exist, which have successfully been employed to reduce approach-biases toward addictive stimuli and to increase efficacy of conventional cessation interventions [8, 9] (for a review see: [2]).
We have recently examined approach-biases for smoking-related and naturally rewarding cues in smokers by means of the AAT [10]. We demonstrated that smoking is associated with a stronger approach-bias for smoking-related pictures relative to naturally rewarding cues, in particular pictures of highly palatable food [10]. Although imaging studies already suggested a decrease in natural-reward responsivity in the course of various addictions, our findings provide the first behavioral evidence for a shift in responsivity to drug cues at the expense of naturally rewarding stimuli in smokers [11, 12]. Research on the functional significance and the underlying neuronal mechanisms mediating this shift in reward reactivity in addiction is still limited. However, it has been proposed that adaptations in meso-corticolimbic dopamine signaling are likely to contribute to a decrease in motivational and behavioral responses to drugs and natural rewards in the course of an addiction [13–15]. For instance, a diminished activation of meso-striatal and meso-corticolimbic brain regions in response to natural reinforcers in detoxified cocaine addicts has been demonstrated [16]. Likewise, monetary rewards which activate typical dopaminergic regions including the striatum and the prefrontal cortex in non-smokers are ineffective in activating the same reward circuits in smokers [17].
Since chronic drug use is accompanied with a progressive downregulation of dopamine D2 receptors (DRD2) in the meso-striatal brain regions [18, 19] and since DRD2 have been strongly implicated in the processing of naturally rewarding stimuli and drugs [20], a decreased DRD2 density in addicts might account for the diminished responsivity toward natural rewards as a consequence of chronic substance use [18]. In this instance, it is well documented that polymorphisms of the DRD2 gene might represent susceptibility factors for various addictive phenotypes [20, 21]. The B1 allele of the DRD2 Taq1B polymorphism in either heterozygosity or homozygosity is associated with less DRD2 density [22]. Subjects carrying the B1 allele exhibit an increased vulnerability to smoking [23, 24] and other addictive behaviors [25, 26] (for a review, see: [27]) probably due to alterations in reward sensitivity [19]. With respect to processes related to smoking cessation in particular, a prominent role of the DRD2 Taq1B polymorphism has been confirmed [20, 21]. Compared to smokers homozygous for the B2 allele, smokers with the minor B1 allele show fewer attempts to quit and stronger withdrawal symptoms after quitting smoking [28, 29], and are younger at the onset of smoking [23, 24, 30] which is inversely correlated to tobacco dependence [31] and to more difficulties to quit later in life [32].
Given the important role of dopaminergic neurotransmission in reward processing of natural stimuli [16] and drugs [18] and the genetic modulation of DRD2 functionality in tobacco dependence [20, 21], we sought to determine whether the Taq1B polymorphism of the DRD2 gene affects differences in smokers’ and non-smokers’ approach-avoidance biases toward smoking versus natural-reward stimuli in the AAT. To this end, we reanalyzed behavioral and self-report data from our previous study examining approach-avoidance tendencies in smokers and non-smokers [10]. We expected that depending on the smoking status, carriers of the B1 allele and homozygous carriers of the B2 allele would show differences in responsivity toward smoking-related and natural-reward stimuli in the AAT. Based on the previous findings on the association between the DRD2 Taq1B polymorphism and smoking behavior, a diminished approach-bias for natural rewarding cues in smokers carrying the B1 allele might be expected. Likewise, our previous finding on a stronger approach-bias for smoking-related pictures relative to naturally rewarding cues in smokers [10] should be mediated by the DRD2 Taq1B polymorphism and be more pronounced in smokers carrying the B1 allele.
Materials and methods
Self-report and behavioral measures obtained from participants in the Machulska et al. [10] study were reanalyzed to examine the effect of the Taq1B polymorphism of the DRD2 gene on these measures. All subjects were genotyped at the beginning of the study. The final sample comprised 90 smokers [mean age 26.6; 44% female; mean Fagerström Test for Nicotine Dependence Score (FTND) 3.4], and 49 non-smokers (mean age 23.3; 59% female). Each participant provided written informed consent for the experimental procedure and the study was approved by the local Ethics Committee of the Ruhr-Universität Bochum.
Self-report measures
Each participant completed an extensive set of questionnaires concerning her/his: (1) Current smoking status, (2) subjective cigarette craving [ranging from 0 (“not at all”) to 5 (“very high”)], (3) degree of nicotine dependence (FTND with a score of 0 indicating no or very weak dependence and a score of 10 indicating very high nicotine dependence [33]; German version: [34]), (4) attitude toward smoking (items ranging from −3 and +3; [35]), and (5) smoking abstinence motivation (Stages of Change Scale [36]; German version: [37]). For full description of all questionnaires, see Machulska and colleagues [10].
Automatic approach and avoidance tendencies
Automatic approach and avoidance tendencies were assessed with an adapted version of the Nicotine-Approach-Avoidance Task (N-AAT). For a detailed task description, see: [10]. Briefly, during the AAT, discrete pictures from four different categories were displayed on a computer screen: (a) smoking-related pictures, (b) shape- and color-matched pictures of tooth-cleaning, (c) pictures of highly palatable food (e.g., pizza, ice cream, etc.), and (d) shape- and color-matched neutral pictures (i.e., empty dishes). Each picture was either rotated 3° to the left or 3° to the right. Participants were instructed to pull pictures rotated to the left and to push pictures rotated to the right, as quickly and accurately as possible using a joystick which was connected to the computer. Upon a pull movement, picture size increased, whereas upon a push movement, picture size decreased, creating a zooming effect [3]. Each picture from the four picture categories was presented for a total of six times (three times in pull-closer format and three times in push-away format), resulting in 192 trials.
Genotyping
All participants were informed to refrain from eating food and drinking beverages apart from water approximately 60 min prior to the study. DNA samples were collected using Oragene saliva kits (DNA Genotek, Ottawa, Canada). DNA extraction and genotyping was performed using established procedures according to the manufacturer’s protocol. The DRD2 Taq1B polymorphism was genotyped by LGC Genomics (Hoddesdon, UK) using KASP technology with validated arrays. Five participants (all smokers) could not be genotyped, giving a total sample of 134 participants and a genotyping success rate of 96.4%.
Data preparation and statistical analysis
The Hardy–Weinberg exact test was used (https://www.cog-genomics.org/software/stats) to analyze whether genotype distribution was in Hardy–Weinberg equilibrium. Chi-square tests were used for the statistical analysis of allele frequencies and the distribution of genotypes in smokers and non-smokers.
Genotype was defined using a dominant model: Homozygotes for the minor B1 allele (B1/B1) were grouped together with heterozygotes (B1/B2) and compared to homozygotes for the major B2 allele (B2/B2).
Individual AAT-bias scores were calculated for each participant. First, error trials were removed and AAT-bias scores were calculated by subtracting median reaction times (RTs) for pulling a picture from median RTs for pushing a picture for each of the four picture categories, separately (median RTpush—median RTpull; see: [10]).
To examine whether the genotype contributed to differences in smokers’ and non-smokers’ AAT-bias scores, a 2 (genotype: B1 allele carriers versus B2 homozygotes) × 2 (smoking status: smoker versus non-smoker) × 4 (picture category: nicotine-related versus tooth-cleaning versus food-related versus neutral pictures) mixed design ANOVA was conducted. Significant main effects and/or first-order (two-way) interactions were investigated with simple effect analyses. To investigate the second-order (three-way) interaction, two separate 2 × 4 ANOVAS were conducted with genotype removed and smoking status (smoker versus non-smoker) as the main between-subjects factor. To account for multiple testing, a more conservative level of significance was applied, using the Bonferroni correction for multiple (n) testing (p corrected = p uncorrected × n). Separate univariate ANOVAS were used to determine genetic influences on smokers’ smoking history and behavior, i.e., subjective craving, degree of nicotine dependence, motivation to quit smoking, and attempts to quit smoking during the last 12 months. Again, Bonferroni correction was used to ensure that the cumulative Type I error was below α = 0.05. Analyses were performed using IBM SPSS Statistics for Windows 23.
Results
Genotyping
Genotyping resulted in two subjects (both smokers) homozygous for the B1 allele, 39 subjects with the heterozygous B1B2 genotype (26 smokers and 13 non-smokers), and 93 subjects homozygous for the major B2 allele (57 smokers and 36 non-smokers). Allele frequencies were 0.15 for the B1 allele (for smokers 0.18, for non-smokers 0.13) and 0.84 for the B2 allele (for smokers 0.82, for non-smokers 0.87), respectively. No significant differences in allele frequencies were found between smokers and non-smokers (p’s > 0.33). No significant deviations from Hardy–Weinberg equilibrium were detected (p = 0.52). Sample characteristics according to smoking status and genotype are summarized in Table 1.
Automatic approach and avoidance tendencies
Mean AAT reaction times per genotype and smoking status for pulling versus pushing a picture are summarized in Table 2. To test the effect of the DRD2 Taq1B polymorphism on automatic approach-avoidance tendencies assessed with the AAT, a 2 × 2 × 4 mixed design ANOVA with smoking status (smoker versus non-smoker) and genotype (B1 allele carriers versus B2 homozygotes) as between-subjects factors and picture category (nicotine-related versus tooth-cleaning versus food-related versus neutral pictures) as the within-subjects factor was conducted. As published previously [10], there was a significant main effect of picture category, F(3, 128) = 10.54, p < 0.001, η 2 = 0.2., and a significant picture category x smoking status interaction, F(3, 128) = 5.29, p = 0.002, η 2 = 0.11. Furthermore, a significant picture category × genotype interaction was evident, F(3, 128) = 5, p = 0.003, η 2 = 0.11. Irrespective of smoking status, simple effect analyses indicated that B1 allele carriers showed a larger avoidance bias toward tooth-cleaning pictures (M = −26, SD = 11) as compared to nicotine-related (M = 3, SD = 12; p = 0.05), neutral (M = 21, SD = 11; p < 0.001), and, by trend, food-related pictures (M = 1, SD = 10; p = 0.075). Furthermore, B2 homozygotes showed a higher approach-bias toward nicotine-related pictures (M = 24, SD = 8) relative to tooth-cleaning (M = −10, SD = 7; p < 0.001) and relative to neutral pictures (M = −2, SD = 7; p < 0.001). In addition, B2 homozygotes showed a larger approach-bias toward food-related pictures (M = 11, SD = 6) relative to tooth-cleaning pictures (p = 0.01).
Smoking status differentially affected the effect of genotype on AAT biases for the different picture categories, as the smoking status × DRD2 genotype × picture category interaction approached significance (F(3,128) = 2.63, p = 0.053, η 2 = 0.06). To obtain an accurate picture of the three-way interaction, we conducted two 2 × 4 ANOVAS for each genotype separately and with smoking status (smoker versus non-smoker) as the between-subjects factor.
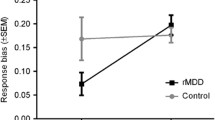
For the B1 allele, Bonferroni corrected analyses revealed a main effect of picture category, F(3, 37) = 5.84, p corrected = 0.004, η 2 = 0.32, qualified by a significant smoking status × picture-category interaction, F(3, 37) = 4.95, p corrected = 0.01, η 2 = 0.29. Specifically, on a between-group level, simple effect analyses revealed that smokers carrying the B1 allele showed less approach for food images than non-smokers carrying the B1 allele (M smokers+B1 = -16, SD = 9, M non−smokers+B1 = 18, SD = 13, p = 0.03) (see Fig. 1). No other between-group differences reached significance (for smoking pictures: p = 0.10, for tooth-cleaning pictures: p = 0.08, for neutral pictures: p = .62). Furthermore, on a within-group level, genotype affected approach-biases in smokers in particular, evidenced by a decreased approach-bias for food images (M food = −16, SD = 9) relative to nicotine-related pictures (M nicotine = 20, SD = 12; p = 0.03) and relative to neutral pictures (M neutral = 16, SD = 11; p = 0.02) in smokers carrying the B1 allele. Furthermore, non-smokers with the B1 allele expressed a stronger avoidance bias for tooth-cleaning images relative to food images (M tooth−cleaning = −45, SD = 18; M food = 18, SD = 13, p = 0.005) and relative to neutral images (M neutral = 26, SD = 16, p = 0.002).
Finally, no group differences in response to the four picture categories occurred for B2 homozygotes as evidenced by a non-significant interaction between smoking status and picture category (F(3, 89) < 1, p corrected = 0.86, η 2 = 0.03).
Self-report measures
The DRD2 Taq1B polymorphism had no effect on craving or nicotine addiction severity (FTDN score) in smokers (see Table 1 for statistics; all ps corrected ≥ 0.20; separate one-way ANOVAs with genotype as the between-subjects factor). However, the DRD2 Taq1B polymorphism had an influence on abstinence motivation in smokers (Stages of change scale; see Table 1): Smokers homozygous for the B2 allele indicated that they had made twice as many quit attempts in the last 12 months than smokers with the B1 allele (M B2smokers = 1.9, SD = 1.4; M B1smokers = 1, SD = 1.3; F(1,82) = 8.82, p corrected = 0.02, η 2 = 0.1).
Discussion
The present study sought to determine the role of the DRD2 Taq1B polymorphism on approach-avoidance biases toward smoking-related and natural-reward stimuli in smokers and non-smokers. To this end, we reanalyzed data from our recent study [10] to examine the contribution of the DRD2 gene on approach-avoidance tendencies in smokers and non-smokers.
While we did not find a genotype-mediated difference in approach-avoidance behavior in the entire sample, we found genotype × smoking status interactions with respect to specific approach-biases towards smoking-related relative to natural-reward related stimuli. In particular, smokers carrying the B1 allele showed a reduced approach behavior for natural rewarding (food) stimuli compared to non-smokers with the same allele. The DRD2 Taq1B polymorphism, however, did not influence responsivity toward different picture categories in the AAT in non-smokers. Interestingly, in the group of smokers, a higher responsivity toward smoking-related relative to food-related pictures in the AAT was found in carriers of the B1 allele. Such a pattern was not found in smokers homozygous for the B2 allele. This pattern of findings suggests that the B1 allele in combination with smoking behavior is associated with a decreased sensitivity to naturally rewarding stimuli (i.e., pictures of highly palatable food). Furthermore, as an important addition to previous results [10] that indicated a shift in approach-bias toward smoking-related stimuli relative to natural-reward stimuli, we found that this shift was limited to smokers with the B1 allele. Our findings are indicative of a genetic contribution to individual variability in approach-avoidance behavior towards naturally rewarding and smoking-related stimuli in smokers similar to previous findings in hazardous drinkers [6].
Several previous studies confirmed a close relation between polymorphisms in the DRD2 gene and tobacco addiction. In this instance, both the B1 allele of the Taq1B polymorphism of the DRD2 gene and the minor A1 allele of the adjacent ankyrin repeat and kinase domain containing 1 (ANKK1) gene are found in higher frequency among polysubstance abusers [38, 39], cocaine-dependent subjects [40, 41], and smokers relative to non-smokers [23]. A reduced density of dopamine receptors has been reported for both, the minor A1 allele of the ANKK1 gene and the minor B1 allele of the DRD2 gene [22]. Reduced DRD2 availability has been linked to the reward deficiency syndrome [42] which is characterized by an increased likelihood to develop impulsive or addictive behaviors [20], but also to more difficulties to abstain from addictive behavior. Here, we add new data suggesting that differences in approach-avoidance tendencies might contribute to these previous findings regarding the relationship between DRD2 availability and nicotine addiction.
The previous imaging studies have already suggested an increased threshold for activation of reward circuits in response to monetary [17] or food reward in tobacco smokers [43]. Our results indicate that such altered responsivity to natural rewards can also be detected on the behavioral level (by means of the AAT) which, however, is related to individual differences in DRD2 availability. A reduced sensitivity to food-related pictures was only found in smokers carrying the B1 allele which is associated with lower DRD2 availability. Similar to other drugs, chronic tobacco use leads to a dysregulation of dopaminergic neurotransmission in meso-corticolimbic areas [15]. These include increases in dopamine cellular activity after acute tobacco consumption, but also a downregulation of dopaminergic activity in response to natural reinforcers [15]. Neuroimaging studies [44] suggest that the orbitofrontal cortex is a central structure responsible for an increased salience attribution to drug cues at the expense of natural rewards in the course of addictions. Interestingly, reductions in DRD2 go along with decreased metabolism in prefrontal cortical regions [45]. Thus, in smokers, a reduction in DRD2 density in combination with a decreased prefrontal activity might lead to an aberrant salience attribution toward drug cues versus food cues representing an important neuroadaptive change in the mesolimbic dopaminergic function [15]. However, our findings only partially support this conclusion, since smokers with the B1 allele did not show a reduced responsivity (approach tendency) towards smoking-related cues. This might be due to the fact that we used a sample of moderate smokers with a mean FTND score of 3.4. Since the AAT is a measure of impulsive tendencies and the prefrontal cortex has been linked to impulse control [2], a disruption of prefrontal control due to reduced DRD2 availability might lead to a greater imbalance between executive and impulsive instances in heavy smokers only [1]. This, in turn, could lead to a more pronounced approach-bias toward smoking cues compared to other cues. Indeed, evidence from animal and human data suggests a strong negative association between DRD2 availability and control of impulsivity [46]. Future studies combining AAT and imaging techniques [47] in heavy smokers genotyped for the Taq1B polymorphism of the DRD2 gene could be helpful to get more insight into the possible neuronal underpinnings.
A major limitation of the current study is the small sample size which might have limited the power to detect overall group differences. In particular, the smoking status × DRD2 genotype × picture category approached borderline statistical significance (p = 0.053). According to discriminatory power analyses which we conducted a posteriori, power was sufficient for detecting main effects and two-way interactions (1 − β > 0.80); however, the power to detect a three-way interaction was, indeed, very small (1 − β = 0.65). Thus, the current findings can be considered as promising, but tentative, and in need of replication with a larger sample. Furthermore, it would be valuable to investigate the contribution of other dopaminergic pathway genes on complex smoking behavior phenotypes, since it is likely that a single-nucleotide polymorphism has only small effects on smoking.
Nevertheless, our results may have implications for the development of more optimized smoking cessation interventions. For instance, specific training programs based on the AAT have successfully been employed to change maladaptive approach-biases and to enhance efficacy of psychological cessation interventions in smokers [48, 49]. However, not all participants profit equally well from these interventions and a large proportion of ex-smokers experience relapse phenomena after successful treatment [29] (see: [50] for a review). The basic rationale of AAT modification paradigms is to incorporate nicotine-related cues as a category of stimuli to be avoided, while cues corresponding to natural rewards such as palatable food or pictures of pleasant activities should be approached. Thus, in AAT retraining studies for smokers, participants could be trained to abolish approach behavior towards nicotine stimuli, but could concomitantly be provided with an alternative behavior, i.e., approaching naturally rewarding stimuli, or stimuli which are at least less toxic or detrimental. Hence, from a theoretical perspective, training to approach naturally rewarding stimuli is equally important as training to avoid smoking stimuli. Understanding the genetic/biological basis of these respective approach-biases in smokers (vs. non-smokers) is, therefore, of high interest. Based on the findings from the present study, it could be concluded that AAT training programs which aim to increase tendencies to approach naturally rewarding stimuli (as an alternative category to smoking-related stimuli) in smokers would be less efficient in B1 allele carriers or that a more extensive retraining protocol would be needed for those participants. However, it remains to be determined whether this would also be associated with a less efficient treatment outcome in smokers carrying the B1 allele relative to those homozygous for the B2 allele. Nevertheless, we found that smokers with the B1 allele underwent fewer attempts to quit smoking compared to smokers homozygous for the B2 allele which, indeed, suggests a more persistent course of smoking behavior. The latter finding corroborates existing literature showing a negative influence of the B1 allele of the Taq1B polymorphism on smoking severity and the ability to abstain from smoking [28, 29].
In conclusion, our results indicate a reduced natural-reward brain reactivity in smokers with the B1 allele of the DRD2 Taq1B polymorphism as evidenced with the AAT. Such a genetically determined decrease in dopaminergic activity (i.e., reduction of DRD2 availability) might result in a different outcome after psychological cessation interventions in smokers [48], which, however, needs to be explored in future research.
References
Deutsch R, Strack F (2006) Reflective and impulsive determinants of addictive behavior. In: Wiers RW, Stacy AW (eds) Handbook of implicit cognition and addiction. Sage Publications, Thousand Oaks, pp 45–57
Wiers RW, Gladwin TE, Hofmann W, Salemink E, Ridderinkhof KR (2013) Cognitive bias modification and cognitive control training in addiction and related psychopathology: Mechanisms, clinical perspectives, and ways forward. Clin. Psychol Sci 20:1–21. doi:10.1177/2167702612466547
Rinck M, Becker ES (2007) Approach and avoidance in fear of spiders. J Behav Ther Exp Psy 38:105–120
Zhou Y, Li X, Zhang M, Zhang F, Zhu C, Shen M (2012) Behavioral approach tendencies to heroin-related stimuli in abstinent heroin abusers. Psychopharmacology (Berl) 221:171–176. doi:10.1007/s00213-011-2557-0
Cousijn J, Goudriaan AE, Wiers RW (2011) Reaching out toward cannabis: approach-bias in heavy cannabis users predicts changes in cannabis use. Addiction 106:1667–1674. doi:10.1111/j.1360-0443.2011.03475.x
Wiers RW, Rinck M, Dictus M, van den Wildenberg E (2009) Relatively strong automatic appetitive action-tendencies in male carriers of the OPRM1 G-allele. Genes, Brain Behav 8:101–106. doi:10.1111/j.1601-183X.2008.00454.x
Wiers CE, Kühn S, Javadi AH, Korucuoglu O, Wiers RW, Walter H et al (2013) Automatic approach bias toward smoking cues is present in smokers but not in ex-smokers. Psychopharmacology (Berl) 229:187–197. doi:10.1016/j.jpsychires.2015.11.015
Wiers RW, Eberl C, Rinck M, Becker ES, Lindenmeyer J (2011) Retraining automatic action tendencies changes alcoholic patients’ approach bias for alcohol and improves treatment outcome. Psychol Sci 22:490–497. doi:10.1177/0956797611400615
Eberl C, Wiers RW, Pawelszack S, Rinck M, Becker ES, Lindenmeyer J (2013) Approach bias modification in alcohol dependence: do clinical effects replicate and for whom does it work best? Dev Cogn Neurosci 4:38–51. doi:10.1016/j.dcn.2012.11.002
Machulska A, Zlomuzica A, Adolph D, Rinck M, Margraf J (2015) “A cigarette a day keeps the goodies away”: smokers show automatic approach tendencies for smoking—but not for food-related stimuli. Plos One 10:1–15. doi:10.1371/journal.pone.0116464
Robinson TE, Berridge KC (2008) The incentive sensitization theory of addiction: some current issues. Philos T Roy Soc B 363:3137–3146. doi:10.1098/rstb.2008.0093
Robinson T, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev 18:247–291
Ernst M, Matochik JA, Heishman SJ, Van Horn JD, Jons PH, Henningfield JE et al (2001) Effect of nicotine on brain activation during performance of a working memory task. Proc Natl Acad Sci 98:4728–4733
Goldstein RZ, Volkow ND (2002) Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159:1642–1652
Wise RA (2002) Brain reward circuitry: insights from unsensed incentives. Neuron 36:229–240
Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R et al (1997) Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 386:830–833
Martin-Sölch C, Magyar G, Künig G, Missimer J, Schultz W, Leenders KL (2001) Changes in brain activation associated with reward processing in smokers and nonsmokers. A positron emission tomography study. Exp Brain Res 139:278–286
Volkow ND, Fowler JS, Wang GJ (2002) Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies. Behav Pharmacol 13:355–366
Johnson PM, Kenny PJ (2010) Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci 13:635–641. doi:10.1038/nn.2519
Noble EP (2000) Addiction and its reward process through polymorphisms of the D2 dopamine receptor gene: a review. Eur Psychiatry 15:79–89
Noble EP (1998) The DRD2 gene, smoking, and lung cancer. J Natl Cancer Ins 90:343–345
Jönsson EG, Nöthen MM, Grünhage F, Farde L, Nakashima Y, Propping P et al (1999) Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry 4:290–296
Spitz MR, Shi H, Yang F, Hudmon KS, Jiang H, Chamberlain RM et al (1998) Case–control study of the D2 dopamine receptor gene and smoking status in lung cancer patients. J Natl Cancer Inst 90:358–363
Comings DE, Ferry L, Bradshaw-Robinson S, Burchette R, Chiu C, Muhleman D (1996) The dopamine D2 receptor (DRD2) gene: a genetic risk factor in smoking. Pharmacogenetics 6:73–79
Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Cull JG et al (1996) The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J R Soc Med 89:396–400
Robinson JD, Versace F, Lam CY, Minnix JA, Engelmann JM, Cui Y (2013) The CHRNA3 rs578776 variant is associated with an intrinsic reward sensitivity deficit in smokers. Front Psychiatry 4:1–11. doi:10.3389/fpsyt.2013.00114
Bowirrat A, Oscar-Berman M (2005) Relationship between dopaminergic neurotransmission, alcoholism and reward deficiency syndrome. Am J Med Genet Part B 132:29–37
Clague J, Cinciripini P, Blalock J, Wu X, Hudmon KS (2010) The D2 dopamine receptor gene and nicotine dependence among bladder cancer patients and controls. Behav Genet 40:49–58. doi:10.1007/s10519-009-9301-0
Robinson JD, Lam CY, Minnix JA, Wetter DW, Tomlinson GE, Minna JD et al (2007) The DRD2 Taq1-B polymorphism and its relationship to smoking abstinence and withdrawal symptoms. Pharmacogenomics 7:266–274
Wu X, Hudmon KS, Detry MA, Chamberlain RM, Spitz MR (2000) D2 dopamine receptor gene polymorphisms among African–Americans and Mexican–Americans: a lung cancer case–control study. Cancer Epidemiol Biomarkers Prev 9:1021–1026
DeBry SC, Tiffany ST (2008) Tobacco-induced neurotoxicity of adolescent cognitive development (TINACD): a proposed model for the development of impulsivity in nicotine dependence. Nicotine Tob Res 10:11–25. doi:10.1080/14622200701767811
Morales AM, Ghahremani D, Kohno M, Hellemann GS, London ED (2014) Cigarette exposure, dependence, and craving are related to insula thickness in young adult smokers. Neuropsychopharmacology 39:1816–1822. doi:10.1038/npp.2014.48
Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO (1991) The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Brit J Addict 86:1119–1127
Bleich S, Havemann-Reinecke U, Kornhuber J (2002) Fagerström-Test für Nikotinabhängigkeit. Beltz Test, Göttingen
Swanson JE, Rudman LA, Greenwald AG (2001) Using the implicit association test to investigate attitude-behavior consistency for stigmatised behavior. Cognition Emotion 15:207–230 doi:10.1080/0269993004200060
Prochaska JO, Velicer WF, DiClemente CC, Guadagnoli E, Rossi JS (1991) Patterns of change: dynamic typology applied to smoking cessation. Multivar Behav Res 26:83–107. doi:10.1207/s15327906mbr2601_5
Jäkle C, Keller S, Baum E, Basler HD (1999) Scales for the measurement of self-efficacy and decisional balance in the process of behavioral change in smokers. Diagnostica 45:138–146
O’Hara BF, Smith SS, Bird G, Persico AM, Suarez BK, Cutting GR et al (1993) Dopamine D2 receptor RFLPs, haplotypes and their association with substance use in black and Caucasian research volunteers. Hum Hered 43:209–218
Smith SS, O’Hara BF, Persico AM, Gorelick DA, Newlin DB, Vlahov D et al (1992) Genetic vulnerability in drug abuse: The dopamine D2 receptor Taq1 B RFLP is more frequent in polysubstance abusers. Arch Gen Psychiatry 49:723–727
Noble EP, Blum K, Khalsa ME, Ritchie T, Montgomery A, Wood RD et al (1993) Allelic association of the D2 dopamine receptor gene with cocaine dependence. Drug Alcohol Depend 33:271–278
Persico AM, Bird G, Gabbay FH, Uhl GR (1996) D2 dopaminereceptor gene Taq1 A1 and B1 restriction fragment length polymorphism: enhanced frequencies in psychostimulant preferring polysubstance abusers. Biol Psychiatry 40:776–784
Blum K, Gardner E, Oscar-Berman M, Gold M (2012) “Liking” and “wanting” linked to reward deficiency syndrome (RDS): Hypothesizing differential responsivity in brain reward circuitry. Curr Pharm Des 18:113–118
Peters J, Bromberg U, Schneider S, Brassen S, Menz M, Banaschewski T et al (2011) Lower ventral striatal activation during reward anticipation in adolescent smokers. Am J Psychiat 168:540–549
Volkow ND, Fowler JS, Wang GJ, Swanson JM (2004) Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry 9:557–569
Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J et al (2008) Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage 42:1537–1543. doi:10.1016/j.neuroimage.2008.06.002
Volkow ND, Wang G-J, Fowler JS, Tomasi D, Telang F, Baler R (2010) Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays 32:748–755
Wiers CE, Ludwig VU, Gladwin TE, Park SQ, Heinz A, Wiers RW et al (2015) Effects of cognitive bias modification training on neural signatures of alcohol approach tendencies in male alcohol-dependent patients. Addict Biol 20:990–999. doi:10.1111/adb.12221
Machulska A, Zlomuzica A, Rinck M, Margraf J, Assion H-J (2016) Approach bias modification in inpatient psychiatric smokers. J Psychiat Res 76:44–51. doi:10.1016/j.jpsychires.2015.11.015
Kong G, Larsen H, Cavallo DA, Becker D, Cousijn J, Salemink E et al (2015) Re-training automatic action tendencies to approach cigarettes among adolescent smokers: a pilot study. Am J Drug Alcohol Abuse 4:425–432. doi:10.3109/00952990.2015.1049492
Holmes S, Zwar N, Jiménez-Ruiz CA, Ryan PJ, Browning D, Bergmann L et al (2004) Bupropion as an aid to smoking cessation: a review of real-life effectiveness. Int J Clin Pract 58:285–291
Acknowledgements
This work was supported by the Alexander von Humboldt-Professorship—International Award for Research in Germany granted to Jürgen Margraf. This study presents independent research part-funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. The authors thank Helen Copeland-Vollrath for her editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the local Ethics Committee of the Ruhr-Universität Bochum and was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki.
Informed consent
All participants gave written informed consent prior to their inclusion in the study.
Rights and permissions
About this article
Cite this article
Zlomuzica, A., Machulska, A., Roberts, S. et al. The dopamine D2 receptor mediates approach-avoidance tendencies in smokers. Eur Arch Psychiatry Clin Neurosci 268, 261–268 (2018). https://doi.org/10.1007/s00406-017-0793-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-017-0793-y