Abstract
The aim of the present study was to evaluate the impulsivity and brain correlates of response inhibition and error processing among subjects with Internet gaming disorder (IGD). We evaluated the response inhibition and error processing by functional magnetic resonance imaging (fMRI) in subjects with IGD and controls. Twenty-six men with IGD for at least 2 years and 23 controls with no history of IGD were recruited as the IGD and control groups, respectively. All subjects performed the event-related designed Go/No-go task under fMRI and completed questionnaires related to Internet addiction and impulsivity. The IGD group exhibited a higher score for impulsivity than the control group. The IGD group also exhibited higher brain activation when processing response inhibition over the left orbital frontal lobe and bilateral caudate nucleus than controls. Both the IGD and control groups exhibited activation of the insula and anterior cingulate cortex during error processing. The activation over the right insula was lower in the subjects with IGD than the control group. Our results support the fact that the fronto-striatal network involved in response inhibition, and the salience network, anchored by the anterior cingulate and insula, contributes to error processing. Further, adults with IGD have impaired insular function in error processing and greater activation of the fronto-striatal network in order to maintain their response inhibition performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Loss of control over Internet use, resulting in negative psychosocial consequences, has been newly defined as Internet gaming disorder (IGD) in the Diagnostic and statistical manual of mental disorders, fifth edition (DSM 5), and has been suggested to be deserving of future study [1]. As Internet use increases worldwide, IGD is now prevalent, not only in Western, but also in Eastern societies [2]. Due to the rapid rise in Internet use, clarification of the mechanisms of IGD is important for the development of effective interventions and preventive schedules. The core behavior criterion of IGD is loss of control of Internet use and is represented as persistence in online gaming use despite awareness that it is directly harmful to psychosocial functioning. Recently, researchers have begun to investigate the cognitive, neurobiological, and neuropsychological characteristics of IGD with the aim of understanding the mechanism of the loss of control [3–8]. Most sufferers of IGD portray similar characteristics to individuals with substance use disorders and demonstrate a deficit with regard to inhibitory control.
Impulsivity is one of the most important factors that contribute to loss of control in addictive behavior [9]. It has been reported to be associated with and to predict IGD [10, 11]. A deficit in suppressing the prepotent motor responses is an important dimension of impulsivity, which has been repeatedly tested with the Go/No-go task to represent the function of response inhibition [12]. Enhanced response inhibition in the Go/No-go task predicts improvement in adaptive social–emotion function. A deficit in response inhibition is involved in poor decision-making in risk situations. Furthermore, impaired response inhibition contributes to addictive behavior, as does impulsivity. One previous study demonstrated impaired response inhibition in subjects with IGD [13]; however, another study revealed a contradictory result [14]. Thus, further study to understand the function of response inhibition in subjects with IGD is necessary.
Functional magnetic resonance imaging (fMRI) is a technique used to record cerebral hemodynamic changes in specific tasks to demonstrate possible brain mechanisms for specific neurocognitive functions [15]. The event-related design of an fMRI study allows the separation of the Go, the successful No-go and the failed inhibited No-go trials in simple mixed designs, and has been preferred for assessing the functional anatomy of response inhibition and error processing [16]. Converging evidence from previous studies has suggested a critical role of regions of the fronto-striatal network, such as the orbital frontal lobe, supplement motor area [17], and caudate nucleus [18], in inhibiting prepotent responses. It is involved in the mechanism whereby humans prevent themselves from becoming subservient to reflex, habit and motivational impulses [19]. Dysfunction of response inhibition of the inferior orbital frontal lobe has been suggested to be one important mechanism to explain loss of control in substance use [20, 21].
Error monitoring is a metacognitive process by which we are able to detect and signal errors as soon as a response is made. This process plays a crucial role in adaptive human behavior, allowing our actions to be shaped by their outcomes [22]. Further, it also contributes to self-regulation [23]. Error processing can be evaluated by investigating the brain response to failed inhibited responses in the Go/No-go task in an event-related fMRI study. The anterior cingulate cortex is one of the most important areas involved in error processing, especially error negativity in event-related potentials [24–26]. Further, the insula is another important area contributing to error processing [25, 26]. Previous researchers have found a deficit in error processing in disorders with higher impulsivity, such as substance use disorder [27].
There are many services, such as online gaming, available online to satisfy users. People might be attracted by these activities and forget to do necessary tasks. If excessive Internet use results in negative consequences, error processing can alert users to pay attention to the negative consequences, and response inhibition will control or prevent these inappropriate Internet activities. However, subjects with IGD have been reported to have poor impulse control [10, 11]. An fMRI study conducted on the Stroop task suggested that the IGD group had significantly greater activity in the anterior and posterior cingulate cortices during the inhibition of the proponent response [3]. However, previous event-related potential studies conducted on the Go/No-go task demonstrated that the IGD group had a lower activation during the conflict detection stage and need to engage in more cognitive endeavors to complete the inhibition task [4]. These two studies, which had limited subject numbers, 12 cases and 12 controls, demonstrated altered brain activation and evoked potentials during response inhibition. A deficit in response function in the Go/No-go task has been reported in previous behavioral [13] and evoked potential studies [4]. However, fMRI has not been used to investigate the brain mechanisms of response inhibition in the Go/No-go task in an adequate number of subjects with IGD.
Error-related negativity (ERN) is a component of an event-related potential (ERP) and is observed when errors are committed in response tasks. Reduced ERN has been reported in borderline personality disorder, which is characterized by impulsivity [28, 29]. A recent event-related potential study focused on error processing and demonstrated reduced error-related negativity amplitudes in the Go/No-go task in excessive computer game players [7]. This study suggested that error processing is an important candidate mechanism for explaining the loss of control of gaming. However, no fMRI study has focused on error processing to demonstrate the brain mechanisms in subjects who fulfill the diagnosis of IGD.
As subjects with IGD have a higher impulsivity which might be associated with impaired response inhibition and error processing, we hypothesized that these subjects will have impaired response inhibition and error processing functions. Based on previous reports on substance use disorder [21, 30], we hypothesized that subjects with IGD might exhibit altered brain activation during response inhibition and error processing over the fronto-striatal network and anterior cingulate and insula, respectively. Thus, the aim of the study was to evaluate the brain correlates of response inhibition and error processing in the Go/No-go task using functional MRI in subjects with IGD and controls.
Methods
Participants
Male right-handed participants were recruited by an advertisement posted on a university campus. All subjects in the IGD group were interviewed by a psychiatrist to confirm a diagnosis of IGD for more than 2 years according to the diagnostic criteria for Internet addiction (DCIA) [31]. At the time of the study, the subjects were addicted to online gaming and spent an average of 4 or more hours/day on weekdays and an average of 8 or more hours/day on weekends participating in online gaming. In the control group, psychiatric interviews and reviews of psychiatric history were performed to confirm that none had ever fulfilled the DCIA. The exclusion criteria were as follows: current use of psychotropic medication and any history of substance use disorder (excluding nicotine dependence), major depressive episode, bipolar I disorder, psychotic disorder, neurological illness or injury, mental retardation, or poor tolerance of magnetic resonance imaging. A total of 26 male subjects with IGD and 23 male controls were recruited in this study. The demographic data in Table 1 shows that the IGD and control groups did not significantly differ in age. The control group had a higher educational level than the IGD group; however, the difference was limited (<1 educational level). After having received a detailed explanation of the study, all subjects gave written informed consent. The study was approved by the Kaohsiung Medical University Institutional Review Board and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Image acquisition
The fMRI experiments were performed with a 3 Tesla General Electric MR scanner (Signa VH/I, software version 4.0). The magnetic resonance (MR) sequence for functional imaging was a gradient-recalled echo planar imaging (EPI) sequence [64 × 64 matrix; 24-cm field of view, echo time (TE) = 35 ms; repetition time (TR) = 2 s; and 4-mm-thick slices with a 0-mm gap]. Twenty-eight image planes were collected parallel to the anterior commissure and posterior commissure (AC–PC) line with the aid of sagittal localizer images. T1-weight anatomical images were obtained using an FSPGR (Spoiled Gradient Echo) sequence (256 × 256 matrix; 24-cm field of view; TE = 2.8 ms; TR = 6.6 ms; and 1-mm-thick slices with a 0.47-mm gap) before the fMRI scanning to provide anatomical registration. Subjects observed stimuli on a visual display and responded using a hand-held mouse with fiber-optic connections to a computer in the control room running presentation software. Head motion was corrected during post-processing using SPM5 (Wellcome Department of Cognitive Neurology, London, UK).
Process
All invited participants were interviewed by a psychiatrist to confirm their diagnoses of IGD and screened for the exclusion criteria based on the Mini-International Neuropsychiatric Interview (MINI) [32]. Before scanning, all subjects were assessed according to the Chen Internet Addiction Scale (CIAS) [33], the Barratt Impulsivity Scale 11(BIS-11) [34, 35] and Dickman’s Impulsivity Scale [36]. The CIAS indicates the severity of Internet addiction. The BIS-11, subscale of lack of self-control, and Dickman’s impulsivity scale indicate the severity of impulsivity. Functional MR images were then acquired during the Go/No-go task using an event-related design.
The behavior task: Go/No-go task
The Go/No-go task requires subjects to press a button as quickly as possible when observing Go stimuli and to refrain from doing so when observing No-go stimuli. Three sections of the Go/No-go task with the same design, but a different sequence, were processed in this study. Each section consisted of 180 trials (150 go trials and 30 no-go trials) shown as numbers 1–9 with 200-ms durations and 1,425-ms inter-stimulus intervals. The participants were instructed to press the button as quickly as possible for all numbers (1, 3–9) except for the number 2. In order to prevent a familiarity effect for the Go trial or a novelty effect that was specific to the No-go trial, no number was identical to the previous number. The higher percentage of the Go trial (83.3 %) was designed to drive the prepotent response and increase the task difficulty. The correct reaction to No-go stimuli (2) was the withholding of a finger press. The frequencies of numbers 2 and 1 were equal in order to compute the contrast between the responses to the No-go and Go trials. The three sections were separated by two 16-second resting periods. The task resulted in a total of 540 trials, lasting 932 s, and 466 volumes. After scanning, the participants completed the posttest task to evaluate the self-perceived difficulty level of the task.
Data analysis
All time-series data exported from the GE system were converted into a statistical parametric mapping (SPM) format using the DICOM import in SPM5. Image preprocessing and statistical analysis were then performed using the SPM5 package. Each image was realigned to correct for motion, and each high-resolution T1 image was coregistered to the mean motion-corrected functional image for each participant. For normalization purposes, high-resolution individual T1-weighted images that had been coregistered to the individual mean EPI obtained during spatial realignment were used. The realigned datasets were then normalized to the Montreal Neurological Institute (MNI) space and resliced to a 2 × 2 × 2 mm3 voxel size. An 8-mm full-width-half-maximum Gaussian kernel was used to smooth the data. One image per contrast was computed for each participant; the data were subjected to a high-pass filter of 8 s with no global scaling.
To identify brain activity related to successful response inhibition and failed response inhibition, a corresponding [successfully inhibited No-go trials minus go trials] contrast and [unsuccessfully inhibited No-go trials minus go trail] one, respectively, were formulated as signed t-contrast for each participant. A general linear model was used to combine the two types of contrast from individual subjects into a full factor model with a within-subject factor (error effect: contrast for successful versus failed response inhibition) and a between-subject factor (IGD effect: IGD versus Control). After including the contrast of all participants, the activation for the successful response inhibition was demonstrated for each group with a significant threshold of p < 0.05 FDR (false discovery rate) corrected at the voxel level. The differences in contrast to successful response inhibition between the IGD and control groups were analyzed with a significant threshold of p < 0.05 with a small volume family-wise error (FWE) correction in the regions of interest.
Many previous studies evaluated error by subtracting correct Go trials from unsuccessfully inhibited No-go trials. However, response inhibition was generated in No-go trials, but not in Go trials; therefore, this method demonstrates not only the response to error processing, but also that for response inhibition. In the present study, the brain activation for error processing was determined by the error effect (calculated by subtracting contract of successful response inhibition from that of failed successful response inhibition). Subjects experienced the same visual stimuli, no-go trials, in successful and failed No-go trials. The error processing response was generated only in failed No-go trials, but not in successful No-go trials. Thus, the subtraction described isolated the response for error processing without the biasing effect of the Go trials [37]. The error effect demonstrated the brain activation during error processing with a significant threshold of p < 0.05 FDR corrected at the voxel level. However, the motor response in failed inhibited No-go trials might also bias the interpretation of the contrast of error effect.
The interaction between the error effect and the IGD effect reveals the differences in brain activation during error processing between the IGD and control groups, with a significant threshold of p < 0.05 in small volume FWE correction in individual regions of interest.
The regions of interest were selected as essential brain areas for response inhibition or error processing, as identified by a literature review. Further, they were also significantly activated in the group-level analysis in this study.
For region-of-interest (ROI) analysis, ROIs were defined as a significantly activated cluster in the IGD group, the control group, or a two-sample t test. Activations for the (successfully inhibited No-go trials minus go trials) contrast were calculated by MarsBaR [38]. Pearson’s correlation was used to test ROIs for correlations with correct responses in the Go/No-go task, reaction time, score on the BIS-11, lack of self-control subscale, and score on Dickman’s impulsivity scale. A p value lower than 0.05 was considered to be significant.
Results
The results of the behavior task (Table 1)
In the behavior task, subjects with IGD successfully inhibited 80.7 ± 13.8 % of No-go trials, compared with 86.1 ± 13.2 % in controls. There was no significant difference in the correct response rate or reaction time in the Go/No-go task between the subjects with IGD and controls. This result suggests that there was no difference in the performance in the Go/No-go task between the two groups. Further, the results also demonstrated that both groups followed the guild to complete the task. Subjects with IGD perceived a higher difficulty of the task than the controls but this was not statistically significant. The IGD group had significantly higher scores on the CIAS, BIS-11, lack of self-control subscale, and dysfunctional impulsivity tests than the control group. These results indicate that the subjects with IGD had a higher impulsivity and poorer self-control than the controls.
The activation of response inhibition among the IGD and control groups
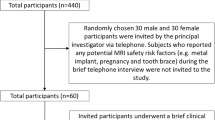
Table 2 and Fig. 1 show that the IGD group activated the bilateral orbital frontal lobe, anterior cingulate, caudate, left putamen, right dorsolateral prefrontal cortex (DLPFC) and middle temporal lobe to response inhibition with a threshold of p < 0.05 FDR corrected at the voxel level. The control group activated the right DLPFC for response inhibition with the same threshold.
The brain activation of response inhibition among Internet gaming disorder (IGD) group and control group. a The IGD group activated the cortico-Striatal network for response inhibition; b The control group activated right dorsolateral prefrontal cortex for response inhibition; c IGD group had a significantly higher activation over the left orbital frontal lobe and bilateral caudate than control group
Previous reviews of response inhibition have suggested that the fronto-striatal network is essential for response inhibition [19]. In these studies, the bilateral orbital frontal lobe, caudate nucleus, and anterior cingulate cortex were significantly activated for response inhibition. Thus, these regions were selected as ROIs for small volume correction in the between-group analysis of brain activation of response inhibition. These anatomical ROIs were created from the AAL library as found in WFU Pickatlas v 2.4 [39]. A further comparative analysis demonstrated that the subjects with IGD had significantly higher activation over the left orbital frontal lobe and bilateral caudate nucleus than controls, with a p value <0.05 FWE correction in the regions of interest. The Pearson’s correlation analysis demonstrated that the contrast values of the bilateral caudate nucleus and left orbital frontal lobe were significantly correlated with the score of lack of self-control among all subjects (x, y, z = −10,2,20: r = 0.32, p = 0.024; x, y, z = 10, 14, 6: r = 0.35, p = 0.014; x, y, z = −28, 28, −4: r = 0.35, p = 0.015, Fig. 1c). Further, the contrast value of the bilateral caudate nucleus was significantly correlated with the score of lack of self-control among the IGD group (Fig. 1c; x, y, z = −10, 2, 20: r = 0.56, p = 0.003; x, y, z = 10, 14, 6: r = 0.44, p = 0.026), but not among controls. This result indicates that subjects with poorer self-control had higher brain activation over the bilateral caudate nucleus during response inhibition among IGD group. To exclude the effect of outliers, a further Spearman’s correlation analysis was carried out and was found to support the significant correlations mentioned above.
The activation for error processing in IGD and control groups
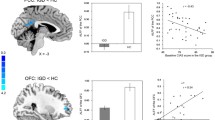
Table 3 and Fig. 2 show that the IGD group activated the bilateral insula and anterior cingulate cortex for error processing at a threshold of p < 0.05 FDR corrected at the voxel level. The control group activated the same areas along with the bilateral inferior frontal lobe for error processing at the same threshold.
The brain activation of error processing among Internet gaming disorder (IGD) group and control group. a, b Both IGD and control group activated the bilateral insula and anterior cingulate for error processing; c The IGD group had higher brain activation over right insula for error processing than control group
Both the anterior cingulate cortex [22, 25, 26] and insula [25, 26, 40] have been previously reported to contribute to error processing. In this study both were significantly activated for error processing (error effect) in both the IGD and control groups. The clusters of activity over the anterior cingulate cortex and insula for error processing in the control group were selected to be ROIs in the small volume correction in the between-group analysis. A further analysis of the interaction between the error effect and the IGD effect demonstrated that the controls had higher activation over the right insula when processing errors than the subjects with IGD.
Discussion
In line with previous studies [7], this study revealed that subjects with IGD had higher scores on the Barratt impulsivity scale, the lack of self-control subscale, and the dysfunctional impulsivity scale. These results indicate that subjects with IGD have a higher impulsivity and poorer self-control. There was no significant difference in terms of performance in the Go/No-go task between the IGD and control groups. However, there were differences in brain activations for response inhibition and error processing between the IGD group and the control group.
The altered brain activation of response inhibition among adults with IGD
Group analysis demonstrated that adults with IGD activate extensive brain areas, such as the DLPFC, inferior orbital frontal lobe, anterior cingulate, caudate, and putamen, for response inhibition. These activated brain regions in the fronto-striatal network correspond to the brain correlates of response inhibition reported in previous studies [17, 18]. The inferior frontal lobe is critical for executive control of response inhibition, especially for the No-go trial [17, 41]. The DLPFC supports inhibition of the preparatory responses and is suggested to be indicator of capacity for response inhibition [42, 43]. Then, frontal lobe projects to caudate, which was found to activate for stop trials and is likely to be involved in the stop process [44]. After the motor response is complete in each trial, the anterior cingulate cortex is involved in error detection in executive functioning and interference monitoring [45]. Our results for the IGD group support the role of the fronto-striatal network in response inhibition described above. However, the control group activated only the DLPFC for response inhibition.
Further comparative analysis demonstrated that the subjects with IGD had higher brain activation over the left inferior orbital frontal lobe and bilateral caudate when processing response inhibition than the controls. Previous studies suggest that subjects with IGD need to be more engaged in cognitive endeavors to complete the Go/No-go task [4]. In the present study, subjects with IGD perceived the task to have a higher difficulty score than the controls, but this was not significant. The lateral orbital frontal lobe has been found to have increased activation and was positively correlated with impulsivity in subjects with borderline personality disorder [46], which was characterized by impulsivity [28, 29]. Further, subjects with prenatal exposure to cocaine had greater activation in the inferior cortex and caudate nucleus during response inhibition [47]. These results suggest that the fronto-striatal networks thought to mediate inhibitory control are vulnerable to the effects of various substances [48].
In the present study, participants with poorer self-control had higher activation over the left orbital frontal lobe and bilateral caudate nucleus. Further, the poorer self-control of IGD subjects, the greater activation over the bilateral caudate nucleus they had. Since subjects with IGD had poorer self-control than controls, the higher brain activation over the fronto-striatal network might suggest that they needed to expend more effort to inhibit the prepotent response in No-go trials. This result suggests that altered fronto-striatal network activation mediated the impaired response inhibition of subjects with IGD. However, the causal relationship between the altered fronto-striatal network activation of response inhibition and IGD could not be confirmed in this cross-section study. Further, a previous study suggested that the IGD group had significantly greater activity in the anterior and posterior cingulate cortices during the inhibition of semantic interference [3]. In the present study, subjects were required to inhibit the motor response, but not semantic interference, in the Go/No-go task. The difference in the tasks might explain why we did not demonstrate a significant difference in the anterior and posterior cingulate cortices in between the IGD and control groups.
The brain activation for error processing in both the IGD and control groups
The present study demonstrated insula and anterior cingulate cortex activation for error processing in both the IGD and control groups. The insula has been previously shown to be consistently activated during performance monitoring, and modulated by error awareness [49]. A meta-analysis study of brain imaging suggested that the anterior insula and the adjacent orbitofrontal cortex are engaged in error awareness [50]. Thus, they play an important role in error processing in terms of adjusting the human behavior.
The anterior cingulate cortex contributes to cognitive and affective regulation, motivation, and feedback monitoring [51]. It is also involved in cognitive control and error awareness during response inhibition [30, 52]. The insula and anterior cingulate cortex have been found to be co-activated in a variety of cognitive control tasks [49]. Both functional and structural connectivity have been demonstrated between the insula and the anterior cingulate cortex, and this is described as a salience network [53]. In the present study, both the IGD and control groups activated the bilateral insula and anterior cingulate cortex for error processing. This result supports the essential role of the salience network in error processing.
The anterior cingulate cortex has been found to contribute to error processing-related negativity in event-related potential studies [24, 26]. The decreased activation of error processing over this region has been repeatedly reported in substance use disorder [30, 54]. Although a previous study suggested a lower event-related negativity in subjects with excessive computer gaming [7], the present study of subjects with IGD did not indicate differences in the anterior cingulate cortex activation for error processing. Aside from the differences in the subject recruitment criteria, the limited subject numbers and weaker BOLD signals in the event-related design might contribute to the lack of differences found in the involvement of the anterior cingulate cortex. Further, a recent review suggested that the lateral prefrontal cortex and the insula might also contribute to error-related negativity. It is also possible that the reduced error-related negativity amplitudes are attributed to another key brain area, such as the insula; however, this claim should be examined in further study.
The lower activation over the right insula among subjects with IGD
Decreased insular activation during error processing has been previously found in subjects with substance use disorder, such as those with chronic cannabis use or alcohol intoxication [30, 54]. The insula plays an important role in assessing risk [40]. A lack of insight might be related to a deficient monitoring process and reduced self-awareness, as impaired insular function in error processing has been reported in psychiatric disorders with a lack of insight, such as ADHD or substance use disorder [30, 55]. Impairment of its function might result in lack of insight into the negative consequences of addictive behavior, such as alcohol dependence [56].
In line with previous results of subjects with substance use disorder [30, 54], our results demonstrated reduced brain activation over the insula for error processing in subjects with IGD. A lower gray matter density of the insula has also been found in subjects with IGD [57], demonstrating limited functioning of this brain region in these individuals. Our results indicate that the reduced activation of the insula when processing error might support the claim that subjects with IGD have a limited insular function, which might result in impaired function of error processing in IGD.
Clinical implications
To the best of our knowledge after searching PubMed, this is the first fMRI study to demonstrate increased fronto-striatal network activation for response inhibition and reduced insular activation for error processing in subjects with IGD in comparison with a control group. The insula needs to be activated for the winning experience of gaming [58], potentially at the expense of interoceptive signals that assess the negative consequences of heavy Internet use [30]. Online gaming links are usually easily accessible on the desktop or in the favorites list when adults with IGD are using a computer for work purposes. The deficit in the error processing function of the insula might enable subjects to go online immediately without thinking of it as being incongruent to their original intention. The impaired response inhibition over the fronto-striatal network might contribute to difficulty in controlling online gaming activity when negative consequences are perceived. This might partly explain why adults with IGD continue their Internet use despite their knowledge of the problems caused by excessive Internet use. Thus, more attention should be paid to the altered function in response inhibition and error processing among subjects with IGD. Impulsivity or deficits in response inhibition should be investigated in order to determine strategies to prevent the negative consequences of excess Internet use. Further, altering tools such as alarm clocks, pop-up reminders, or blocking inappropriate links might be necessary to assist subjects with IGD to activate their response inhibition or error processing systems to regulate their excessive online gaming use. Further, therapy that emphasizes the negative consequences of IGD is also necessary to compensate for the deficit in error monitoring. However, the contribution of the insula in error processing and the fronto-striatal network in response inhibition to insight and control of online gaming, respectively, should be confirmed in future studies.
Limitations
Several limitations of this study should be noted. First, only men were included in the study. Second, as cases that were comorbid with substance and other major psychiatric disorders were excluded in this study, there is a limitation in terms of generalizing the results to those with IGD with other substance use disorders or major psychiatric disorders. Third, there was a significant difference in the educational level between the IGD group and the control group. However, the difference was limited to less than one level. Further, we searched on PubMed and did not find a study showing a significant effect of educational level on error processing. Fourth, without prospective investigation, the causal relationship between the reduced insular function in error processing and IGD could not be confirmed in this study. Fifth, details of the duration of illness were not recorded in this study. Therefore, any association between the deficit in error processing and the duration of IGD could not be confirmed in this study. Sixth, the error correlated contrast was evaluated by (failed inhibited No-go trials minus successfully inhibited No-go trials). The motor response in failed inhibited No-go trials could not be controlled in calculation and might have biased our results. Seventh, depression was not assessed in the study and was therefore not controlled for in the data analysis.
Conclusions
This study demonstrates the neurobiological mechanisms of altered response inhibition and error processing in subjects with IGD. Our results suggest that subjects with IGD exhibit increased activation of the fronto-striatal network when processing response inhibition and reduced activation of the insula when processing errors. Further, the subjects with IGD had a higher impulsivity and poorer self-control than controls. These results suggest that the altered activation of the fronto-striatal network during response inhibition and the deficit in error processing by the insula might represent the brain mechanisms that explain the loss of control of online gaming in subjects with IGD. Finally, the deficits in response inhibition and error processing in IGD should be the focus of interventions and deserve further research.
References
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Association, Arlington
Yen CF, Yen JY, Ko CH (2010) Internet addiction: ongoing research in Asia. World Psychiatry 9(2):97
Dong G, Devito EE, Du X, Cui Z (2012) Impaired inhibitory control in ‘Internet addiction disorder’: a functional magnetic resonance imaging study. Psychiatry Res 203(2–3):153–158
Dong G, Zhou H, Zhao X (2010) Impulse inhibition in people with Internet addiction disorder: electrophysiological evidence from a Go/NoGo study. Neurosci Lett 485(2):138–142
Ko CH, Liu GC, Hsiao S, Yen JY, Yang MJ, Lin WC, Yen CF, Chen CS (2009) Brain activities associated with gaming urge of online gaming addiction. J Psychiatr Res 43(7):739–747
Ko CH, Liu GC, Yen JY, Chen CY, Yen CF, Chen CS (2011) Brain correlates of craving for online gaming under cue exposure in subjects with Internet gaming addiction and in remitted subjects. Addict Biol 18(3):559–569
Littel M, van den Berg I, Luijten M, van Rooij AJ, Keemink L, Franken IH (2012) Error processing and response inhibition in excessive computer game players: an event-related potential study. Addict Biol 17(5):934–947
Yen JY, Yen CF, Chen CS, Tang TC, Huang TH, Ko CH (2011) Cue-induced positive motivational implicit response in young adults with Internet gaming addiction. Psychiatry Res 190(2–3):282–286
Winstanley CA, Eagle DM, Robbins TW (2006) Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev 26(4):379–395
Lee HW, Choi JS, Shin YC, Lee JY, Jung HY, Kwon JS (2012) Impulsivity in Internet addiction: a comparison with pathological gambling. Cyberpsychol Behav Soc Netw 15(7):373–377
Gentile DA, Choo H, Liau A, Sim T, Li D, Fung D, Khoo A (2011) Pathological video game use among youths: a two-year longitudinal study. Pediatrics 127(2):e319–e329
Torregrossa MM, Quinn JJ, Taylor JR (2008) Impulsivity, compulsivity, and habit: the role of orbitofrontal cortex revisited. Biol Psychiatry 63(3):253–255
Zhou Z, Yuan G, Yao J (2012) Cognitive biases toward Internet game-related pictures and executive deficits in individuals with an Internet game addiction. PLoS ONE 7(11):e48961
Sun DL, Chen ZJ, Ma N, Zhang XC, Fu XM, Zhang DR (2009) Decision-making and prepotent response inhibition functions in excessive Internet users. CNS Spectr 14(2):75–81
Tejado Lde A, Ruiz RM, Trebbau H, Diaz-Marsa M, Perera JL (2010) Functional magnetic resonance studies in eating behavior disorders. Actas Esp Psiquiatr 38(3):183–188
Criaud M, Boulinguez P (2013) Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neurosci Biobehav Rev 37(1):11–23
Chambers CD, Garavan H, Bellgrove MA (2009) Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev 33(5):631–646
Li CS, Yan P, Sinha R, Lee TW (2008) Subcortical processes of motor response inhibition during a stop signal task. Neuroimage 41(4):1352–1363
Isoda M, Hikosaka O (2011) Cortico-basal ganglia mechanisms for overcoming innate, habitual and motivational behaviors. Eur J Neurosci 33(11):2058–2069
Everitt BJ, Hutcheson DM, Ersche KD, Pelloux Y, Dalley JW, Robbins TW (2007) The orbital prefrontal cortex and drug addiction in laboratory animals and humans. Ann NY Acad Sci 1121:576–597
Goldstein RZ, Volkow ND (2002) Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159(10):1642–1652
Yeung N, Summerfield C (2012) Metacognition in human decision-making: confidence and error monitoring. Philos Trans R Soc Lond B Biol Sci 367(1594):1310–1321
Shiels K, Hawk LW Jr (2010) Self-regulation in ADHD: the role of error processing. Clin Psychol Rev 30(8):951–961
Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK (2005) Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci 25(50):11730–11737
Hester R, Fassbender C, Garavan H (2004) Individual differences in error processing: a review and reanalysis of three event-related fMRI studies using the GO/NOGO task. Cereb Cortex 14(9):986–994
Taylor SF, Stern ER, Gehring WJ (2007) Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist 13(2):160–172
Luijten M, van Meel CS, Franken IH (2011) Diminished error processing in smokers during smoking cue exposure. Pharmacol Biochem Behav 97(3):514–520
de Bruijn ER, Grootens KP, Verkes RJ, Buchholz V, Hummelen JW, Hulstijn W (2006) Neural correlates of impulsive responding in borderline personality disorder: ERP evidence for reduced action monitoring. J Psychiatr Res 40(5):428–437
Ruchsow M, Walter H, Buchheim A, Martius P, Spitzer M, Kachele H, Gron G, Kiefer M (2006) Electrophysiological correlates of error processing in borderline personality disorder. Biol Psychol 72(2):133–140
Hester R, Nestor L, Garavan H (2009) Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology 34(11):2450–2458
Ko CH, Yen JY, Chen SH, Yang MJ, Lin HC, Yen CF (2009) Proposed diagnostic criteria and the screening and diagnosing tool of Internet addiction in college students. Compr Psychiatry 50(4):378–384
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Herqueta T, Baker R, Dunbar GC (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(Suppl 20):22–33; (quiz: 34–57)
Chen SH, Weng LC, Su YJ, Wu HM, Yang PF (2003) Development of Chinese Internet addiction scale and its psychometric study. Chin J Psychol 45(3):279–294
Li CS, Chen SH (2007) Obsessive-compulsiveness and impulsivity in a non-clinical population of adolescent males and females. Psychiatry Res 149(1–3):129–138
Patton JH, Stanford MS, Barratt ES (1995) Factor structure of the Barratt impulsiveness scale. J Clin Psychol 51(6):768–774
Dickman SJ (1990) Functional and dysfunctional impulsivity: personality and cognitive correlates. J Pers Soc Psychol 58(1):95–102
Graf H, Abler B, Freudenmann R, Beschoner P, Schaeffeler E, Spitzer M, Schwab M, Gron G (2011) Neural correlates of error monitoring modulated by atomoxetine in healthy volunteers. Biol Psychiatry 69(9):890–897
Brett M, Anton JL, Valabregue R, Poline JB (2002) Region of interest analysis using an SPM toolbox. Dissertation, 8th International conference on functional mapping of the human brain
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15(1):273–289
Bossaerts P (2010) Risk and risk prediction error signals in anterior insula. Brain Struct Funct 214(5–6):645–653
Aron AR, Poldrack RA (2005) The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol Psychiatry 57(11):1285–1292
Kadota H, Sekiguchi H, Takeuchi S, Miyazaki M, Kohno Y, Nakajima Y (2010) The role of the dorsolateral prefrontal cortex in the inhibition of stereotyped responses. Exp Brain Res 203(3):593–600
Asahi S, Okamoto Y, Okada G, Yamawaki S, Yokota N (2004) Negative correlation between right prefrontal activity during response inhibition and impulsiveness: a fMRI study. Eur Arch Psychiatry Clin Neurosci 254(4):245–251
Boehler CN, Appelbaum LG, Krebs RM, Hopf JM, Woldorff MG (2010) Pinning down response inhibition in the brain–conjunction analyses of the stop-signal task. Neuroimage 52(4):1621–1632
Blasi G, Goldberg TE, Weickert T, Das S, Kohn P, Zoltick B, Bertolino A, Callicott JH, Weinberger DR, Mattay VS (2006) Brain regions underlying response inhibition and interference monitoring and suppression. Eur J Neurosci 23(6):1658–1664
Wolf RC, Thomann PA, Sambataro F, Vasic N, Schmid M, Wolf ND (2012) Orbitofrontal cortex and impulsivity in borderline personality disorder: an MRI study of baseline brain perfusion. Eur Arch Psychiatry Clin Neurosci 262(8):677–685
Sheinkopf SJ, Lester BM, Sanes JN, Eliassen JC, Hutchison ER, Seifer R, Lagasse LL, Durston S, Casey BJ (2009) Functional MRI and response inhibition in children exposed to cocaine in utero. Preliminary findings. Dev Neurosci 31(1–2):159–166
Fryer SL, Tapert SF, Mattson SN, Paulus MP, Spadoni AD, Riley EP (2007) Prenatal alcohol exposure affects frontal-striatal BOLD response during inhibitory control. Alcohol Clin Exp Res 31(8):1415–1424
Menon V, Uddin LQ (2010) Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214(5–6):655–667
Klein TA, Endrass T, Kathmann N, Neumann J, von Cramon DY, Ullsperger M (2007) Neural correlates of error awareness. Neuroimage 34(4):1774–1781
Botvinick MM (2007) Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci 7(4):356–366
Agam Y, Joseph RM, Barton JJ, Manoach DS (2010) Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders. Neuroimage 52(1):336–347
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27(9):2349–2356
Anderson BM, Stevens MC, Meda SA, Jordan K, Calhoun VD, Pearlson GD (2011) Functional imaging of cognitive control during acute alcohol intoxication. Alcohol Clin Exp Res 35(1):156–165
Klein TA, Ullsperger M, Danielmeier C (2013) Error awareness and the insula: links to neurological and psychiatric diseases. Front Hum Neurosci 7:14
Li CS, Luo X, Yan P, Bergquist K, Sinha R (2009) Altered impulse control in alcohol dependence: neural measures of stop signal performance. Alcohol Clin Exp Res 33(4):740–750
Zhou Y, Lin FC, Du YS, Qin LD, Zhao ZM, Xu JR, Lei H (2011) Gray matter abnormalities in Internet addiction: a voxel-based morphometry study. Eur J Radiol 79(1):92–95
Katsyri J, Hari R, Ravaja N, Nummenmaa L (2013) The opponent matters: elevated fMRI reward responses to winning against a human versus a computer opponent during interactive video game playing. Cereb Cortex 23(12):2829–2839
Acknowledgments
The present study was supported by Grants from the National Science Council, Taiwan (NSC 98-2410-H-037-007), and the Kaohsiung Municipal Hsiao-Kang Hospital (KMHK-98-001).
Conflict of interest
Dr. Chih-Hung Ko received research grants from the National Science Council, Kaohsiung Medical University, and Kaohsiung Municipal Hsiao-Kang Hospital. These institutions had no role in the design, process, analyses, and production of the present study. The other authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ko, CH., Hsieh, TJ., Chen, CY. et al. Altered brain activation during response inhibition and error processing in subjects with Internet gaming disorder: a functional magnetic imaging study. Eur Arch Psychiatry Clin Neurosci 264, 661–672 (2014). https://doi.org/10.1007/s00406-013-0483-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-013-0483-3