Abstract
Via influencing brain plasticity, aerobic exercise could contribute to the treatment of schizophrenia patients. As previously shown, physical exercise increases hippocampus volume and improves short-term memory. We now investigated gray matter density and brain surface expansion in this sample using MRI-based cortical pattern matching methods. Comparing schizophrenia patients to healthy controls before and after 3 months of aerobic exercise training (cycling) plus patients playing table football yielded gray matter density increases in the right frontal and occipital cortex merely in healthy controls. However, respective exercise effects might be attenuated in chronic schizophrenia, which should be verified in a larger sample.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In schizophrenia, brain structural differences can be observed in vivo via magnetic resonance imaging [7] or post-mortem analyses [1]. These changes occur early, demonstrating some degree of progression [3, 25] and following a specific and more severe pattern compared to bipolar disorders [7, 13]. Whether these changes are merely a consequence of the illness [1], treatment related [11] or due to life style remains unresolved. Structural changes may be rather related to neuropil components such as synapses than neurodegeneration [8, 23] and reversible by nature.
Based on the aforementioned findings we recently initiated a clinical trial studying the influence of aerobic exercise on hippocampal structure and function in schizophrenia. Compared with pre-treatment baseline values, we observed a significantly increased relative hippocampal volume at post-treatment follow-up in patients (12 %) and healthy subjects (16 %), compared to patients not participating in aerobic exercise (−1 %) [18]. Currently, the effect of regular exercise on cortical volumes in schizophrenia is unknown. However, interestingly, it has been shown that cardiovascular fitness increases cortical brain volumes during aging in subjects without psychiatric disorders [4]. Therefore, to determine whether aerobic training leads to more widespread neocortical changes affecting also regions outside the hippocampus in schizophrenia patients, we used MRI-based cortical pattern matching methods. We hypothesize that exercise induces gray matter density and cortical expansion increases as a sign of neuroplastic processes in schizophrenia patients and healthy controls.
Materials and methods
A randomized controlled three-armed study was performed to determine whether exercise increases gray matter density and surface expansion. This study is presenting additional MRI analyses from a randomized controlled three-armed study which has been previously performed by our group and revealed increased hippocampal volumes after aerobic exercise training [18]. Male patient’s with schizophrenia attending a day hospital program or an outpatient clinic were randomized to either aerobic exercise training (cycling) (N = 8, age 32.9 ± 10.6 years) or table football as control group (N = 8, age 37.4 ± 8.1 years) for 3 × 30 min weekly over a period of 3 months. All patients underwent treatment with either first or second generation antipsychotics with no differences in dosage or duration of illness between groups. Cycling at heart rate corresponding to a blood lactate concentration of 1.5−2 mmol/L was performed on standardized bikes in a local gym and the amount of exercise was monitored and adjusted by measuring power (W/kg) heart rate and gas exchange (VO2, carbon dioxide output) [18].
Both group of patients were compared to an age- and gender-matched group of healthy subjects engaged in aerobic exercise (cycling) for the same period (N = 8, age 34.8 ± 10.2 years). As primary outcome parameter, magnetic resonance imaging of the cortex (1.5 T) and, as secondary outcome parameter of the present study, assessment of neuropsychological (Rey Auditory Verbal Learning Test, Corsi block tapping test) and clinical symptoms (PANSS) were performed at baseline and after a 3 months training period with raters blinded for treatment arms. In an additional analysis, we compared brain structural changes of exercising schizophrenia patients and healthy controls to schizophrenia patients playing table football using algorithms matching cortical pattern to achieve accurate inter-subject cortical registrations [24]. Gray matter density (GMD) and brain expansion were measured to determine local longitudinal brain changes during the training period. After automated tissue segmentation, anatomical landmark curves following major sulci were manually traced. Cortical surfaces and curves were elastically warped to each other by matching individual curves to their average positions. Co-registered surface models were transformed back to each individual scan’s native space for sampling gray matter density and cortical surface expansion. Local GMD was calculated as the proportion of gray matter volume within a sphere of 15 mm radium at each surface point. Surface expansion was defined as the distance between baseline and follow-up surface models at corresponding surface points, which were assigned to the surface models. The obtained GMD and surface expansion maps were transformed to the standard space for statistical analysis. The study was approved by the local Ethics Committee [18].
Results
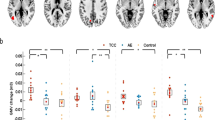
After exercise training, healthy controls showed a significant increase in gray matter density in the right frontal and occipital pole regions (p = 0.032 in permutation tests, Fig. 1). Figure 2 represents uncorrected p values across the cortical surface (red indicating p < 0.05, white indicating p < 0.01). For surface expansion, in controls we noticed a trend to show greater expansion in the left lateral surface compared with schizophrenia patients playing table football, although this difference did not reach significance in permutation tests (p = 0.088, Fig. 3). Overall, aerobic exercise had no significant effect on cortical regions in schizophrenia patients. The maximum power (W/kg) and oxygen capacity (VO2/kg) after study and the ratios before/after study did not differ between patients and controls and were not correlated with GMD, SIENA-based expansion and radial distance change across the cortex. Patients improved in short-term memory and PANSS total symptoms after exercise. [18].
Discussion
While in the same sample Pajonk et al. [18] were able to demonstrate volume expansion in the hippocampus of chronic schizophrenia patients after physical exercise, the present investigation of cortical areas found no exercise-induced changes. As our sample size in the aerobic exercise condition was small, it is possible that effects in favour of cortical gray matter increases could be detected with larger patient samples. Despite an equivalent sample size, our adult healthy controls showed significantly increased gray matter densities in the right frontal and occipital cortex in response to exercise, suggesting that any exercise effects on cortical gray matter are likely to be attenuated in chronic schizophrenia. As opposed to schizophrenia patients with disturbances of synaptic plasticity [22], we hypothesize a better cortical neuroplastic capacity in healthy probands, which probably are more sensitive to effects of exercise. Our results in healthy young adults confirm earlier findings in healthy elderly individuals demonstrating an association between physical activity and increases in local gray matter volumes of the prefrontal cortex [21] as well as larger superior frontal cortex volumes in individuals with higher levels of exercise engagement [2]. In contrast, regular exercise had no effects on these cortical areas in a group of young healthy students [10]. However, such individuals also show high levels of cognitive training and intellectual activities, which is known to influence gray matter volumes [6]. With respect to the occipital cortex, a previous 1H-MRS study detected increased lactate levels and increased glutamate and glutamine signals following exercise [17], suggesting that biochemical alterations may underlie gray matter density alterations in healthy probands. We could not confirm alterations in the right anterior insula as suggested by findings of increased volume in this region in healthy young subjects with higher functional aerobic capacity [19], since the insula region cannot be measured with our method.
Gray matter density alterations in brain areas may be related to functional consequences. Accordingly, a functional near-infrared spectroscopy study in young adults revealed that exercise enhances activation of the left dorsolateral prefrontal cortex associated with Stroop interference [28]. In older adults, activation of the right frontopolar area was enhanced after exercise [12]. Within this context muscle contractions are known to activate a cortical network including the prefrontal area [15]. Consistent with results obtained in older adults, functional MRI studies in obese children also reveal increased bilateral prefrontal cortex activity after exercise [5]. Finally, physical activity has shown to improve memory encoding in healthy elderly subjects and to induce an increase of cerebral gray matter volume in the prefrontal cortex [9]. In monkeys, regular exercise improved working memory performance and vascularization of the cortex [20]. Despite the absence of cortical volume changes in our group of chronic schizophrenia patients, this group may benefit from brain activation due to exercise such as improvement of short-term memory and symptoms [18]. These results are in accordance with previous studies in a larger group of patients showing that functional exercise capacity correlates with improvements in global functioning, total PANSS scores, positive-, negative- and cognitive symptoms [14, 26, 27].
In summary, we found targeted exercise leading to increased right frontal and occipital cortical gray matter density in healthy subjects, but not in schizophrenia patients. However, due to the lacking relationship between changes in aerobic fitness and gray matter densities, the effects of exercise in healthy controls should be interpreted with caution and validated in a larger sample. Nevertheless our results indicate that aerobic exercise may affect the hippocampus, but not the cortex in persons suffering from schizophrenia. Antipsychotic medication might have some influence on cortical, but not so much on hippocampal volumes [11, 16] and regeneration capacity may be attenuated in patients. In light of the limitations of our study, i.e. the small number of cases, effects of physical exercise on volumes of cortical regions should be investigated in larger samples of patients with mental illness to more exhaustively explore regenerative mechanisms of this therapeutic regime. Additionally, parallel MR-spectroscopy should be performed to assess neurochemical compounds in the regions which have been identified here.
References
Bogerts B, Falkai P, Haupts M, Greve B, Ernst S, Tapernon-Franz U, Heinzmann U (1990) Post-mortem volume measurements of limbic system and basal ganglia structures in chronic schizophrenics. Initial results from a new brain collection. Schizophr Res 3:295–301
Bugg JM, Head D (2011) Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiol Aging 32:506–514
Cahn W, Rais M, Stigter FP, van Haren NE, Caspers E, Hulshoff Pol HE, Xu Z, Schnack HG, Kahn RS (2009) Psychosis and brain volume changes during the first five years of schizophrenia. Eur Neuropsychopharmacol 19:147–151
Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF (2003) Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sc 58:176–180
Davis CL, Tomporowski PD, McDowell JE, Austin BP, Miller PH, Yanasak NE, Allison JD, Naglieri JA (2011) Exercise improves executive function and achievement and alters brain activation in overweight children: a randomized, controlled trial. Health psychol 30:91–98
Eack SM, Hogarty GE, Cho RY, Prasad KM, Greenwald DP, Hogarty SS, Keshavan MS (2010) Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia: results from a 2-year randomized controlled trial. Arch Gen Psychiatry 67:674–682
Ellison-Wright I, Bullmore E (2010) Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr Res 117:1–12
Falkai P, Honer WG, David S, Bogerts B, Majtenyi C, Bayer TA (1999) No evidence for astrogliosis in brains of schizophrenic patients. A post-mortem study. Neuropathol Appl Neurobiol 25:48–53
Floel A, Ruscheweyh R, Kruger K, Willemer C, Winter B, Volker K, Lohmann H, Zitzmann M, Mooren F, Breitenstein C, Knecht S (2010) Physical activity and memory functions: are neurotrophins and cerebral gray matter volume the missing link? Neuroimage 49:2756–2763
Gondoh Y, Sensui H, Kinomura S, Fukuda H, Fujimoto T, Masud M, Nagamatsu T, Tamaki H, Takekura H (2009) Effects of aerobic exercise training on brain structure and psychological well-being in young adults. J Sports Med Phys Fit 49:129–135
Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V (2011) Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry 68:128–137
Hyodo K, Dan I, Suwabe K, Kyutoku Y, Yamada Y, Akahori M, Byun K, Kato M, Soya H (2012) Acute moderate exercise enhances compensatory brain activation in older adults. Neurobiol Aging
Kempton MJ, Geddes JR, Ettinger U, Williams SC, Grasby PM (2008) Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry 65:1017–1032
Knochel C, Oertel-Knochel V, O’Dwyer L, Prvulovic D, Alves G, Kollmann B, Hampel H (2012) Cognitive and behavioural effects of physical exercise in psychiatric patients. Prog Neurobiol 96:46–68
Kwon YH, Park JW (2011) Different cortical activation patterns during voluntary eccentric and concentric muscle contractions: an fmri study. Neurorehabilitation 29:253–259
Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, Keefe RS, Green AI, Gur RE, McEvoy J, Perkins D, Hamer RM, Gu H, Tohen M (2005) Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry 62:361–370
Maddock RJ, Casazza GA, Buonocore MH, Tanase C (2011) Vigorous exercise increases brain lactate and glx (glutamate + glutamine): a dynamic 1 h-mrs study. Neuroimage 57:1324–1330
Pajonk FG, Wobrock T, Gruber O, Scherk H, Berner D, Kaizl I, Kierer A, Muller S, Oest M, Meyer T, Backens M, Schneider-Axmann T, Thornton AE, Honer WG, Falkai P (2010) Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry 67:133–143
Peters J, Dauvermann M, Mette C, Platen P, Franke J, Hinrichs T, Daum I (2009) Voxel-based morphometry reveals an association between aerobic capacity and grey matter density in the right anterior insula. Neuroscience 163:1102–1108
Rhyu IJ, Bytheway JA, Kohler SJ, Lange H, Lee KJ, Boklewski J, McCormick K, Williams NI, Stanton GB, Greenough WT, Cameron JL (2010) Effects of aerobic exercise training on cognitive function and cortical vascularity in monkeys. Neuroscience 167:1239–1248
Ruscheweyh R, Willemer C, Kruger K, Duning T, Warnecke T, Sommer J, Volker K, Ho HV, Mooren F, Knecht S, Floel A (2011) Physical activity and memory functions: an interventional study. Neurobiol Aging 32:1304–1319
Schmitt A, Hasan A, Gruber O, Falkai P (2011) Schizophrenia as a disorder of disconnectivity. Eur Arch Psychiatry Clin Neurosci 261(Suppl 2):S150–S154
Schmitt A, Steyskal C, Bernstein HG, Schneider-Axmann T, Parlapani E, Schaeffer EL, Gattaz WF, Bogerts B, Schmitz C, Falkai P (2009) Stereologic investigation of the posterior part of the hippocampus in schizophrenia. Acta Neuropathol 117:395–407
Thompson PM, Hayashi KM, Sowell ER, Gogtay N, Giedd JN, Rapoport JL, de Zubicaray GI, Janke AL, Rose SE, Semple J, Doddrell DM, Wang Y, van Erp TG, Cannon TD, Toga AW (2004) Mapping cortical change in alzheimer’s disease, brain development, and schizophrenia. Neuroimage 23(Suppl 1):S2–18
van Haren NE, Schnack HG, Cahn W, van den Heuvel MP, Lepage C, Collins L, Evans AC, Hulshoff Pol HE, Kahn RS (2011) Changes in cortical thickness during the course of illness in schizophrenia. Arch Gen Psychiatry 68:871–880
Vancampfort D, Probst M, Scheewe T, Knapen J, De Herdt A, De Hert M (2012) The functional exercise capacity is correlated with global functioning in patients with schizophrenia. Acta Psychiatr Scand 125:382–387
Wolff E, Gaudlitz K, von Lindenberger BL, Plag J, Heinz A, Strohle A (2011) Exercise and physical activity in mental disorders. Eur Arch Psychiatry Clin Neurosci 261(Suppl 2):S186–S191
Yanagisawa H, Dan I, Tsuzuki D, Kato M, Okamoto M, Kyutoku Y, Soya H (2010) Acute moderate exercise elicits increased dorsolateral prefrontal activation and improves cognitive performance with stroop test. Neuroimage 50:1702–1710
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Peter Falkai and Berend Malchow contributed equally.
Rights and permissions
About this article
Cite this article
Falkai, P., Malchow, B., Wobrock, T. et al. The effect of aerobic exercise on cortical architecture in patients with chronic schizophrenia: a randomized controlled MRI study. Eur Arch Psychiatry Clin Neurosci 263, 469–473 (2013). https://doi.org/10.1007/s00406-012-0383-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-012-0383-y