Abstract
Low-frequency repetitive transcranial magnetic stimulation (LF-rTMS) of the left temporo-parietal cortex (LTPC) has been proposed as a useful therapeutic method for auditory hallucinations (AHs). Stereotactic neuronavigation enables the magnetic coil to be targeted according to the individual parameters obtained from neuroimaging. Individualized rTMS neuronavigated according to 18-fluorodeoxyglucose positron emission tomography (18FDG PET) allows us to focus the coil explicitly on a given area with detected maxima of specific abnormalities, thus presuming a higher therapeutic effect of the method. The objective of this study is to test clinical efficacy of neuronavigated LF-rTMS administered according to the local maxima of 18FDG PET uptake of LTPC and to compare it with treatment effects of standard and sham rTMS. In a double-blind, sham-controlled design, patients with AHs underwent a 10-day series of LF-rTMS using (1) 18FDG PET-guided “neuronavigation,” (2) “standard” anatomically guided positioning, and (3) sham coil. The effect of different rTMS conditions was assessed by the Auditory Hallucinations Rating Scale (AHRS) and the Positive and Negative Syndrome Scale (PANSS). Fifteen patients were randomized to a treatment sequence and ten of them completed all three treatment conditions. The intention-to-treat analysis of AHRS score change revealed superiority of the 18FDG PET-guided rTMS over both the standard and the sham rTMS. The analyses of the PANSS scores failed to detect significant difference among the treatments. Our data showed acute efficacy of 18FDG PET-guided rTMS in the treatment of AHs. Neuronavigated rTMS was found to be more effective than standard, anatomically guided rTMS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The clinical effect of low-frequency repetitive transcranial magnetic stimulation (LF-rTMS) (≤1 Hz) on auditory hallucinations (AHs) in schizophrenia has been documented by several controlled trials [1–5]. The rationale of this treatment is based on the fact that LF-rTMS inhibits the cortical excitability and leads to a weakening of the synaptic transfer [6–8]. This assumption inspired Hoffman et al., who reported that the excessive activation of speech perception areas of the cerebral cortex connected with the symptomatology of AHs [9] could be suppressed by 1 Hz rTMS [8]. This initial report of the beneficial effects of rTMS on hallucinations was followed by subsequent studies, which found low-frequency rTMS focused on the supposed hyper-activated temporo-parietal area to be effective in the treatment [10, 11]. These studies also documented the influence of rTMS on regional brain metabolism [12] in the given area and its successive modulations in transsynaptically connected areas (cortical and subcortical regions except temporo-parietal regions in the dominant hemisphere), which were previously demonstrated in PET studies [12–22].
The majority of rTMS studies on AHs were based on the assumption that the cortical overactivity is in a region below the coil placed over the scalp according to the skull coordinates of the international 10/20 EEG electrode system (midway between the left T3 and P3 sites). This area is assumed to be represented by the posterior border of the superior temporal gyrus (STG) (Wernicke area) and in our article is denoted as a “standard” coil position for anatomically guided intervention trials [1–5, 7, 8, 23].
However, there is a substantial gap between this standard anatomic positioning of rTMS and recent neuroimaging (fMRI) findings in Ahs [9, 24–27]. These studies documented that the individual local maxima of cortical activity are not entirely explicitly registered in the given area below the standard coil positioning but are often registered in areas that are a part of the auditory cortico-subcortical network, such as the right/left middle temporal gyrus [9, 25], Broca’s area [9], the left inferior parietal gyrus [26], the anterior cigulate [27], or the right thalamus and hippocampus [9, 27]. Hence, the main challenge in rTMS is the precise targeting of the coil in the cortical area of maximal functional changes underlying the psychopathology.
Stereotactic neuronavigation is a technology enabling the coil to be aimed with a high (millimeter) degree of anatomic accuracy [28, 29] together with the ability to target the coil according to individual physiological parameters obtained from functional neuroimaging [30]. Neuronavigated rTMS targeted according to brain metabolism detected by 18fluoro-deoxyglucose (18FDG) positron emission tomography (PET) could be a suitable method to specifically modulate the metabolic abnormalities [15, 16, 31] related anatomically to the changes in cortical areas of auditory processing [15]. PET studies in schizophrenia patients document time consistent findings of regional brain metabolism [32], which reflect the association with long-lasting clinical findings and resting hallucination-related activity detected even at times with an absence of hallucinations while scanning [18].
The primary objective of our double-blind, sham-controlled study was to prove the clinical effect of rTMS targeted according to the local maxima of resting regional brain metabolism (18FDG PET) in comparison with standard positioning of the rTMS coil and inactive “sham” stimulation in the treatment of AHs. We limited the neuronavigated rTMS to the left acoustic-linguistic cortex considering three critical aspects: (a) the clinical efficacy within this region was documented in previous non-navigated rTMS studies, (b) this region is accessible to scalp-administered rTMS [8], and c) rTMS administered to this region is suitable for double-blind trials. In the consequent analyses, we also confirmed the increased metabolism in this area of interest, that is, the left temporo-parietal cortex (LTPC). Finally, we evaluated the anatomical distance between the cortical area underlying the standard coil position and the local maxima of metabolism in all individual patients.
Methods
Subjects
The group of 15 patients (Table 1) with paranoid schizophrenia diagnosed according to DSM-IV criteria (American Psychiatric Association 1994) by two experienced psychiatrists was recruited from Prague Psychiatric Centre (Czech Republic). All patients fulfilled the criteria for treatment-resistant AHs defined as non-response to at least one conventional and one atypical antipsychotic adequate trial and at least 5 episodes of AHs per day during the past month [12]. They received the following antipsychotics in stable doses: (1) antipsychotics in monotherapy: olanzapine N = 2, clozapine N = 1, haloperidol N = 3, flupentixol N = 1, amisulpride N = 1, (2) combination of antipsychotics: olanzapine + haloperidol N = 1, clozapine + haloperidol N = 1, clozapine + risperidone N = 1, risperidone + haloperidol N = 1, flupentixol + quetiapine N = 1, clozapine + flupentixol + chlorpromazine N = 1 and haloperidol + levomepromazine N = 1. Other concomitant treatment included: (1) antidepressants: SSRI N = 7, tricyclic ADs N = 1; (2) anticonvulsants: valproate N = 1, carbamazepine N = 1; (3) anxiolytics: clonazepam N = 6. Standard physical examination, medical history evaluation, biochemistry, ECG, and EEG were performed to exclude neurological or medical illness, and drug or alcohol abuse. All participants signed an informed consent in accordance with the latest version of the Declaration of Helsinki, and the local ethics committee approved the study.
Study protocol and neuroimaging
Using a double-blind, sham-controlled cross-over design, we compared the clinical effect of LF-rTMS using (1) neuronavigated positioning of the coil, (2) standard stimulation with coil positioning according to scull anatomy [8], and (3) inactive (sham) rTMS. The setting order of three rTMS series was randomized in two steps. In the first step, the patients (N = 15) were randomized into standard (N = 6) or sham (N = 9) rTMS to exclude placebo responders and subjects who maintained response after the defined washout period following the standard treatment. The PET scanning was arranged between the first and second randomization step. This approach enabled effective blinding of patients with a shorter period between brain imaging and the neuronavigated rTMS. Those patients who maintained a response after the defined washout period were excluded from the second step randomization. In the second step, the standard initiated treatment was followed by a sham → navigation or navigation → sham sequence. The sham initiated treatment was followed by a sequence of standard → navigation or navigation → standard. Neuronavigated rTMS as the second step in a sequence of rTMS treatments was used because of its hypothesized therapeutic superiority (Fig. 1).
Each series of rTMS consisted of 10 rTMS sessions over 10 consecutive working days within 2 weeks. During rTMS, antipsychotic medications were continued without dose changes for at least 4 weeks before and throughout the rTMS treatment period. The washout period (T) between therapeutic trials of separate rTMS series was classified as a minimum of 6 weeks (median T1/T2 = 157.5 days, T2/T3 = 63.5 days), with respect to (1) an individual effect duration of separate rTMS series [33, 34] and also with respect to (2) current knowledge of the long-term efficiency (over a number of weeks) administrated in further rTMS studies [2, 4]. The double-blind design was concepted that the study participants and clinical raters remained blind to allocated condition and allocation parameters. The physician applying rTMS was not blind to the treatment conditions.
The study was approved by the local ethics committee and included as the subproject of project 1M0517 from the Ministry of Education, the Czech Republic.
rTMS administration
Standard rTMS
Parameter settings for each rTMS series were as follows: 0.9 Hz of low-frequency rTMS at 100 % motor threshold, 1080 pulses/each session, 10 rTMS sessions over 10 days within 2 weeks. We chose the frequency 0.9 Hz (instead of 1 Hz) to ensure an inhibitory effect on the brain cortex. The inhibitory effect of 0.9 Hz rTMS on cortical connectivity or functional coupling has been clearly documented by EEG and 18FDG PET as well [12].
Motor threshold was assessed as the lowest strength of TMS needed to elicit 5 or more electromyographic responses (EMG, Neurosign 400) ≥50 μV within ten trials [35]. The coil was administered over the left temporo-parietal region defined as the midway between the T3 and the P3 sites according to the international 10/20 EEG electrode system for “standard” anatomically guided positioning [1, 7]. For the rTMS treatment, we used Magstim Super Rapid equipment (Magstim, Whitland, UK) with an air-cooled, figure-of-eight 70-mm coil.
Neuronavigated rTMS
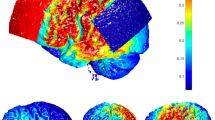
The same parameters of rTMS as in the standard stimulation were used for neuronavigated trial, except the positioning of the coil, which was administered with the assistance of the Brainsight Frameless Neuronavigation System (Rogue Research Inc., Montreal, Canada), designed specifically for rTMS. This stereotactic system consists of a 3D infrared camera detecting triangularly arranged trackers with a reflective surface mounted on the head of the subject and on the coil. Each session started by a co-registration of three modalities: (1) the subject’s head position, (2) the coil placement, and (3) the 3D graphic model representing the overlay of MRI and local maxima of 18FDG PET uptake. This approach enabled real-time stereotactic monitoring of the coil location and gave us exact information on the coil position on the head of the subject during the entire stimulation. The focus point of the coil was targeted to the individual cluster contrasting the highest metabolic activity (in comparison with control group) within the region of LTPC (Fig. 2). LTPC has been anatomically defined as a surface region over the superior, middle, and inferior temporal gyrus, and the supramarginal and angular gyrus.
Cortical location targeted by neuronavigated rTMS, where the coil was focused according to individual local maxima in the area of left temporo-parietal cortex. Cortical location focused by neuronavigational-guided rTMS: Response to neuronavigated rTMS with reduction ≥50 % (N = 6), Response to neuronavigated rTMS with reduction ≤50 % (N = 6), Cortical location focused by standard rTMS coil placement (T3–P3)
Sham rTMS
Sham stimulation was administered to the same location, strength, and frequency as the standard positioning, but during the sham condition, the coil was angled 90 degrees away from the skull in a single-wing tilt position. This method reproduced sound similar to the active stimulation and some somatic sensations with minimal direct brain effects [8].
Psychometric measurement
The psychometric assessments were conducted before the first stimulation started (W0), after the first week (W1) and after 2 weeks of treatment, that is, at the end of each rTMS series (W2), or in the case of withdrawal from the study. The clinical effect was assessed by the Auditory Hallucination Rating Scale (AHRS) [1, 36] as a primary outcome measure and the Positive and Negative Syndrome Scale (PANSS) [37, 38] by a rater blind to the treatment condition. The separate items for the AHRS are represented as AHRS 1 = frequency, AHRS 2 = reality, AHRS 3 = loudness, AHRS 4 = number of voices, AHRS 5 = length, AHRS 6 = attentional salience, and AHRS 7 = distress level. A responder was defined by a reduction in total AHRS score by least 50 % of the baseline [39].
Magnetic resonance imaging (MRI) investigation
Magnetic resonance images were obtained with a 3T system Siemens Trio (Siemens, Erlangen, Germany). Subjects were scanned using a T1-weighted (T1 W) 3D-MPRAGE sequence (repetition time TR of 2,000 ms; echo time TE of 4.4 ms; bandwidth 130 Hz/pixel; field of view 256 mm; matrix 256 × 256; slice thickness 1 mm; 160 contiguous sagittal slices; voxel size of 1 × 1×1 mm and total acquisition time 8 min).
18FDG PET investigation and pre-processing
Patients were examined with PET during time period between the beginning of neuronavigated rTMS and the previous standard or sham rTMS (median = 7 month). The participants (patients and controls) were fasted for at least 6 h before the PET investigation. In a dimly lit and quiet room, 3 MBq/kg of 18FDG was administered via a peripheral vein catheter. The participants rested for 30 min in a specified condition that is described as random episodic silent thinking (REST). The REST produces favorable reliability in PET findings for a schizophrenic population [40]. The data were acquired using the ECAT EXACT 922 (CTI/Siemens, Knoxville, Tenn., USA) PET scanner. The 2D ‘hot’ transmission scans were immediately followed by 3D emission scanning which lasted 15 min. The data were reconstructed by an iterative OS-EM algorithm (matrix 1,282, brain mode 47 slices, zoom 2, subsets 16, iterations 6, Hann filter 5 mm) and implemented using ECAT 7.2 software.
The pre-processing (and data analysis) was performed using Statistical Parametric Mapping, SPM5 (http://www.fil.ion.ucl.ac.uk/spm) implemented in Matlab (MathWorks, Natick, Mass., USA). The PET scans were normalized into standard stereotactic space and smoothed with an isotropic Gaussian filter (full width at half maximum of 12 mm).
Individualized regional cerebral metabolism (18FDG PET) analysis
To detect the local maxima of brain metabolism within an area of temporo-parietal cortex, we used individualized subject-versus-group 18FDG PET analysis based on the general linear model [41]. This approach has been validated to detect individual characteristics of regional brain metabolism in psychiatric and neurological disorders [42]. In a typical example, the individualized SPM analysis of 18FGD uptake showed the excellent sensitivity in the detection of epileptogenic foci [41–43].
For individualized 18FDG PET analyses, we used a group of 19 control subjects (M/F = 10/9, mean age = 37,0, SD = 12,2) from the Prague Psychiatric Center PET database of control subjects screened for the absence of any neuropsychiatric disorder and absence of any medication. The selection of control group was performed after the completion of the first step of randomized rTMS (vs. sham) treatment. At that time, the demographic characteristics for the final sample of patients with acoustic hallucination who continued with neuronavigated (vs. sham or standard) rTMS were defined. Subsequently, the sample of control subjects was selected from a similar sociodemographic background (Caucasians only form the urban area of Prague only) and respecting the age (t = 0.25, p = 0.80) and gender characteristics (χ2 = 0.19, p = 0.67) of the whole schizophrenia sample. To detect the local maxima of brain metabolism within an area of temporo-parietal cortex, we used individualized subject-versus-group PET analysis comparing each patient with schizophrenia with the whole control sample.
The group of control subjects, patients with AHs, and covariate effects were estimated according to the general linear model at the entire brain volume. The global activity, age, and chlorpromazine equivalents were used as confounding covariates [41]. The t statistics was used to detect the difference between the mean regional glucose utilization of the control group and the regional glucose utilization of the subject with AHs, divided by the error estimated with the control group data (after correcting for confounding covariates). The p values at a voxel level of ≤0.05 with FDR (false discovery rate) correction with a minimum of 10 voxels per cluster (extent threshold) were considered statistically significant for the predefined region of the temporo-parietal cortex. The global intensity differences were corrected by proportional scaling (global mean to 50, analysis threshold 0.8), and a global calculation was performed by the mean voxel value.
In the next step, we co-registered the individual T-map with a native (non-normalized) MRI image for the purpose of brain navigation. Both the MRI and the T-map in the patient’s native space were overlaid in the brain navigation software, and the rTMS coil was targeted at the local maxima of brain metabolism within the region of LTPC.
Measurement of individual distance between stimulated cortical areas
We measured the individual distance between the standard coil positioning and the coil neuronavigated according to PET contrast for the local maxima of 18FDG uptake as follows: Firstly, Brainsight Frameless Neuronavigation System was used for three-dimensional brain reconstruction generated from the T1 W MRI examination. All subsequent analyses were also performed on a 3D reconstructed brain using the Brainsight software. Secondly, this approach enabled to identify the cortical projection of the focus of rTMS coil during standard positioning treatment was registered on the reconstructed brain surface. Thirdly, the T-maps of SPM5 PET analysis were co-registered with MRI, and the cortical projection of coil position used for neuronavigated rTMS was registered. Finally, the coordinates for cortical projections of standard and neuronavigated coil positioning were exported, and the individual distances were measured using the following equation |AB| = (x a − x b )2 + (y a − y b )2 + (z a − z b )2 (Table 2).
Statistical analyses
The primary efficacy analyses were based on an intent-to-treat (ITT) data set (N = 15), which was defined as the subset of patients who completed a baseline and at least one post-baseline visit (evaluation) with the last observation analysis (LOAN) method. The treatment difference was estimated in a pairwise fashion by the difference in the least square means with 95 % confidence intervals. The primary efficacy measure (AHRS) as well as PANSS total, positive, and negative subscales scores changes over the treatment conditions were tested using a repeated measure of analyses of variance (RM-ANOVA) with the Greenhouse–Geisser adjustment. The sequence as a between-subjects factor and treatment, time and period as the within-subjects factors were considered. Once ANOVA revealed a significant difference, the analysis continued with a Bonferroni post hoc test to discern where specific differences between treatments existed. The differences between treatments were expressed in terms of Cohen’s d as well. Furthermore, the same analyses were performed in the subsample of treatment completers (patients who completed all treatment conditions).
Response to rTMS treatment was defined as ≥50 % reduction in AHRS within a particular treatment period. Relationships between AHs severity changes (AHRS) and neuronavigated-standard position distances were assessed by Spearman’s rank correlation coefficient.
The sample size was calculated using the following parameters: RM-ANOVA, within-between interaction, alpha = 0.05, power = 0.8, number of groups (treatment) = 3, measurement repetitions per treatment = 3, correlations among repeated measures = 0.5, non-sphericity correction ε = 0.6. Under this calculation, the sample size needed to detect the large effect size (f ≤ 0.4) is 12 subjects. All statistical analyses were performed using the STATISTICA 9.0 (Statsoft Inc., Tulsa, OK) software; all tests were two-sided; and p < 0.05 was regarded as being statistically significant.
Results
Subjects
Fifteen patients were enrolled to the study and randomized to a treatment sequence. Ten patients completed all three treatment conditions (Table 1). One patient withdrew because AHs completely and persistently disappeared after standard rTMS (sequence sham-standard-navigated), one patient was excluded due to non-compliance during the first treatment period (sham), and three patients did not met the criteria of stabile antipsychotic medication between the second and the third treatment period (one after standard and two after navigated rTMS) (Fig. 1). Overall, 13 patients completed sham rTMS, 14 patients standard rTMS, and 12 patients finalized the navigated rTMS period. Treatment was generally well tolerated, and no serious side effects were detected.
Measurement of anatomical distance between standard coil positioning and local maxima of 18FDG uptake (PET)
Neuronavigation revealed that the centers of the coil targeting according to the standard procedure (TP3) in thirteen patients were located over Wernicke’s area (N = 5), angular gyrus (N = 4), supramarginal gyrus (N = 3), and superior temporal gyrus (N = 1). Local maxima indicated by PET were found on the superior temporal gyrus (N = 6), the middle temporal gyrus (N = 3), the angular gyrus (N = 1), the supramarginal gyrus (N = 1), Wernicke’s area (N = 1), and the primary auditory cortex (N = 1) (Table 2). When measuring the discrepancy between the area focused by standard coil positioning and PET local maxima for individual patients, we found an overall mean difference of 35.9 mm (95 %CI 27.9–44.0 mm).
Intent-to-treat analysis
The mean change from baseline AHRS total score at the end of treatment was 11.4 (95 %CI 6.8–16.1) in navigated rTMS, 6.3 (95 %CI 2.9–9.6) in standard rTMS, and 0.3 (95 %CI–0.4–1.1) in sham rTMS (Table 3). RM-ANOVA revealed treatment effect (F(2,26) = 7.94, p = 0.002), time effect (F(2,26) = 21.68, p < 0.001) and treatment x time interaction (F(4,52) = 6.26, p < 0.001) irrespectively to sequence (F(1,13) = 0.73, p = 0.41), and period (F(2,26) = 0.08, p = 0.90). In post hoc analysis, superiority of both active treatments over sham (standard vs. sham: p = 0.02; Cohen’s d = 0.79 and navigated vs. sham: p < 0.001; d = 1.52) and a better outcome in navigated compared to standard rTMS period (p = 0.005; d = 1.00) at week 2 was detected (Fig. 3). Subanalysis of particular AHRS items revealed significant differences between navigated rTMS and sham in item 5 “length” (F = 4.16, p = 0.03) and 7 “distress level” (F = 5.02, p = 0.02), but not between active treatment. Concerning the response to the completed treatment series (≥50 % score reduction), we identified no responders after 13 series of sham rTMS, two responders after 14 series (29 %) of standard rTMS, and six responders after twelve (50 %) navigated rTMS series. Coil positions of responders and non-responders to navigated rTMS are shown in Fig. 2.
When analyzing the PANSS positive subscale change scores, we found significant differences among treatments (F(2,26) = 4.66, p = 0.02) but non-significant treatment x time interaction (F(4,52) = 0.67, p = 0.61), despite the absence of a carryover effect. Negative results were found for PANSS total and negative subscale scores.
Completer analysis
The mean change in AHRS total score was 11.6 (95 %CI 5.6–17.6) in navigated rTMS, 6.3 (95 %CI 1.2–11.4) in standard rTMS, and 0.4 (95 %CI 0.5–1.3) in sham rTMS (Table 3). RM-ANOVA detected treatment effect (F(2,16) = 5.86, p = 0.01) time effect (F(2,16) = 14.73, p < 0.001) and treatment x time interaction (F(4,32) = 3.49, p = 0.02) but not the sequence (F(1,8) = 0.26, p = 0.62) or period (F(2,16) = 1.03, p = 0.38) effect. In post hoc analysis, only the superiority of navigated rTMS over sham (p = 0.004; Cohen’s d = 1.44) was found. Differences between other treatment conditions did not achieve statistical significance (navigated vs. standard: p = 0.06; d = 0.96; standard vs. sham: p = 0.09; d = 0.76). A similar analysis did not reveal a significant difference for the PANSS total and subscales scores.
Additionally, we found no significant associations between neuronavigated-standard position distances or the AHRS total change score after neuronavigated period (rho = 0.12) and neuronavigated-standard difference in AHRS score change (rho = 0.10) in twelve patients who completed both active treatment conditions (Table 2).
Discussion
This is the first controlled study confirming the acute effect of rTMS targeted according to the local maxima of brain metabolism within left temporo-parietal region according to 18FDG PET co-registered with structural imaging (MRI). Our findings demonstrated the effect of neuronavigated rTMS in alleviating AHs as measured by the AHRS. Compared to standard and sham rTMS, we found neuronavigated algorithm the most effective of the trial.
Resting 18FDG PET primarily reflects the regional glutamate turnover at the synaptic (particularly, presynaptic) level and provides a probe for relative synaptic strength and consequent metabolic activity [46, 47]. Hence, the 18FDG increased uptake in AHs confirmed by several PET studies [22, 48] is related to the long-term over-activity with consequent changes at the synaptic level.
According to the hypothesis of a fronto-temporal disconnectivity [49], the specific changes represented by increased 18FDG uptake could reflect the hyperactive hallucination-related network. This condition could be efficiently affected by rTMS guided to the local maxima of 18FDG uptake. Our approach to target rTMS according to the local maxima of 18FDG uptake is in accordance with the supposed long-term depression phenomenon induced by LF-rTMS responsible for the inhibitory effect [50, 51] and our previous finding of decreased metabolism in the cortex underlying the coil in LF-rTMS treatment [12].
The post hoc comparison of regional brain metabolism in the whole group of hallucinating patients with controls confirmed the over-metabolism within left temporal and temporo-parietal cortex used as the target for rTMS treatment (Fig. 4).
The increase in regional brain metabolism in the group of schizophrenia patients with auditory hallucinations investigated prior to neuronavigated rTMS in comparison with controls. The most pronounced increase in 18FDG PET uptake was found in the cluster consisting of lentiform nucleus, thalamus and left parahippocampal gyrus bilaterally, bilateral postcentral gyrus and right superior frontal gyrus. Within the area of interest (left acoustic-linguistic cortex), we found the increased metabolism in the middle temporal gyrus, and temporo-parietal junction including supramarginal and angular gyrus (arrows). No regions with decreased metabolism were detected in the group of hallucinating patients. Significant results exceeding the height threshold T = 4.09 (p ≤ 0.05, FDR corrected, cluster ≥100 voxels) are displayed on study-specific 3D template (upper images) and mean image slices (lower images). L or R left or right hemisphere
By measuring the distance between cortical coil projection (focus of magnetic field) in standard positioning and local maxima of metabolism used in our neuronavigated algorithm, we found an average distance of 3.51 ± 0.63 cm. Respecting the fact that the standard range of electromagnetic field in rTMS does not cover the distance of 2 cm [52], we suggest this finding (application of magnetic field away from the place with the highest contrast of metabolic activity) could be a plausible explanation for negative results in previous controlled studies with standard rTMS positioning [23, 53].
Except for various rTMS studies on hallucinating patients which used a neuronavigational system for individual anatomic and functional targeting [24, 33, 39, 54], the magnetic coil was administrated predominantly over the area of standard positioning [1, 2, 7, 23].
Also, the fact that previous controlled studies of neuronavigated rTMS guided by individual fMRI in hallucinatory conditions (except one controlled study using a different algorithm with high-frequency rTMS [39]) revealed negative results [24, 33, 54] supported our idea of using 18FDG PET. Comparing with fMRI, 18FDG PET represents a physiologically highly relevant method to detect and directly quantify the metabolic abnormalities [15, 16, 31] related to AHs [15].
In addition, PET studies with schizophrenic patient document time consistent findings of regional brain metabolism [32]. The resting hallucination-related over-activity is detectable by PET even at periods with an absence of hallucinations while scanning [18]. These findings are connected also with the fact that the temporal resolutions of 18FDG are much longer (45 min.) than those of fMRI (approx. 7 s) or PET using 15O (approx. 2 min.). 18FDG PET reflects longer time period and thus could detect more stable brain abnormality that underlies hallucinations [55]. Moreover, in our previous study, we documented that low-frequency rTMS inhibits the regional 18FDG uptake in AHs [12].
In spite of the mechanism of the rTMS effect, we do not have data on 18FDG uptake after rTMS and so we cannot certainly claim, whether successful reduction in AH is really related to reduction in metabolism at the local maxima of 18FDG uptake. However, our previous findings from the non-navigated LF-rTMS implicate that the effect of rTMS is connected with decreased metabolism in the cortex underlying the rTMS site with propagation by inter- and intrahemispheric connections [12]. These PET and our complementary EEG results indicate that the neuroplastic changes affect the functional laterality and provide the substrate for a metabolic effect. In this context, it is of interest that 18FDG PET-guided rTMS studies in the treatment of tinnitus do not provide evidence for superiority of neuronavigational coil positioning [56] and the clinical effects of rTMS on tinnitus are not related to changes in PET activity [57].
Subanalysis of particular AHRS items also revealed significant differences between navigated and sham rTMS. Analyses of PANSS total and subscales scores did not reveal a significant difference over time. Our findings are in agreement with the results from recent open studies [39, 58], but compared to previous controlled studies [2, 23, 24, 33, 54], we found rTMS more efficient on hallucination withdrawal.
The limitation of our study was the processing of two-step randomization (Fig. 1). However, analysis of order effect did not demonstrate a significant effect on the final results. A potential source of false-positive ratings overestimating the navigated trial was represented by the standard initiated group (standard → navigation → sham), where the treatment order of two consecutive active stimulations (standard → navigation) would potentially overvalue the effect of rTMS navigation. Nevertheless, this fact was minimized by the number of patients from the standard initiated group (N = 6), where only four patients additionally completed the study. The concept of the sham initiated group (N = 9) between the first and the second treatment periods (sham → navigation/standard) was without the risk of false-positive results in favor of neuronavigated rTMS.
Another limitation is the absence of monitoring of long-time effects, coupled with clinical monitoring of patients, at least for the next 6 months after rTMS application. Based on the evaluation of long-term effects of rTMS, we would be able to design a suitable algorithm for maintenance of rTMS in patients with AHs in the future, which may represent another breakthrough in the treatment of treatment-resistant schizophrenia.
In conclusion, our study documents the acute clinical effect of neuronavigated rTMS in treatment-resistant AHs. Our findings are also congruent with the hypothesis that local neuroplastic and metabolic changes provide a substrate for the clinical effect. Taking into account the individual differences in brain metabolism, neuronavigated rTMS allows an individualized approach to the treatment of patients with pharmaco-resistant AHs.
References
Hoffman RE, Hawkins KA, Gueorguieva R et al (2003) Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch Gen Psychiatry 60:49–56
Hoffman RE, Gueorguieva R, Hawkins KA et al (2005) Temporoparietal transcranial magnetic stimulation for auditory hallucinations: safety, efficacy and moderators in a fifty patient sample. Biol Psychiatry 58:97–104
Lee SH, Kim W, Chung Y et al (2005) A double blind study showing that two weeks of daily repetitive TMS over the left or right temporoparietal cortex reduces symptoms in patients with schizophrenia who are having treatment-refractory auditory hallucinations. Neurosci Lett 376:177–181
Chibbaro G, Daniele M, Alagona G et al (2005) Repetitive transcranial magnetic stimulation in schizophrenic patients reporting auditory hallucinations. Neurosci Lett 383:54–57
Brunelin J, Poulet E, Bediou B et al (2006) Low frequency repetitive transcranial magnetic stimulation improves source monitoring deficit in hallucinating patients with schizophrenia. Schizophr Res 81(1):41–45
George MS, Nahas Z, Kozel F et al (2002) Mechanisms and state of the art of transcranial magnetic stimulation. J ECT 18:170–181
Hoffman RE, Boutros NN, Berman RM et al (1999) Transcranial magnetic stimulation of left temporoparietal cortex in three patients reporting hallucinated “voices”. Biol Psychiatry 46(1):130–132
Hoffman RE, Boutros NN, Hu S et al (2000) Transcranial magnetic stimulation and auditory hallucinations in schizophrenia. Lancet 355:1073–1075
Dierks T, Linden DE, Jandl M et al (1999) Activation of Heschl’s gyrus during auditory hallucinations. Neuron 22:615–621
Aleman A, Sommer IE, Kahn RS (2007) Efficacy of slow repetitive transcranial magnetic stimulation in the treatment of resistant auditory hallucinations in schizophrenia: a meta-analysis. J Clin Pychiatry 68:416–421
Cordes J, Arends M, Mobascher A et al (2006) Potential clinical targets of repetitive transcranial magnetic stimulation treatment in schizophrenia. Neuropsychobiology 54:87–99
Horacek J, Brunovsky M, Novak T et al (2007) Effect of low-frequency rTMS on electromagnetic tomography (LORETA) and regional brain metabolism (PET) in schizophrenia patients with auditory hallucinations. Neuropsychobiology 55:132–142
Paus T, Jech R, Thompson CJ et al (1997) Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J Neurosci 17(9):3178–3184
Siebner HR, Peller M, Willoch F et al (2000) Lasting cortical activation after repetitive TMS of the motor cortex: a glucose metabolic study. Neurology 54(4):956–963
Langguth B, Eichhammer P, Zowe M et al (2006) Neuronavigated transcranial magnetic stimulation and auditory hallucinations in a schizophrenic patient: monitoring of neurobiological effects. Schizophr Res 84(1):185–186
Langguth B, Eichhammer P, Wiegand R et al (2003) Neuronavigated rTMS in a patient with chronic tinnitus. Effects of 4 weeks treatment. NeuroReport 14:977–980
Silberswieg DA, Stern E, Frith C et al (1995) A functional neuroanatomy of hallucinations in schizophrenia. Nature 378:176–179
Copolov DL, Seal ML, Maruff P et al (2003) Cortical activation associated with the experience of auditory hallucinations and perception of human speech in schizophrenia: a PET correlation study. Psychiatry Res 122:139–152
Weiss AP, Heckers S (2001) Neuroimaging of declarative memory in schizophrenia. Scand J Psychol 42:239–250
McGuire PK, Shah GM, Murray RM (1993) Increased blood flow in Broca’s area during auditory hallucinations in schizophrenia. Lancet 342:703–706
Cleghorn JM, Garnett ES, Nahmias C et al (1990) Regional brain metabolism during auditory hallucinations in chronic schizophrenia. Br J Psychiatry 157:562–570
Szechtman H, Woody E, Bowers KS, Nahmias C (1998) Where the imaginal appears real: a positron emission tomography study of auditory hallucinations. PNAS 95(4):1956–1960
Fitzgerald PB, Benitez J, Daskalakis JZ et al (2005) A double-blind sham-controlled trial of repetitive transcranial magnetic stimulation in the treatment of refractory auditory hallucinations. J Clin Psychophramacol. 25(4):358–362
Schonfeldt-Lecuona C, Gron G, Walter H et al (2004) Stereotaxic rTMS for the treatment of auditory hallucinations in schizophrenia. NeuroReport 15:1669–1673
Bentaleb LA, Bearegard M, Liddle P, Stip E (2002) Cerebral activity associated with auditory verbal hallucinations: a functional magnetic resonance imaging case study. J Psychiatry Neurosci 27:110–115
Lennox BR, Park SB, Medlez I, Morris PG, Jones PB (2000) The functional anatomy of auditory hallucinations in schizophrenia. Psychiatry Res 100:13–20
Shergill SS, Brammer MJ, Williams SCR et al (2000) Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry 57:1033–1038
Herwig U, Padberg F, Unger J, Spitzer M, Schonfeldt-Lecuona C (2001) Transcranial magnetic stimulation in therapy studies: examination of the reliability of “standard“coil positioning by neuronavigation. Biol Psychiatry 50:58–61
Herwig U, Schönfeldt-Lecuona C, Wunderlich AP et al (2001) The navigation of transcranial magnetic stimulation. Psychiatry Res Neuroimaging 108(2):123–131
Schonfeldt-Lecuona C, Thielscher A, Freudenmann RW et al (2005) Accuracy of stereotaxic positioning of transcranial magnetic stimulation. Brain Topogr 17(4):253–259
Herwig U, Lampe Y, Juengling FD et al (2003) Add-on rTMS for treatment of depression: a pilot study using stereotaxic coil-navigation according to PET data. J Psychiatr Res 37:267–275
Fernandez-Egea E, Parellada E, Lomeña F et al (2010) 18FDG PET study of amygdalar activity during facial emotion recognition in schizophrenia. Eur Arch Psychiatry Clin Neurosci 260(1):69–76
Sommer IEC, Slotema CW, de Weijer AD, Blom JD, Daalman K, Neggers SF, Somers M, Hoek HW, Aleman A, Kahn RS (2007) Can fMRI-guidance improve the efficacy of rTMS treatment for auditory verbal hallucinations? Schizophr Res 93(1–3):406–408
Rosa MO, Gattaz WF, Rosa MA, Rumi DO, Tavares H, Myczkowski M, Sartorelli MC et al (2007) Effects of repetitive transcranial magnetic stimulation on auditory hallucinations refractory to clozapine. J Clin Psychiatry 68(10):1528–1532
Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH et al (1994) Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91:79–92
Poulet E, Brunelin J, Bediou B, Bation R, Forgeard L, Dalery J, d’Amato T, Soud M (2005) Slow transcranial magnetic stimulation can rapidly reduce resistant auditory hallucinations in schizophrenia. Biol Psychiatry 57(2):188–191
Fitzgerald P, Sritharan A, Benitez J, Daskalakis Z, Jackson G, Kulkarni J, Egan G (2007) A preliminary fMRI study of the effects on cortical activation of the treatment of refractory auditory hallucinations with rTMS. Psychiatry Res: Neuroimaging 155(1):83–88
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276
Montagne-Larmurier A, Etard O, Razafimandimby A, Morello R, Dollfus S (2009) Two-day treatment of auditory hallucinations by high frequency rTMS guided by cerebral imaging: a 6 month follow-up pilot study. Schizophr Res 113:77–83
Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Ponto LL, Hichwa RD (1995) Remembering the past: two taceta of episodic memory explored with positron emission tomography. Am J Psychiatry 152:1576–1585
Signorini M, Paulesu E, Friston K, Perani D, Colleluori A (1999) Rapid assessment of regional cerebral metabolic abnormalities in single subjects with quantitative and nonquantitative [18F]FDG PET: a clinical validation of statistical parametric mapping. NeuroImage 9(1):63–80
Lee DS, Lee JS, Kang KW, Jang MJ, Lee SK, Chung JK (2001) Disparity of perfusion and glucose metabolism of epileptogenic zones in temporal lobe epilepsy demonstrated by SPM/SPAM analysis on 15O water PET, [18F]FDG-PET, and [99mTc]-HMPAO SPECT. Epilepsia 42(12):1515–1522
Kopeček M, Brunovský M, Bareš M, Španiel F (2005) Regional cerebral metabolic abnormalities in individual patients with nonquantitative 18FDG PET and qEEG (LORETA). Psychiatrie 9(Suppl 3):56–63
Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC (2010) Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry 67(3):255–262
Woods SW (2003) Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 64(6):663–667
Shulman RG (2001) Functional imaging studies: linking mind and basic neuroscience. Am J Psychiatry 158:11–20
Rocher AB, Chapon F, Blaizot X, Baron JC, Chavoix C (2003) Resting-state brain glucose utilization as measured by PET is directly related to regional synaptophysin levels: a study in baboons. Neuroimage 20:1894–1898
Kaplan RD, Szechtman H, Franco S, Szechtman B, Nahmias C (1993) Three clinical syndromes of schizophrenia in untreated subjects: relation to brain glucose activity measured by position emission tomography (PET). Schizophr Res 11(1):47–54
Nickl-Jockschat T, Schneider F, Pagel AD, Laird AR, Fox PT, Eickhoff SB (2011) Progressive pathology is functionally linked to the domains of language and emotion: meta-analysis of brain structure changes in schizophrenia patients. Eur Arch Psychiatry Clin Neurosci 261(Suppl 2):S166–S171
Chen R, Classen J, Gerloff C et al (1997) Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 48:1398–1403
Pascual-Leone A, Tarazona F, Keenan J, Tormos JM, Hamilton R, Catala MD (1999) Transcranial magnetic stimulation and neuroplasticity. Neuropsychologia 37:207–217
George MS (2001) Summary and future directions of therapeutic brain stimulation: neurostimulation and neuropsychiatric disorders. Epilepsy Behav 2(3):95–100
Herwig U, Satrapi P, Schonfeldt-Lecuona C (2003) Using the international 10–20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr 16(1):95–99
Slotema CW, Blom JD, deWeijer AD et al (2010) Can Low-frequency repetitive transcranial magnetic stimulation really relieve medication-resistant auditory verbal hallucinations? Negative results from a large randomized controlled trial. Biol Psychiatry 69(5):450–456
Kopecek M, Spaniel F, Novak T, Tislerova M, Belohlavek O, Horacek J (2012) 18FDG PET in hallucinating and non-hallucinating patients. Neuro Endocrinol Lett 28(1):53–58
Langguth B, Klienjung T, Landgrebe M, de Ridder D, Hajak G (2010) rTMS for the treatment of tinnitus: the role of neuronavigation for coil. Clin Neurophysiol 40(1):45–58
Mennemeier M, Chlette KC, Allen S, Bartel TB, Triggs W (2011) Variable changes in PET activity before and after rTMS treatment for tinnitus. Laryngoscope 121(4):815–822
d’Alfonso A, Aleman A, Kessels R et al (2002) Transcranial magnetic stimulation of left auditory cortex in patients with schizophrenia—effects on hallucinations and neurocognition. J Neuropsychiatry Clin Neurosci 14:77–79
Acknowledgments
This work was supported by Grant No. 1M0517 from the MEYS Czech Republic, VZ0021620816, PRVOUK P 34, and “2009 J. Horacek’s NARSAD Independent Investigator Award.” We thank Craig Hampson for language correction and Prof. Martin Alda for helpful suggestions and comments.
Conflict of interest
All authors confirmed their agreement to submission and declared that they have no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klirova, M., Horacek, J., Novak, T. et al. Individualized rTMS neuronavigated according to regional brain metabolism (18FGD PET) has better treatment effects on auditory hallucinations than standard positioning of rTMS: a double-blind, sham-controlled study. Eur Arch Psychiatry Clin Neurosci 263, 475–484 (2013). https://doi.org/10.1007/s00406-012-0368-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-012-0368-x