Abstract
The presence of the metabolic syndrome is an important risk factor for cardiovascular disease and diabetes. The short- and long-term metabolic safety of sertindole was compared to that of risperidone in a subset of patients enrolled in the sertindole cohort prospective (SCoP) study, an open randomized study. In 261 randomized patients, there were moderate increases in mean weight, BMI, and waist circumference during treatment with either sertindole or risperidone; after 12 weeks, the increase in weight was 1.3 and 1.1 kg, respectively, and after 36 weeks, it was 2.2 and 2.0 kg, respectively. From baseline to last assessment (up to 60 weeks), weight gains of 1.8 and 1.7 kg for sertindole and risperidone, respectively, were observed. Similar proportions of patients (sertindole: 17% versus risperidone: 16%) had weight increases ≥7% from baseline to last assessment. The mean changes from baseline in triglycerides, total cholesterol, HDL-cholesterol, LDL-cholesterol, plasma glucose and blood pressure were small and not clinically relevant in both treatment groups. No patient in either of the groups developed type 2 diabetes during the study. At last assessment, the prevalence of metabolic syndrome (International Diabetes Federation) was 17% in the sertindole group and 26% in the risperidone group and the incidence of metabolic syndrome was 7% in the sertindole group and 10% in the risperidone group. Treatment with either sertindole or risperidone did not appear to be associated with an increased comparative risk of developing metabolic syndrome. In general, the metabolic effects of sertindole and risperidone were similar.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
People with severe mental illness have nearly twice the normal risk of dying from cardiovascular disease (CVD) [16, 23, 27, 40, 41, 44, 54]. There is a growing concern about CVD risk in people with schizophrenia [6, 9, 12, 28, 34].
Metabolic syndrome (MetS) is a risk predictor for diabetes and cardiovascular disease [5, 19, 23, 24, 55]. Two recent reviews highlight the consistent high prevalence rates of MetS in patients with schizophrenia [13, 35]. Rates of MetS in people with schizophrenia are at least twice that of the general population of similar age [7, 10, 38]. The underlying reasons for the high prevalence of metabolic abnormalities are complex, including the mental illness, sedentary lifestyle, food choices, poverty, poor access to physical screening, smoking, alcohol, and substance use. Antipsychotic medication, to varying extents, can induce weight gain and have a negative impact on different CVD risk factors [2, 8, 11, 18, 36, 39, 45, 46, 49, 51].
Sertindole is an atypical, efficacious, and non-sedating antipsychotic [25, 26, 29–31] associated with placebo-level extrapyramidal symptoms [21, 56]. Sertindole was developed in the 1990s, was taken off the European market because of concerns that a known QTc prolongation would translate into an increased mortality. Sertindole has been reintroduced in Europe since 2006. There are few data in the literature on possible metabolic side effects of sertindole.
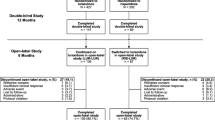
The sertindole cohort prospective (SCoP) study was carried out to confirm the short- and long-term safety of sertindole. The primary results from the SCoP have recently been published [42, 43, 52]. With 9,809 patients enrolled, the SCoP study is one of the largest prospective, randomized studies ever conducted in patients with schizophrenia. Here, we present the assessment of metabolic variables in a subset of patients enrolled in the SCoP study.
Methods
Study design
The overall objective of the SCoP study was to compare the safety of sertindole with that of risperidone under normal conditions of use. The design of the SCoP study is described in more detail elsewhere [43]. Briefly, the SCoP study was a multinational, multicenter, parallel-group, random allocation, open-label study. The study included patients with schizophrenia, who were at least 18 years of age, for whom a new antipsychotic or a change in antipsychotic treatment was indicated. Following the discussion with the FDA, it was decided to amend the SCoP protocol to include metabolic assessments in order to evaluate the effect of short- and long-term treatment with sertindole or risperidone on metabolic parameters. A metabolic substudy was incorporated in all sites of actively ongoing recruiting countries (Poland, Russia, and Ukraine). Patients who gave their informed consent for the assessment of metabolic variables were randomized (1:1) to treatment with sertindole or risperidone. Investigators were not blind to the allocated treatment and managed their patients according to their usual clinical practice. The initial and maintenance dosages as well as dose titration were set by the investigator, in accordance with the respective labels. Sertindole and risperidone tablets were administered orally. The study was carried out between September 2006 and December 2007. Treatment duration for an individual patient was not limited.
The study was designed and conducted in accordance with the principles of the Declaration of Helsinki (1964 and later amendments), and any relevant local laws. The study protocol adhered to the guidelines for Good Clinical Practice and was approved by the appropriate Independent Ethics Committee. Patients were only allowed to participate in the study after their written informed consent was obtained.
Metabolic assessments
Metabolic assessments were carried out at weeks 8 and 12 and every 3 months thereafter.
Weight, waist circumference, and blood pressure
Patients were weighed wearing light clothing and no shoes. The patient’s waist was measured around the bare abdomen at its narrowest. Two waist measurements were made; if the two measurements differed by 0.5 cm or more, a third measurement was made. Waist circumference was the average of all the measurements made. Systolic and diastolic blood pressures were measured after the patient had rested in a sitting position for at least 5 min. Blood pressure was the average of two measurements.
Fasting laboratory tests: fasting plasma glucose and fasting serum lipids
In addition to fasting plasma glucose, the following fasting serum lipids were assayed: triglycerides, total cholesterol, HDL-cholesterol, and LDL-cholesterol [for patients who had triglycerides below 4.4 mmol/l, LDL-cholesterol was calculated using the Friedewald formula: LDL-cholesterol = total cholesterol − (HDL-cholesterol) − (triglycerides/5)].
The patients were asked to fast overnight (≥8 h) before the blood tests; they were asked whether they were fasting or not. Data from the fasting blood samples (approximately 7 ml) were used for the metabolic evaluation. All the blood samples for serum lipid profile and plasma glucose were analyzed at Quintiles Laboratories Europe, Livingstone, Scotland, United Kingdom.
The International Diabetes Federation (IDF) definition of MetS was used, whereby a person is defined as having MetS if he/she has abdominal obesity (waist circumference): men ≥94 cm, women ≥80 cm, and two of the following factors: triglycerides ≥1.7 mmol/l (150 mg/dl) or specific treatment for this lipid abnormality, HDL-cholesterol: men <1.03 mmol/l (40 mg/dl), women <1.29 mmol/l (50 mg/dl) or specific treatment for this lipid abnormality, blood pressure: systolic ≥130 mmHg diastolic or treatment of previously diagnosed hypertension ≥85 mmHg, and fasting glucose: ≥5.6 mmol/l (100 mg/dl) or previously diagnosed type 2 diabetes [1, 20].
In the analysis of the fasting serum lipid profile, the potentially clinically significant thresholds correspond to those set out by the National Cholesterol Education Program (NCEP-ATP III) for clinically abnormal values and are as follows: triglycerides ≥2.2 mmol/l (200 mg/dl), total cholesterol ≥6.216 mmol/l (240 mg/dl), HDL-cholesterol <1.036 mmol/l (40 mg/dl), LDL-cholesterol ≥4.144 mmol/l (160 mg/dl) [15, 20].
Safety assessments
Adverse events were assessed every 4 weeks, up to week 12 and every 3 months thereafter. Serious adverse events were collected on an ongoing basis. A safety assessment was undertaken 30 days after discontinuing the randomized treatment. Patients were followed up after discontinuation of the randomized treatment until the end of the study.
Statistical analysis
The only randomized treatment period (i.e., from the date of prescription of randomized treatment until randomized treatment was stopped or the date of start of add-on antipsychotic(s), whichever occurred first) constituted the period for metabolic assessments. All randomized patients who received treatment at least until week 8 and who had an assessment at the week 8 visit or later were used for the analysis and reporting of metabolic variables. Further, the analysis of metabolic variables was based on those patients who had not received medications for triglycerides or cholesterol abnormalities, type 2 diabetes, or hypertension from 3 months prior to inclusion up until the week 8 visit. Any patient who started taking concomitant medications for triglycerides or HDL-cholesterol abnormalities, type 2 diabetes, or hypertension after the week 8 visit was excluded from the respective per-protocol analysis from the time they started taking such concomitant medication.
The baseline characteristics and demographics were compared between treatments using t-test and Chi square test. The effect of treatment on the changing status of MetS and individual MetS criteria from baseline to last assessment was compared using Chi square/Fisher’s exact tests. Logistic regression analysis of MetS (adjusted for baseline values of the metabolic variables and for sex and center) was used to compare prevalence and incidence. Gender differences were tested using Chi square/Fisher’s exact tests. All statistical calculations were performed using SAS®, version 9.1.
Results
Baseline characteristics and patient disposition
There were 261 randomized patients in three countries (Russia, Ukraine, and Poland). All patients received at least one dose of study treatment; 131 for sertindole and 130 for risperidone. The patients were in the chronic stable phase of the disease.
More than half of the patients (55% in the sertindole group and 72% in the risperidone group) were in the study for at least 24 weeks. The mean exposure was approximately 1 month longer (P = 0.014) in the risperidone group (214 days) than in the sertindole group (179 days). Previous antipsychotic treatment was similar in sertindole and risperidone patients (Table 1) though significantly (P = 0.027) more in the risperidone group received zuclopenthixol. The most common reason for withdrawal from treatment was study completion; 53% in the sertindole group and 59% in the risperidone group (Table 1).
The randomized treatment daily dose was maintained stable during the course of the study and was in the recommended dose range with a preference for the lowest dose (for about 80% of the patients); 12 mg for sertindole treatment and 4 mg for risperidone (Table 1).
Baseline demographics
Women comprised a slight majority of both groups (51% in the sertindole group and 54% in the risperidone group). Women were older than men (women; 36.9 and 40.1 years and men 33.9 and 32.8 years in sertindole and risperidone groups, respectively) though not statistically significantly so.
The mean BMI of 25.5 kg/m2 was indicative of a slightly overweight population. The patients in the risperidone group were slightly older and heavier than the patients in the sertindole group (Tables 1, 2).
Weight, BMI, and waist circumference
There were moderate increases in mean weight, BMI, and waist circumference during treatment with either sertindole or risperidone; after 12 weeks, the increase in weight was 1.3 and 1.1 kg, respectively, and after 36 weeks, it was 2.2 and 2.0 kg, respectively (Table 2). The change in weight from baseline to last assessment was 1.8 and 1.7 kg for sertindole and risperidone, respectively. In both treatment groups, similar proportions of patients (sertindole: 17% and risperidone: 16%) had weight increases ≥7% from baseline to last assessment (up to 60 weeks).
Fasting serum lipids and fasting plasma glucose
At baseline, the mean triglyceride levels were 1.25 and 1.52 mmol/l in the sertindole and risperidone groups, respectively, (Table 3) and 6% of sertindole and 13% of risperidone-treated patients had triglyceride levels ≥2.2 mmol/l. Total cholesterol levels at baseline were 4.8 and 5.0 mmol/l for sertindole and risperidone, respectively, and 10.1 and 16.5% had a total cholesterol of ≥6.216 mmol/l. At baseline, the mean HDL-cholesterol levels were 1.28 and 1.26 mmol/l for sertindole and risperidone, respectively, and 19 and 25% had levels of <1.036 mmol/l. Mean LDL-cholesterol levels at baseline were 3.1 and 3.1 mmol/l in the sertindole and risperidone groups, respectively, and 11 and 13% had levels >4.144 mmol/l. There were no statistical differences between treatments for any of the fasting serum lipids at baseline. The mean changes from baseline to each assessment in triglycerides, total cholesterol, HDL-cholesterol, and LDL-cholesterol were small and not clinically relevant in both treatment groups (Table 3). No patient in either treatment group took concomitant medications for triglycerides or HDL-cholesterol during the study. In both treatment groups, the mean changes from baseline in plasma glucose were small. No patient in either group developed type 2 diabetes during the course of the study (Table 3).
Blood pressure
At baseline, the mean (±SD) blood pressure was 117 (±10.8) mmHg systolic and 74.6 (±8.1) mmHg diastolic for the sertindole-treated patients and 120 (±9.6) mmHg systolic and 76.6 (±8.4) mmHg diastolic for the risperidone-treated patients. The mean changes from baseline to weeks 12, 24, and 36 in systolic and diastolic blood pressure were small (at most 2 mmHg) in both treatment groups. Concomitant medication for hypertension was taken by five sertindole patients (three women and two men) in the period from 3 months prior to inclusion up until week 8 and were not included in metabolic variables’ analyses. After week 8, one man in the sertindole group and one woman in the risperidone group received concomitant medication for hypertension and they were excluded from the analyses set from the time they started taking the medication. Hypertension was reported as an adverse event for five patients (four in the sertindole group and one patient in the risperidone group).
MetS and individual MetS criteria
At baseline, the prevalence of MetS was lower in the sertindole group (13%) than in the risperidone group (21%) (Table 4). The MetS status in the majority of patients remained the same throughout the study; 80% of the patients in the sertindole group and 69% in the risperidone group did not have MetS at baseline nor at last assessment, and 8% of the patients in the sertindole group and 12% in the risperidone group had MetS at baseline and at last assessment. During the study, the incidence with MetS was similar in both treatment groups (Table 4).
At last assessment, the prevalence of MetS was 17% in the sertindole group and 26% in the risperidone group, and the incidence of MetS in patients without MetS at baseline was 7% in the sertindole group and 10% in the risperidone group.
A logistic regression analysis of both the observed case (OC) and the last observation carried forward of prevalence of MetS (adjusted for baseline values of the metabolic variables and for sex and center) at each visit and at last assessment showed that there were no statistically significant differences between the treatment groups at any time point up to week 48, when there were too few OC patients to allow a statistical comparison. Similar analyses of incidence data revealed no statistically significant differences between the treatment groups at week 8. At later time points, the incidence of MetS was too low to allow statistical comparison between treatment groups.
For MetS criteria, there were no significant differences between the two treatments. For both treatments, between baseline and last assessment, the percentage of patients who developed central obesity was higher than who lost it. For both treatment groups, more patients developed abnormal glucose levels than normalized.
For triglycerides and HDL-cholesterol, there appeared to be differences between the treatment groups. The percentage of patients whose triglyceride levels shifted from normal at baseline to abnormal at last assessment was higher in the sertindole-treated patients than in the risperidone-treated patients. The percentage of patients whose HDL-cholesterol levels shifted from normal at baseline to abnormally low at last assessment was higher in the risperidone-treated patients than in the sertindole-treated patients. These differences were not statistically significant.
None of the metabolic effects were dose dependent for either the sertindole or the risperidone group.
Influence of gender on metabolic parameters
At baseline, significantly more women than men had MetS (24% of women and 10% of men; P = 0.01). There was no significant difference in the change of metabolic status between men and women. Slightly more women (not significant) developed central obesity and slightly (not significant) more men developed elevated blood pressure and elevated triglycerides.
Safety evaluation
There were no metabolic serious adverse events reported. The incidence of non-serious metabolic adverse events was low (less than 4%) in both treatment groups. The non-serious metabolic adverse events comprised hypertension, hypercholesterolemia, and blood triglycerides increased; weight changes, although not elicited, were also reported as adverse events. There were no study withdrawals as a result of metabolic adverse events.
Two patients died during the study: a risperidone-treated patient committed suicide and a sertindole-treated patient died during the follow-up period (between end of treatment and end of the study). Both events were assessed by the investigators as not related to treatment. The incidence of serious adverse events was 2.3% (n=3) for sertindole and 1.5% (n=2) for risperidone. All serious adverse events were assessed by the investigators as not related to treatment.
Discussion
In this study, the metabolic effects of sertindole were compared with those of risperidone in a subset of patients from the SCoP study. Risperidone was chosen as the comparator as it is the most extensively used antipsychotic worldwide.
The mean treatment exposure was approximately 7 months in the risperidone group and 6 months in the sertindole group. Thus, the extent of exposure was adequate to evaluate metabolic changes occurring in the first months of treatment.
Based on the mean changes from baseline in each of the metabolic variables, the only potentially clinically relevant changes observed were in weight, BMI, and waist circumference, all of which increased over time. These changes occurred to a similar extent in both treatment groups. The increase in waist circumference was reflected in a slight increase in the incidence of central obesity (as defined by the IDF). At last assessment, weight gain was modest for both agents (risperidone 1.7 kg and sertindole 1.8 kg) and the study confirmed that these changes were not dose related [48]. In the literature, the estimated short-term weight gain for risperidone-treated patients with schizophrenia ranges from 2.0 to 5.6 kg [39, 51]. Within this range in first episode, treatment-naive schizophrenia patients showed more pronounced weight gain. In this study, the patients were in the chronic phase of the disease and were not treatment-naïve, thus the weight gains observed agree with the comparative patients in literature. Patients did not participate in any weight management plans as this was not part of normal clinical practice in the participating centers of this study.
In both treatment groups, the mean changes from baseline to last assessment in each of the metabolic variables (weight, BMI, waist circumference, blood pressure, triglycerides, total cholesterol, HDL-cholesterol, LDL-cholesterol, and plasma glucose) were similar irrespective of whether the patients had MetS at baseline. The mean changes from baseline in the lipid values were small and not clinically relevant; nevertheless, potentially clinically significant lipid values were reported in both treatment groups and, in general, the occurrence was similar.
Equal proportions of patients treated with either sertindole or risperidone lost or developed MetS indicating that the changes in either direction were probably due to background variation and were not attributable to treatment. This pattern of changes was also recently observed in a naturalistic cohort with treatment with different antipsychotics [47].
The MetS neutral effect over time of risperidone was confirmed in a prospective analysis of CATIE phase 1 data [33].
The major strengths of this study are the randomization, the comprehensive assessment of metabolic changes, the duration of the study, and the number of patients included. The limitations are the open-label design, the limited number of countries involved, and the patients included were mainly Caucasian. Although the use of the concept of MetS is currently being debated, the study evaluated a large number of cardiovascular risk factors [17]. Before participating in the study, the patients were informed that there was a risk of developing metabolic syndrome with the use of antipsychotics. There were no specific weight management plans included in the study design since the study was conducted under normal conditions of use. We cannot exclude that some patients may have had particular diet plans as part of normal practice in the participating centers of this study; however, such information was not collected in the study. In addition, the patients were in the chronic stable phase of the disease and were not treatment-naïve, thus the results may not be indicative of those first starting antipsychotic medication.
Assessing the possible effects of antipsychotic treatment on metabolic variables is especially important, given that metabolic abnormalities have historically been associated with schizophrenia and that there is increasing concern that antipsychotic treatment might add to the metabolic burden in patients with schizophrenia.
Patients with schizophrenia die earlier than the general population, in large part due to cardiovascular disease. It has been suggested that schizophrenia is directly associated with weight gain. However, it has also been argued that weight gain is a result of unhealthy diets and sedentary lifestyles (this unhealthy lifestyle being more common than that in the general population), substance abuse, and antipsychotic medication. The potential role of antipsychotic treatment in explaining the increased mortality due to somatic disorders is highly debated. A recent study of death registers in Finland compared the cause-specific mortality in 66,881 patients versus the total population (5.2 million) between 1996 and 2006 [50], which showed no significant differences between any of the examined antipsychotics (clozapine, haloperidol, quetiapine, and risperidone) regarding death due to ischemic heart disease. However, a number of methodological and conceptual issues make the interpretation of these findings problematic and are discussed in detail by De Hert et al. [14].
The evaluation of the metabolic effects of an antipsychotic is also complicated by the presence of confounders such as prior antipsychotic treatment and the impact of baseline BMI. Furthermore, although obesity appears to be linked to metabolic abnormalities, such as dyslipidemia, hyperglycemia, and hypertension, it is not fully understood what other factors might influence whether a patient with schizophrenia will develop metabolic abnormalities such as MetS.
Over recent years, both national and international groups have developed screening and monitoring guidelines [2, 3, 6, 9, 32, 53], but these have not made their way to routine clinical care for patients [4, 22, 36, 37]. Recently, the European Psychiatric Association in collaboration with European cardiologists and diabetologists have proposed a comprehensive guidance for screening and monitoring of metabolic risk in people with severe mental illness [12], and an update of the ADA/APA 2004 consensus document is expected to be published later this year.
Conclusions
Treatment with either sertindole or risperidone for up to 12 months did not appear to be associated with an increased comparative risk of developing MetS as defined by the IDF. In general, the metabolic effects of sertindole and risperidone were similar: both treatments were associated with modest weight gain, and a corresponding increase in BMI and waist circumference.
Neither of the treatments was associated with clinically relevant mean changes in blood pressure, triglycerides, total cholesterol, HDL-cholesterol, LDL-cholesterol, or blood glucose. The incidence of non-serious metabolic adverse events was low (less than 4%) and there were no metabolic serious adverse events reported.
References
Alberti KG, Zimmet P, Shaw J (2006) Metabolic syndrome—a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabetic Med 23:469–480
American Diabetes Association (2004) Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 27:596–601
Barnett AH, Mackin P, Chaudhry I, Farooqi A, Gadsby R, Heald A, Hill J, Millar H, Peveler R, Rees A, Singh V, Taylor D, Vora J, Jones PB (2007) Minimising metabolic and cardiovascular risk in schizophrenia. J Psychopharmacol 21(4):357–373
Buckley PF, Mille DD, Singer B (2005) Clinicians’ recognition of the metabolic adverse effects of antipsychotic medications. Schizophr Res 79:281–288
Byrne CD, Wild SH (2005) The global burden of the metabolic syndrome and its consequences for diabetes and cardiovascular disease. In: Byrne CD, Wild SH (eds) The metabolic syndrome. Wiley, West Sussex, pp 1–42
Casey DE, Haupt DW, Newcomer JW, Henderson DC, Sernyak MJ, Davidson M, Lindenmayer JP, Manoukian SV, Banerji MA, Lebovitz HE, Hennekens CH (2004) Antipsychotic-induced weight gain and metabolic abnormalities: implications for increased mortality in patients with schizophrenia. J Clin Psychiatry 65(Suppl 7):4–18
Correll CU (2007) Balancing efficacy and safety in the treatment with antipsychotics. CNS Spectr 12(Suppl 17):2–20
Daumit GL, Goff DC, Meyer JM, Davis VG, Nasrallah HA, McEvoy JP, Rosenheck R, Davis SM, Hsiao JK, Stroup TS, Lieberman JA (2008) Antipsychotic effects on estimated 10-year coronary heart disease risk in the CATIE schizophrenia study. Schizophr Res 105(1–3):175–187
De Hert M, Van Eyck D, De Nayer A (2006) Metabolic abnormalities associated with second generation antipsychotics: fact or fiction? Development of guidelines for screening and monitoring. Int Clin Psychopharmacol 21(Suppl 2):11–15
De Hert M, van Winkel R, Van Eyck D, Hanssens L, Wampers M, Scheen A, Peuskens J (2006) Prevalence of diabetes, metabolic syndrome and metabolic abnormalities in schizophrenia over the course of the illness: a cross-sectional study. Clin Pract Epidemiol Ment Health 2:14 (online)
De Hert M, Schreurs V, Sweers K, Van Eyck D, Hanssens L, Šinko S, van Winkel R, Wampers M, Peuskens J (2008) Typical and atypical antipsychotics differentially affect long-term incidence rates of the metabolic syndrome in first-episode patients with schizophrenia: a retrospective chart review. Schizophr Res 101(1–3):295–303
De Hert M, Dekker JM, Wood D, Kahl KG, Möller HJ (2009) Cardiovascular disease and diabetes in people with severe mental illness. Position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC). Eur Psychiatry 24(6):412–424
De Hert M, Schreurs V, Vancampfort D, van Winkel R (2009) Metabolic syndrome in people with schizophrenia: a review. World Psychiatry 8(1):15–22
De Hert M, Correll CU, Cohen D (2010) Do antipsychotic medications reduce or increase mortality in schizophrenia? A critical appraisal of the FIN-11 study. Schizophr Res 117(1):68–74
Expert Panel on Detection and Evaluation of Treatment of High Blood Cholesterol in Adults (2001) Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 285:2486–2497
Fleischhacker WW, Cetkovich-Bakmas M, De Hert M, Hennekens CH, Lambert M, Leucht S, Maj M, McIntyre RS, Naber D, Newcomer JW, Olfson M, Osby U, Sartorius N, Lieberman JA (2008) Cormorbid somatic illnesses in patients with severe mental disorders: clinical, policy and research challenges. J Clin Psychiatry 69:514–519
Gale E (2005) The myth of the metabolic syndrome. Diabetologica 48:679–683
Goff DC, Sullivan LM, McEvoy JP, Meyer JM, Nasrallah HA, Daumit GL, Lamberti S, D’Agostino RB, Stroup TS, Davis S, Lieberman JA (2005) A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res 80(1):45–53
Grundy SM (2005) The changing face of cardiovascular risk. JACC 46:173–176
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Spertus JA, Costa F (2005) Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112:2735–2752
Hale A, Azorin JM, Kasper S, Maier W, Syvalahti E, Van Der Burght M, Sloth-Nielsen M, Wehnert A (2000) Sertindole is associated with a low level of extrapyramidal symptoms in schizophrenic patients: results of a phase III trial. Int J Psychiatry Clin Pract 4:47–54
Haupt DW, Rosenblatt LC, Kim E, Baker RA, Whitehead R, Newcomer JW (2009) Prevalence and predictors of lipid and glucose monitoring in commercially insured patients treated with second-generation antipsychotic agents. Am J Psychiatry 166(3):345–353
Hennekens CH, Hennekens AR, Hollar D, Casey DE (2005) Schizophrenia and increased risks of cardiovascular disease. Am Heart J 150(6):1115–1121
Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, Taskinen MR, Groop L (2001) Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 24(4):683–689
Kasper S (2002) Serindole: safety and tolerability profile. Int J Psych Clin Pract 6(Suppl 1):S27–S32
Kasper S, Möller HJ, Hale A (2010) The European post-marketing observational sertindole study: an investigation of the safety of antipsychotic drug treatment. Eur Arch Psychiatry Clin Neurosci 260(1):59–68
Laursen TM, Munk-Olsen T, Agerbo E, Gasse C, Mortensen PB (2009) Somatic hospital contacts, invasive cardiac procedures, and mortality from heart disease in patients with severe mental disorder. Arch Gen Psychiatry 66(7):713–720
Leucht S, Burkard T, Henderson J, Maj M, Sartorius N (2007) Physical illness and schizophrenia: a review of the literature. Acta Psychiatr Scand 116:317–333
Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM (2009) Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 373(9657):31–41
Leucht S, Komossa K, Rummel-Kluge C, Corves C, Hunger H, Schmid F, Asenjo Lobos C, Schwarz S, Davis JM (2009) A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry 166(2):152–163
Lindstrom E, Levander S (2006) Sertindole: efficacy and safety in schizophrenia. Expert Opin Pharmacother 7:1825–1834
Marder SR, Essock SM, Miller AL, Buchanan RW, Casey DE, Davis JM, Kane JM, Lieberman JA, Schooler NR, Covell N, Stroup S, Weissman EM, Wirshing DA, Hall CS, Pogach L, Pi-Sunyer X, Bigger JT, Friedman A, Kleinberg D, Yevich SJ, Davis B, Shon S (2004) Physical health monitoring of patients with schizophrenia. Am J Psychiatry 161(8):1334–1349
Meyer JM, Davis VG, Goff DC, McEvoy JP, Nasrallah HA, Davis SM, Rosenheck RA, Daumit GL, Hsiao J, Swartz MS, Stroup TS, Lieberman JA (2008) Change in metabolic syndrome parameters with antipsychotic treatment in the CATIE schizophrenia trial: prospective data from phase 1. Schizophr Res 101(1–3):273–286
Meyer JM, Nasrallah HA (2003) Medical illness and schizophrenia. American Psychiatric Publishing, Washington, DC
Meyer JM, Stahl SM (2009) The metabolic syndrome and schizophrenia. Acta Psychiatr Scand 119(1):4–14
Nasrallah HA, Meyer JM, Goff DC, McEvoy JP, Davis SM, Stroup TS, Liberman J (2006) Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res 86(1–3):15–22
Newcomer JW, Nasrallah HA, Loebel AD (2004) The atypical antipsychotic therapy and metabolic issues national survey. J Clin Psychopharmacol 24:1–6
Newcomer J (2007) Metabolic syndrome and mental illness. Am J Manag Care 13:170–177
Newcomer JW (2005) Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs 19(Suppl 1):1–93
Osby U, Correia N, Brandt L, Ekbom A, Sparen P (2000) Mortality and causes of death in schizophrenia in Stockholm County, Sweden. Schizophr Res 45(1–2):21–28
Osby U, Correia N, Brandt L, Ekbom A, Sparen P (2000) Time trends in schizophrenia mortality in Stockholm County, Sweden: cohort study. BMJ 321(7259):483–484
Peuskens J, Moore N, Azorin JM, Toumi M, Cochran J (2007) The European sertindole safety and exposure survey: a follow-up study of 8600 patients. Pharmacoepidemiol Drug Saf 16(7):804–811
Peuskens J, Tanghøj P, Mittoux A, Cohort Sertindole (2008) The Sertindole Cohort Prospective (SCoP) study: rationale, design and methodology. Pharmacoepidemiol Drug Saf 17(5):425–433
Saha S, Chant D, Mcgrath J (2007) A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry 64:1123–1131
Scheen A, De Hert M (2007) Abnormal glucose metabolism in patients treated with antipsychotics. Diabetes Metabol 33:169–175
Scheen A, van Winkel R, De Hert M (2008) Traitement neuroleptique et troubles metabolic. Med Mal Metabol 2(6):593–599
Schorr SG, Slooff CJ, Bruggeman R, Taxis K (2008) Incidence of metabolic syndrome and its reversibility in a cohort of schizophrenic patients followed for one year. Schizophr Res 102(Suppl):241
Simon V, van Winkel R, De Hert M (2009) Are weight gain and metabolic side-effects of atypical antipsychotics dose-dependent? A literature review. J Clin Psychiatry 70(7):1041–1050
Smith M, Hokins D, Peveler R, Holt R, Woodward M, Ismail K (2008) First versus second generation antipsychotics and risk for diabetes in schizophrenia: systematic review and meta-analysis. Br J Psychiatry 192(6):406–411
Tiihonen J, Lönnqvist J, Wahlbeck K, Klaukka T, Niskanen L, Tanskanen A, Haukka J (2009) 11-Year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet 374(9690):620–627
Tschoner A, Engl J, Laimer M, Kaser S, Rettenbacher M, Fleischhacker W, Patsch J, Ebenbichler C (2007) Metabolic side effects of antipsychotic medication. Int J Clin Pract 61(8):1356–1370
Thomas SHL, Drici MD, Hall GC, Crocq MA, Everitt B, Lader MH, Le Jeunne C, Naber D, Priori S, Sturkenboom M, Thibaut F, Peuskens J, Mittoux A, Tanghøj P, Toumi M, Moore ND, Mann RD (2010) Safety of sertindole versus risperidone in schizophrenia: principal results of the sertindole cohort prospective study (SCoP). Acta Psychiatr Scand 1–11. doi:10.1111/j.1600-0447.2010.01563.x
van Winkel R, De Hert M, Van Eyck D, Hanssens L, Wampers M, Scheen A, Peuskens J (2006) Screening for diabetes and other metabolic abnormalities in patients with schizophrenia: evaluation of incidence and screening methods. J Clin Psychiatry 67(10):1493–1500
Weinmann S, Read J, Aderhold V (2009) Influence of antipsychotics on mortality in schizophrenia: systematic review. Schizophr Res 113(1):1–11
Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L, INTERHEART Study Investigators (2004) Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case–control study. Lancet 364(9438):937–952
Zimbroff DL, Kane JM, Tamminga CA, Daniel DG, Mack RJ, Wozniak PJ, Sebree TB, Wallin BA, Kaskin KB (1997) Controlled, dose–response study of sertindole and haloperidol in the treatment of schizophrenia. Sertindole Study Group. Am J Psychiatry 154:782–791
Acknowledgments
The authors would like to thank the clinicians who collected the data and the patients who participated in the clinical trial.
Funding source
H Lundbeck A/S was the sponsor of this trial. This trial has been registered in a database available free of charge at: http://www.lundbecktrials.com/.
Clinical trial registration
Safety Study of Sertindole versus Risperidone under Normal Conditions of Use. NCT 00856583 (EudraCT 2004-000213-19) http://clinicaltrials.gov/ct2/results?term=00856583.
Conflict of interest
M. De Hert has been a consultant for, received grant/research support and honoraria from, and been on the speakers/advisory boards of Astra Zeneca, Bristol-Myers Squibb, Eli Lilly, Janssen-Cilag, Lundbeck, Pfizer, and Sanofi-Aventis. M. De Hert did not receive a financial compensation for writing the article. A. Mittoux and Y. He are employees of H. Lundbeck A/S, Copenhagen, Denmark. J Peuskens was the principle investigator of the SCoP study and has acted as a consultant and co-operated in clinical trials with AstraZeneca, Bristol Myers Squibb, Eli Lilly, Janssen-Cilag, Lundbeck, Pfizer, Sanofi Synthélabo. He has also received research grants from AstraZeneca, Janssen-Cilag, Eli Lilly, Lundbeck, and Sanofi-Aventis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Hert, M., Mittoux, A., He, Y. et al. Metabolic parameters in the short- and long-term treatment of schizophrenia with sertindole or risperidone. Eur Arch Psychiatry Clin Neurosci 261, 231–239 (2011). https://doi.org/10.1007/s00406-010-0142-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-010-0142-x