Abstract
Background
Individual randomized clinical trials (RCTs) with cholinesterase inhibitors (ChEIs) aiming to delay the progression from mild cognitive impairment (MCI) to Alzheimer’s disease (AD) have not found significant benefit of their use for this purpose. The objective of this study is to meta-analyze the RCTs conducted with ChEIs in order to assess whether pooled analysis could show the benefit of these drugs in delaying the progression from MCI to AD.
Methods
We searched for references of published and unpublished studies on electronic databases (Medline, Embase, Web of Science, and Clinical Trial Database Registry, particularly the Clinicaltrials.gov—http://www.clinicaltrials.gov). We retrieved 173 references, which yielded three references for data extraction. A total of 3.574 subjects from four RCTs were included in the meta-analysis. Among 1,784 subjects allocated in the ChEI-treatment group, 275 (15.4%) progressed to AD/dementia, as opposed to 366 (20.4%) out of 1,790 subjects in the placebo group. The relative risk (RR) for progression to AD/dementia in the ChEI-treated group was 0.75 [CI95% 0.66–0.87], z = −3.89, P < 0.001. The patients on the ChEI group had a significantly higher all-cause dropout risk than the patients on the placebo group (RR = 1.36 CI95% [1.24–1.49]; z = 6.59, P < 0.001). The RR for serious adverse events (SAE) in the ChEI-treated group showed no significantly statistical difference from the placebo group (RR = 0.95 [CI95% 0.83–1.09], z = −0.72, P = 0.47). The subjects in the ChEI-treated group had a marginally, non-significant, higher risk of death due to any cause than those in the placebo-treated group (RR = 1.04, CI95% 0.63–1.70, z = 0.16, P = 0.86).
Conclusion
The long-term use of ChEIs in subjects with MCI may attenuate the risk of progression to AD/dementia. This finding may have a significant impact on public health and pharmaco-economic policies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cholinesterase inhibitors (ChEIs) were firstly introduced for symptomatic treatment of clinical Alzheimer’s disease (AD) in the early 1990s, with the introduction of the tacrine, donepezil, rivastigmine, and galantamine. These drugs significantly improved cognitive performance and global status of AD patients at early and moderate stages of the disease as compared to placebo in randomized clinical trials (RCTs) [37, 38, 46]. Nonetheless, the improvement in cognition observed in the patients treated with these drugs waned after continued administration of these drugs and the cognitive performance of the patients continued to decline despite drug continuation. Therefore, these drugs were regarded as useful for cognitive symptomatic treatment only, with no or non-significant effects on halting the progression of cognitive deficits in these patients [23]. In addition, they did not show superior therapeutic efficacy in comparison to each other, nor had significant differences in relation to their profile of adverse events. For these reasons, they were regarded as having drug-class effect, thus, being used interchangeably [25, 47].
In spite of the evidence drawn from the double-blind phase of the RCTs, open-label, continuation phase of the studies with long-term administration of ChEIs for up to 5 years have shown a slower cognitive decline loop and better global clinical status in the AD patients taking ChEIs in comparison to AD patients who were not on ChEIs or the expected cognitive decline for the subjects who never used ChEIs [11, 34, 42]. Also, long-term use of ChEIs was associated with neurobiological changes in cascades associated with the physiopathology of Alzheimer’s disease [18, 24]. Such evidences suggested that these drugs would have disease-modifying properties for long-term use in AD patients, thus, delaying the progression of the disease to more advanced stages [39].
In view of potential disease-modifying properties of ChEIs in AD, clinical trials were carried out with subjects with mild cognitive impairment (MCI), currently regarded as a pre-dementia stage, to assess whether these drugs could delay or reduce the rate of progression from MCI to AD [14, 33, 49]. If this were true, it would be an unequivocal evidence of their disease-modifying properties. Nevertheless, these studies did not show significant benefits of these drugs in delaying the progression from MCI to AD in the proposed study end-points, nor they demonstrated persistent improvement on cognitive performance of MCI ChEI-treated group. Some explanations to these negative results might be the lack of standardized diagnostic criteria for MCI, different clinical and cognitive assessment protocols for each trial, and, consequently, the recruitment of heterogeneous study population, and also a lower than recommended study drug dose in the case of the InDDEx study [31]. In addition, the disease-modifying properties of ChEIs may be small; thus, the sample size of individual RCTs might have been underpowered to unravel the efficacy of ChEIs in delaying the progression of pre-dementia stages to AD/dementia.
In view of these controversies, the objective of the present study is to meta-analyze the RCTs, placebo-controlled studies, carried out to assess the effects of ChEIs in delaying the progression to AD. We hypothesize that pooled analysis of the published RCTs, including unpublished studies, would increase the sample size of MCI subjects and may reveal a beneficial effect of these drugs to delay the progression to AD.
Methods
Search strategy
Published and unpublished references were electronically searched through the databases Medline, Embase and Web of Science, and Clinical Trial Database Registry, particularly the Clinicaltrials.gov (http://www.clinicaltrials.gov). The search terms used were: ‘mild cognitive impairment’, ‘donepezil’, ‘rivastigmine’, ‘galantamine’, its synonyms and acronyms. The search was done from January 1990 to May 2008, and we did not restrict for language. We did not included the term ‘tacrine’ as it is not routinely used in clinical practice due to its hepatotoxicity.
Inclusion criteria
Published and unpublished studies were included if they were double-blind, placebo controlled, RCTs of ChEIs (donepezil, rivastigmine, galantamine), whose primary outcome was to show the delay of progression from pre-dementia stages, either defined by the Petersen’s MCI criteria [32] or a Clinical Dementia Rating (CDR) of 0.5, to AD or CDR of 1.0.
Exclusion criteria
The studies were excluded if the design was not RCT; the study did not present original data or report secondary analyses of RCTs; the drug was not an ChEI; the criteria for subject selection of the diagnostic criteria of MCI was not clearly stated or was not compatible with Petersen’s criteria [32]; or the criteria for progression to AD or dementia was not clearly stated. Ongoing studies retrieved from Clinical Trials Database Registry were not considered for revision.
Data extraction
We screened the obtained titles, abstracts and protocols. Data were extracted by a standardized methodology developed by the authors. The extracted information included was study drug; mean drug dose; number of subjects enrolled and randomized to study drug or placebo; demographic data from study participants; duration of the trial; progression to AD or to CDR 1.0. We further extracted data for all-cause dropouts, serious adverse events (SAE), and death reported in the double-blind phase of the RCTs. Data from selected trials were retrieved by two investigators independently (MLCG and FMG) and summarized by a third investigator (BSD). If any discrepancy emerged during the data extraction, they were resolved by consensus among all investigators. To assess the quality of the study design/reporting of each selected trial, we used the Jadad scale [20].
Statistical analysis
Meta-analyses were performed using the software R: A Language and Environment for Statistical Computing version 2.4.1 (R Foundation for Statistical Computing, Vienna, Austria) and the software MIX-for-meta-Analysis (Kitasato Clinical Research Center, Evidence Synthesis Division—MIX, Kanagawa, Japan). Due to evidence of class effect for the therapeutic benefit of ChEIs in the treatment of AD, we considered the selected studies altogether, regardless of study drug. We calculated the relative risk, with the Mantel-Haenszel weighting method, of conversion from MCI to AD in the patients who were treated with ChEIs as compared to placebo. We further calculated the relative risk of all-cause dropouts, SAE and death in the patients who were treated with ChEIs as compared to placebo. Due to the small number of studies selected for meta-analysis we used both fixed-effect and random-effect models in the meta-analysis (Table 4). Q-test was performed to test for the heterogeneity of the data between the studies. Statistical significance level was set at α = 0.05 for all statistical analyses.
Results
Study selection
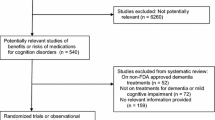
One hundred and fifty-six references from electronic databases and 17 references from clinical trials registry database were retrieved, yielding a total of 173 references. From this amount, three references were selected for data-extraction (three published and no unpublished trials). The flow diagram 1 shows the steps for the study selection in detail. We retrieved data only from the donepezil and placebo arm from the donepezil trial [33]; data from the vitamin E arm were not considered as this arm was regarded as treatment arm. In addition, this study did not clearly state the number or rate of adverse events, and was thus excluded from the sub-analysis of SAEs. Data from the Gal-Int 11/18 Study were retrieved either separate (study 1 and study 2) or in conjunction according to how it was presented in the published report [49]. Tables 1 and 2 show individual information for each study. Table 3 displays the characteristics of the studies excluded in the last step of the selection (Fig. 1).
Meta-analysis
A total of 3,574 subjects of the three RCTs were included in the meta-analysis (1,784 in the ChEI-treated group; 1,790 in the placebo group). Two-hundred and seventy-five subjects (15.4%) progressed to AD/dementia in the ChEI-treated group, and 366 subjects (20.4%) progressed to AD/dementia in the placebo group. The all-cause drop-outs rates were 39.8% (711/1,784) in the ChEI-treated group and 29.2% (524/1,790) in the placebo group. The rates of SAE in the ChEI-treated group was 23% (333/1,445) and 23.7% (349/1472) in the placebo group; death rates in the ChEIs-treated group was 1.7% (31/1784) and 1.6% (30/1790) in placebo group. Table 4 shows the meta-analytical (fixed-effect and random-effect models) for conversion from MCI to AD, all-cause drop-outs, serious adverse events and death (Table 4). Figures 2, 3, 4 and 5 show forest plots for the meta-analysis of progression from MCI to AD/dementia, all-cause dropouts, SAE, and death.
Discussion
In this meta-analysis, we showed that the long-term use of ChEIs significantly reduced the risk of progression of MCI subjects to AD/dementia in 25% in comparison to those subjects in the placebo group. The patients on the ChEI group did not have increased risk of serious adverse events; nonetheless, the ChEI treated-group showed a small, non-significant, increase in all-cause dropout rate and risk of death in comparison to the placebo group, albeit the latter event was reputed unrelated to study drugs. These results were very consistent regardless of the model (fixed effect or random effect) used in the meta-analyses.
Taken together, these results suggest that the long-term use of ChEI drugs may have a beneficial effect on delaying the progression of pre-dementia stages to AD. This is of utmost importance in light of the fast growing of elderly population and, as a consequence, the “epidemics” of AD that follows it, with exponential increase in the prevalence of the AD in the coming years [48]. Interventions that are able to delay the onset of dementia would diminish the prevalence of dementia in the coming years. Brookmeyer et al. [7] have estimated that a delay in the onset of dementia by 2 years would reduce the prevalence of dementia by 23% at 2050. This would have an important impact on public health system and on the pharmaco-economics related to AD care.
There are several lines of evidence to support the potential disease-modifying properties of ChEIs. Pre-clinical studies have shown that the activation of muscarinic receptor may regulate amyloid precursor protein (APP) metabolism. In vitro studies have shown that the stimulation of M1 and M3 receptors stimulates the processing of APP through the α-secretase pathway [8, 29]. This would lead to increased release of sAPP and reduced production of amyloid-β protein [50], what, in turn, would reduce the deleterious effects of β-amyloid production and aggregation in the brain. In addition, the activation of these muscarinic receptors also inhibits GSK-3β activation and tau phosphorylation, other important features of the physiopathology of AD [13, 16, 19].
Other lines of evidence arise from trials with MCI patients. Despite the negative findings of RCTs with MCI patients on the proposed trials end-points, some sub-analysis of these trials may further corroborate the potential disease-modifying properties of these drugs. Petersen et al. [33] have reported a lower rate of progression to AD in the first 24 months of donepezil treatment as compared to placebo; in the APOE ε4 carriers group, a widely recognized genetic risk factor for late-onset AD and a potential therapeutic target for AD [26], MCI subjects treated with donepezil showed lower risk to progress to AD than those who were on the placebo arm, and this effect remained significant on the 12th, 24th, and 36th month of follow-up. Nevertheless, Feldman et al. [14] have not confirmed the positive effect of ChEIs in APOE ε4 carriers. On the other hand, they have shown that whole brain volume decline and ventricular expansion were reduced in women taking rivastigmine as compared to placebo, even though these effects were not associated with lower conversion to AD. The current data suggest that MCI subjects on long-term ChEIs use may decline at a lower pace, delaying the conversion to AD. Such outcome may be secondary to cognitive enhancement per se, which is compatible with a symptomatic effect of ChEI on cognition. On the other hand, one cannot rule out the possibility that the long-term use of ChEIs may also facilitate neurobiological changes, as highlighted above, which may actually hinder the progression of AD-related pathology, suggesting a potential disease-modifying property.
Though not the main subject of the present study, it is worth mentioning that the long-term use of ChEIs in MCI was associated with better cognitive performance, as measured by the ADAS-Cog (modified version), and the MMSE in the Vitamin E and Donepezil Trial [33], and by the Digit Symbol Substitution Test in the Gal-Int 11/18 Trial [49] and better global functioning, as measured by the CDR sum of boxes [33, 49]. These benefits were mostly observed within the first 24 months of treatment in these trials, being more pronounced in the first year of ChEI treatment. This pattern is somewhat similar to those observed in the open-label continuation phase of the ChEI trials with AD patients [42].
Why do individual RCTs failed? Firstly, the MCI concept and diagnostic criteria is under continuous reformulation and there are no standardized assessment procedures for the identification of MCI cases in either clinical and research settings [4]. The concept of MCI relies on a continuum of cognitive performance, ranging from very mild deficits to more severe impairment, but the diagnosis of dementia remains unwarranted. Considering this, the time needed to observe conversion to AD may be greatly variable, what, in turn, may also influence the outcome of RCTs designed to address disease-modifying properties of drugs for AD [30]. Furthermore, the baseline diagnosis of MCI may not be specific to predict dementia progression, as these patients may return to normal cognitive function in the follow-up, may remain stable and decline very slowly over long follow-up periods [6, 36]. Indeed, Visser et al. [44] have shown that the criteria for MCI used in different clinical trials had low to moderate diagnostic accuracy, sensitivity and specificity to predict the conversion to AD in different clinical trials aiming to assess disease-modifying properties of drugs. Taken together, these factors may have led to inclusion of many patients who do not have pre-dementia AD and the exclusion of many who had this condition in these RCTs, what certainly biased the outcome of these trials, i.e. the prevention of AD conversion [15]. Besides, these studies did not incorporate biomarkers of the disease process in the selection criteria of MCI patients to include patients at highest risk of progression to AD, despite strong evidence that CSF amyloid-β42, total and phosphorylated tau protein [10, 17] and hippocampal atrophy [22, 45] are independent strong predictors of conversion to AD in MCI patients. The disease-modifying properties of ChEIs may be of small effect size; therefore, the study sample must be large enough with a long follow-up period to detect such benefit of treatment. These factors altogether may have yielded a heterogeneous sample of MCI subjects, what, in turn, may have had jeopardized the outcomes of these clinical trials. Of note, these issues are also relevant to other psychiatric disorders, e.g. unipolar depressive disorder, in which pharmacotherapy efficacy is highly dependent on the diagnostic criteria employed in RCTs, severity of depression on baseline, and assessment scales of treatment efficacy [28].
Recently, Dubois et al. [12] have proposed a revision of the research diagnostic criteria for AD. It includes, besides evidence of impairment in memory (and optionally in other cognitive domains), the addition of biomarkers of the disease (CSF, RMN hippocampal atrophy, PET scans, genetic markers in specific cases, e.g.: familial AD) in the AD diagnostic workout. Despite it yet lacks appropriate validation for its widespread use; it may be more specific to identify subjects at the highest risk of AD progression or, in other words, identify subjects at pre-clinical stages of the disease, when the introduction of disease-modifying interventions would be more effective. These criteria or their proposed diagnostic workout for AD would be included in the future clinical trial aiming to address the disease-modifying properties of an intervention in the future [9]. Moreover, the inclusion of other study end-points apart from the conversion to AD/dementia, e.g. slower cognitive decline, stability of cognitive deficits or modification on biological parameters, would prove beneficial in establishing disease-modifying properties of an intervention [27, 35, 43].
Several concerns arise in the interpretation of our results. Notwithstanding the fact that ChEI drugs are regarded as having a drug-class effect, each study used a different medication from this class. In view of minor differences in the pharmacological action of these drugs, these differences may have influenced the risk of conversion from MCI to AD/dementia on individual RCTs, what, in turn, may have influenced the results of the pooled analysis of our work. Methodological differences in the case definition at study entry (neuropsychological definition vs. CDR classification), cognitive assessment protocols, and the definition of case-conversion to AD/dementia (neuropsychological definition vs. CDR classification) were not considered in the data extraction process, and, thus, may have biased the pooled analysis of the conversion data.
In conclusion, we showed that the long-term use of ChEIs reduce the risk of progression from MCI to AD. This may have significant impact on public health and pharmaco-economic issues. Nevertheless, further RCTs with ChEIs with newer methodological approaches should be carried out prior to the widespread use of ChEIs in patients with MCI to prevent the progression to AD/dementia in clinical practice.
References
[No author listed] (2004) Efficacy and safety of rivastigmine in patients with mild cognitive impairment. NCT00134953. Available on-line on http://www.clinicaltrials.gov/ct2/show/NCT00134953. Accessed 27 Oct 2008
[No author’s listed]. (2007) A randomized double blind parallel, placebo-controlled trial to examine the efficacy of oral donepezil (5 mg qd for 6 weeks) after single dose and steady state therapy (2 and 6 weeks) in subjects with mild cognitive impairment. NCT00483028. Available on-line on http://clinicaltrials.gov/ct2/show/NCT00483028. Accessed 27 Oct 2008
[No authors listed] (2004) Cognitive enhancers explored with PET imaging. NCT 00042172. Available on-line http://clinicaltrials.gov/ct2/show/NCT00042172. Accessed 27 Oct 2008
Ames D, Petersen RC, Knopman DS, Visser PJ, Brodaty H, Gauthier S (2006) Is mild cognitive impairment a clinically useful concept? Int Psychogeriatr 18:393–414
Anita M (2007) A one-year, multicenter, randomized, double-blind, placebo controlled evaluation of the efficacy and safety of donepezil hydrochloride in subjects with mild cognitive impairment. NCT00293176. Available on line http://clinicaltrials.gov/ct2/show/NCT00293176. Accessed 27 Oct 2008
Bäckman L, Small BJ, Fratiglioni L (2001) Stability of the preclinical episodic memory deficit in Alzheimer’s disease. Brain 124:96–102
Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM (2007) Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement 3:186–191
Buxbaum JD, Oishi M, Chen HI, Pinkas-Kramarski R, Jaffe EA, Gandy SE, Greengard P (1992) Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer beta/A4 amyloid protein precursor. Proc Natl Acad Sci USA 89:10075–10078
Cummings JL, Doody R, Clark C (2007) Disease-modifying therapies for Alzheimer disease: challenges to early intervention. Neurology 69:1622–1634
Diniz BS, Pinto JA Jr, Forlenza OV (2008) Do CSF total tau, phosphorylated tau, and beta-amyloid 42 help to predict progression of mild cognitive impairment to Alzheimer’s disease? A systematic review and meta-analysis of the literature. World J Biol Psychiatry 9:172–182
Doody R, Geldmacher D, Gordon B, Perdomo CA, Pratt RD, Donepezil Study Group (2001) Open-label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Arch Neurol 58:427–433
Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro OJ, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P (2007) Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 6:734–746
Farias GG, Godoy JA, Hernandez F, Avila J, Fisher A, Inestrosa NC (2004) M1 muscarinic receptor activation protects neurons from beta-amyloid toxicity: a role for Wnt signaling pathway. Neurobiol Dis 17:337–348
Feldman HH, Ferris S, Winblad B, Sfikas N, Mancione L, He Y, Tekin S, Burns A, Cummings J, del Ser T, Inzitari D, Orgogozo JM, Sauer H, Scheltens P, Scarpini E, Herrmann N, Farlow M, Potkin S, Charles HC, Fox NC, Lane R (2007) Effect of rivastigmine on delay to diagnosis of Alzheimer’s disease from mild cognitive impairment: the InDDEx study. Lancet Neurol 6:501–512
Forlenza OV, Chiu E (2008) Mild cognitive impairment: a concept ready to move on? Curr Opin Psychiatry 21:529–532
Forlenza OV, Spink JM, Dayanandan R, Anderton BH, Olesen OF, Lovestone S (2000) Muscarinic agonists reduce tau phosphorylation in non-neuronal cells via GSK-3beta inhibition and in neurons. J Neural Transm 107:1201–1212
Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L (2006) Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol 5:228–234
Hashimoto M, Kazui H, Matsumoto K, Nakano Y, Yasuda M, Mori E (2005) Does donepezil treatment slow the progression of hippocampal atrophy in patients with Alzheimer’s disease? Am J Psychiatry 162:676–682
Hooper C, Killick R, Lovestone S (2008) The GSK3 hypothesis of Alzheimer’s disease. J Neurochem 104:1433–1439
Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds JM, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary. Controlled Clin Trials 17:1–12
Koontz J, Baskys A (2005) Effects of galantamine on working memory and global functioning in patients with mild cognitive impairment: a double-blind placebo-controlled study. Am J Alzheimers Dis Other Demen 20:295–302
Korf ESC, Wahlund LO, Visser PJ, Scheltens P (2004) Medial temporal lobe atrophy on MRI predicts dementia in patients with mild cognitive impairment. Neurology 63:94–100
Lanctôt KL, Herrmann N, Yau KK, Khan LR, Liu BA, LouLou MM, Einarson TR (2003) Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysis. CMAJ 169:557–564
Leyhe T, Stransky E, Eschweiler GW, Buchkremer G, Laske C (2008) Increase of BDNF serum concentration during donepezil treatment of patients with early Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci 258:124–128
López-Pousa S, Turon-Estrada A, Garre-Olmo J, Pericot-Nierga I, Lozano-Gallego M, Vilalta-Franch M, Hernández-Ferràndiz M, Morante-Muñoz V, Isern-Vila A, Gelada-Batlle E, Majó-Llopart J (2005) Differential efficacy of treatment with cholinesterase inhibitors in patients with mild and moderate Alzheimer’s disease over a 6-month period. Dement Geriatr Cogn Disord 19:189–195
Mahley RW, Weisgraber KH, Huang Y (2006) Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci USA 103:5644–5651
Mendes CT, Mury FB, de Sá Moreira E, Alberto FL, Forlenza OV, Dias-Neto E, Gattaz WF (2008) Lithium reduces Gsk3b mRNA levels: implications for Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci [Epub ahead of print]
National Institute for Clinical Excellence (NICE) (2004) Management of depression in primary and secondary care. The British Psychological Society and Gaskel. Leicester
Nitsch RM, Slack BE, Wurtman RJ, Growdon JH (1992) Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science 28:304–307
Petersen RC (2006) Conversion. Neurology 67(Suppl. 3):s12–s13
Petersen RC (2007) MCI treatment trials: failure or not? Lancet Neurol 6:473–475
Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E (1999) Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 56:303–308
Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ, Alzheimer’s Disease Cooperative Study Group (2005) Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 352:2379–2388
Pirttila T, Wilcock G, Truyen L, Damaraju CV (2004) Long-term efficacy and safety of galantamine in patients with mild-to-moderate Alzheimer’s disease: multicenter trial. Eur J Neurol 11:734–741
Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JC, DeGroodt W, Mehta P, Craft S (2008) Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology 70:440–448
Ritchie K, Artero S, Touchon J (2001) Classification criteria for mild cognitive impairment: a population-based validation study. Neurology 56:37–42
Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT (1998) A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease: Donepezil Study Group. Neurology 50:136–145
Rösler M, Anand R, Cicin-Sain A, Gauthier S, Agid Y, Dal-Bianco P, Stähelin HB, Hartman R, Gharabawi M (1999) Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. BMJ 318:633–638
Sabbagh MN, Farlow MR, Relkin N, Beach TG (2006) Do cholinergic therapies have disease-modifying effects in Alzheimer’s disease? Alzheimers Dement 2:118–125
Salloway S, Ferris S, Kluger A, Goldman R, Griesing T, Kumar D, Richardson S (2004) Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology 63:651–657
Saykin AJ, Wishart HA, Rabin LA, Flashman LA, McHugh TL, Mamourian AC, Santulli RB (2004) Cholinergic enhancement of frontal lobe activity in mild cognitive impairment. Brain 127:1574–1583
Small GW, Kaufer D, Mendiondo MS, Quarg P, Spiegel R (2005) Cognitive performance in Alzheimer’s disease patients receiving rivastigmine for up to 5 years. Int J Clin Pract 59:473–477
Vellas B, Andrieu S, Sampaio C, Coley N, Wilcock G, for the European Task Force Group (2008) Endpoints for trials in Alzheimer’s disease: a European task force consensus. Lancet Neurol 7:436–450
Visser PJ, Sheltens P, Verhey FRJ (2005) Do MCI criteria in drug trials accurately identify subjects with pre-dementia Alzheimer’s disease? J Neurol Neurosurg Psychiatry 76: 1348–1354
Whitwell JL, Shiung MM, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, Petersen RC, Jack CR Jr (2008) MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology 70: 512–520
Wilcock GK, Lilienfeld S, Gaens E (2000) Efficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: multicentre randomised controlled trial. Galantamine International-1 Study Group. BMJ 321:1445–1449
Wilkinson DG, Passmore AP, Bullock R, Hopker SW, Smith R, Potocnik FC, Maud CM, Engelbrecht I, Hock C, Ieni JR, Bahra RS (2002) A multinational, randomised, 12-week, comparative study of donepezil and rivastigmine in patients with mild to moderate Alzheimer’s disease. Int J Clin Pract 56:441–446
Wimo A, Winblad B, Jönsson L (2007) An estimate of the total worldwide societal costs of dementia in 2005. Alzheimers Dement 3:81–91
Winblad B, Gauthier S, Scinto L, Feldman H, Wilcock GK, Truyen L, Mayorga AJ, Wang D, Brashear HR, Nye JS (2008) Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology 70:2024–2035
Wolf BA, Wertkin AM, Jolly YC, Yasuda RP, Wolfe BB, Konrad RJ, Manning D, Ravi S, Williamson JR, Lee VM (1995) Muscarinic regulation of Alzheimer’s disease amyloid precursor protein secretion and amyloid beta-protein production in human neuronal NT2N cells. J Biol Chem 270:4916–4922
Conflict of interest
Dr. Gattaz has received research support and speakership honoraria from AstraZeneca, Bristol Myers-Squibb, Eli Lilly, and Janssen-Cilag.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Diniz, B.S., Pinto, J.A., Gonzaga, M.L.C. et al. To treat or not to treat? A meta-analysis of the use of cholinesterase inhibitors in mild cognitive impairment for delaying progression to Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci 259, 248–256 (2009). https://doi.org/10.1007/s00406-008-0864-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-008-0864-1