Abstract
The anterior cingulate cortex (ACC) is crucially involved in executive control of attention. Here, seven medication-naïve adult patients suffering from attention deficit/hyperactivity disorder (ADHD) were studied with 2D 1H-magnetic resonance spectroscopic imaging (MRSI) of the ACC [Brodmann areas 24b′–c′ and 32′] twice, once before initiation of stimulant treatment and once after 5–6 weeks of methylphenidate. Upon retest, all patients demonstrated marked clinical improvement. Analysis of regional brain spectra revealed a significantly decreased signal of choline containing compounds as well as increased N-acetyl-aspartate (NAA) levels following treatment with methylphenidate whereas total creatine remained unchanged. Our results add to a growing body of evidence implicating the ACC in the pathophysiology of ADHD and suggest that subtle structural changes might be associated with aspects of clinical improvement under stimulant treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The behavioral complex of hyperactivity, impulsivity and inattention subsumed in the diagnosis of attention-deficit/hyperactivity disorder (ADHD) constitutes a common, chronic and serious mental health problem. ADHD is the most common psychiatric disorder of childhood affecting approximately 5% of school-aged children [33]. A cross-cultural comparison of patients recently found that dimensional symptoms associated with the categorical diagnosis of ADHD are similar in diverse cultural backgrounds [28]. Importantly, symptoms frequently persist into maturity (e.g. [35, 39]) and for some patients, ADHD may even lead to more problem behaviors when the individual is faced with the complex challenges of adolescence and/or adulthood [27, 29].
Through its connections with the prefrontal and parietal cortices as well as the motor system and the frontal eye fields the anterior cingulate cortex (ACC) is a crucial regulator of top-down and bottom-up information processing [26]. Accumulating evidence from both electrophysiological and functional neuroimaging studies has implicated the ACC in the cognitive control deficits associated with ADHD (e.g. [6, 17, 19, 38]). Notably, the neuropsychological deficit profile of patients with structural lesions of the cingulate cortex shows certain similarities with that of ADHD patients. In particular, following bilateral anterior cingulotomy, slower response times and increased interference on the Stroop task have been observed [24]. Similarly, superior medial lesions including the anterior cingulate have also been associated with lengthened reaction times on a variant of the Stroop interference paradigm [1]. Conversely, two spectroscopic studies have recently described subtle structural abnormalities of the ACC in adult medication-free [25] and medication-naïve ADHD patients, respectively [9]. In particular, both studies independently found increased choline resonances indicative of altered membrane turnover/breakdown in the ACC of adult ADHD patients. Slight structural abnormalities associated with membrane turnover/breakdown and myelination agree well with the concept of ADHD as a neuro-developmental disorder (e.g. [2]). The pathophysiological relevance of increased ACC choline in adult ADHD was further supported by a high inverse correlation with hit reaction times on a high-processing load continous performance test [9].
Magnetic resonance spectroscopy is emerging as a powerful novel technique in cognitive neuroscience research. Methylphenidate is the most commonly prescribed medication for the treatment of ADHD [34]. We here addressed the hypothesis that chronic methylphenidate at therapeutic doses would exert a discernible effect on the neurometabolic pattern of the adult ACC. Three markers of brain metabolism, N-acetyl-aspartate (NAA), choline containing compounds (Ch) and total creatine (tCr; i.e., creatine and phosphocreatine) were evaluated in seven adult medication-naïve ADHD patients prior to and during chronic treatment with methylphenidate. NAA is regarded as an indicator of neuronal viability and functioning. The choline resonance is constituted of metabolites of phosphatidylcholine [3]. As a pivotal buffer capacity in the energy metabolism of the cell the creatine signal is relatively stable [32]. In particular, we speculated that the mechanism of action of methylphenidate would involve an attenuation of Ch resonances.
Methods
Data presented here were collected as part of a larger project aimed at defining both cross-sectional and longitudinal neurochemistry in adult ADHD [9]. This is the second paper to be published from this work. Seven adult ADHD patients from the Central Institute of Mental Health ADHD outpatient clinic who were medication-naïve at the time of the first MRI (T1) were re-investigated 5–6 weeks after initiation of methylphenidate treatment (T2). Exclusion criteria included current major depressive episode, anxiety disorder, current or past pharmacotherapy for ADHD, current or past bipolar or psychotic disorder, serious medical or neurological illness, alcohol dependence as well as active use of drugs of abuse. Upon retest, patients received between 20 and 40 mg methylphenidate per day.
Sociodemographic characteristics of the patient sample are given in Table 1. IQ was estimated with the Multiple Word Choice Fluency Test (MWT-B; [22]) and the Leistungspruefsystem-3 (LPS-3; [18]). ADHD symptom severity was assessed using the clinican administered ADHD Symptom Checklist for DSM-IV [13]. Additionally, patients were tested on the continous performance test, identical pairs version (CPT-IP) at T1 and T2. The CPT-IP is a high-processing load CPT that is particularly suitable to detect subtle processing deficits. The procedure has been described in detail previously [9, 11]. The protocol had been approved by the local ethics committee and written informed consent was obtained from each participant according to the Declaration of Helsinki.
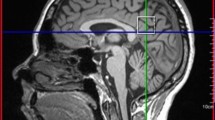
MR-spectroscopic imaging (MRSI) was conducted as described previously [9, 10]. Briefly, data were acquired on a 1.5 T Magnetom VISION™ (Siemens, Erlangen, Germany) using a standard circularly polarized head coil. 2D FLASH images in coronal, saggital, and oblique transverse orientation were acquired. Transverse images were angulated parallel to the anterior cingulate gyrus [14]. A 2D MRSI sequence with PRESS volume selection was used with the PRESS volume angulated parallel to the transverse images and centered on the dorsal anterior cingulate gyrus. For the second scan, voxel placement from the first scan was followed with special reference to anatomical landmarks (Fig. 1). To minimize operator errors, coil placement, signal acquisition, and postprocessing were carried out by the same person. An MRSI field of view of 210 × 210 mm and a PRESS volume thickness of 15 mm was used with circular k-space sampling equivalent to a maximum of 24 × 24 phase encoding steps [21]. Other measurement parameters were: TR = 1.5 s; TE = 135 ms; resultant total measurement time = 11 min.
Typical PRESS spectra acquired from ACC before and after 5–6 weeks of methylphenidate treatment. The saggital view depicts PRESS volume angulated and centered on the anterior cingulate gyrus. Metabolite data are given as raw (black) and fitted material (blue). tCr total creatine, Ch total choline, NAA N-acetylaspartate
Postprocessing of the MRSI data with an automated spectral fitting program has been described previously [36, 42, 43]. This program uses a parametric spectral model with acquisition specific a priori information for NAA, tCr: creatine and phosphocreatine (tCr), and Ch resonances, in combination with a wavelet-based, non-parametric characterization of baseline signals. A k-space apodization resulting in an effective voxel size of approximately 2.4 cm3 and zero filling to 32 × 32 k-space points was applied prior to the spatial Fourier transformation [14]. Zerofilling from 512 to 1,024 time domain data points and Gaussian multiplication corresponding to 0.6 Hz line broadening were carried out prior to the time domain Fourier transformation. Spectral phasing was also performed automatically. The automated spectral analysis procedure was applied to all complex spectra in a user defined region of interest within the PRESS volume in an iterative manner, with each iteration including both the non-parametric baseline characterization and parametric fitting of the metabolite model.
In a previous study [14], it was established that voxels selected from the cingulate gyrus which partially contain white matter showed a significant difference to voxels containing gray matter without partial volume of the corpus callosum. The Ch signal turned out to be significantly lower in the cingulate gray matter than in the corpus callosum. Therefore, great care was taken in the choice of the voxels included in the analysis. Voxels of interest (VOIs) were chosen in the ACC under the supervision of an experienced radiologist. In the axial slice which showed the most anterior boundary of the genu of corpus callosum, the VOI was placed closest to the genu of the corpus callosum. On average, four voxels containing almost pure cortical gray matter from the cognitive division of the anterior cingulate gyrus were selected from each subject’s data set. To minimize partial volume effects, localization of each VOI was carefully checked. Voxels were only included, when there was no cerebrospinal fluid (CSF) on any of the axial MR-slices used for anatomical co-registration. Voxels had to contain pure gray matter on all of the axial MR-slices. Thus the number of included voxels differed from subject to subject (mean number of voxels: 4). However, upon retest, the number of left and right cingulate voxels remained the same in each participant. Furthermore, B0 inhomogeneities are most prominent in the frontal lobes including the cingulate region. Consequently, voxels with linewidths above 7 Hz were excluded from further analysis.
Absolute integral values of the model peaks obtained by the fitting algorithm for NAA, tCr, and Ch were corrected for differential head coil loading by multiplication with the transmitter reference voltage [15]. This yields a semi-quantitative measure so that the ambiguity of metabolite ratios can be avoided [15].
All numerical analyses were performed with StatView 5.0.1 for MacIntosh. Comparisons between the measurements obtained at T1 and T2 were performed with two-tailed Student’s paired t test. P < 0.05 was considered statistically significant.
Results
All patients showed marked clinical improvement on methylphenidate, as judged by the ADHD Symptom Checklist for DSM IV (T1: 31.6 ± 2.4; T2: 17.1 ± 1; t = 9.3, df = 6, P < 0.0001). CPT-IP performance measures at T1 and T2 are given in Table 2. While the percentage of hits increased (t = −2.4, df = 6, P = 0.057, 1 − β = 0.43), patients made signficantly less errors of commission (responses to stimuli other than the target) at T2.
Typical PRESS-spectra from anterior cingulate voxels before and during chronic methylphenidate treatment are given in Fig. 1. tCr concentrations remained unchanged between T1 and T2 (7.2 ± 0.3 and 7.2 ± 0.2, respectively; t = 0.3, df = 6, P = 0.75). NAA concentrations increased significantly and Ch resonances decreased significantly following methylphenidate treatment (Fig. 2).
Discussion
A growing body of evidence implicates a dysfunction in dopaminergic fronto-striatal circuits in the executive control deficit associated with ADHD. In particular, the ACC plays a crucial role in recruiting cognitive control over behavior by predicting error likelihood [5]. Here, within the framework of a pilot study, we investigated seven medication-naïve adult patients suffering from ADHD twice, once before initiation of stimulant treatment and once after 5–6 weeks of methylphenidate. A rigorous protocol was followed for the selection of voxels with special reference to anatomical landmarks upon retest. Importantly, tCr remained unchanged between the voxels investigated at both time points. Despite the small size of the sample, stimulant treatment significantly decreased anterior cingulate choline resonances while it increased NAA in adult methylphenidate-responsive ADHD.
Methylphenidate and other psychostimulants exert profound and enduring neurobiological effects. Methylphenidate affects synaptic plasticity regulatory proteins in the cingulate gyrus [41]. A number of preclinical studies have specifically linked methylphenidate to increased brain energy metabolism. Reactive oxygen species (ROS) are continuously produced as a by-product of normal metabolism. Chronic methylphenidate has been demonstrated to increase ROS production in young rat brains [20]. Furthermore, chronic methylphenidate treatment has been shown to induce mitochondrial respiratory chain enzyme activities, especially in prefrontal cortex and striatum [16]. Our observation of increased NAA concentrations in the ACC following chronic methylphenidate are well compatible with these reports in that NAA is synthesized energy-dependently in the mitochondria and is therefore increasingly regarded as an indicator of neuronal energy status [8].
Whereas methylphenidate boosts brain dopamine levels, typical neuroleptics decrease dopaminergic signaling by blocking D2 receptors. Here, methylphenidate led to a significant increase in ACC NAA. It is therefore interesting to note that a number of studies have described reduced anterior cingulate NAA in schizophrenic patients (e.g. [12, 40]). Importantly, there does not seem to exist a significant relationship between anterior cingulate NAA and duration of untreated psychosis and untreated schizophrenia [37]. Furthermore, reduced anterior cingulate NAA levels have been reported in schizophrenics treated with typical as compared to atypical medications [4]. It needs to be mentioned, however, that a recent preclinical study in rats treated with haloperidol failed to demonstrate an effect on cingulate neurochemistry [7].
Two recent studies have independently described elevated ACC choline resonances in medication-free [25] and medication-naïve [9] adult ADHD patients, respectively. The pathophysiological underpinnings of this empirical finding still remain to be unravelled. However, the decrease in choline concentration detected here following methylphenidate treatment fits well with a recent energetics hypothesis of ADHD. This hypothesis posits insufficient lactate supply to oligodendrocytes leading to impairments in fatty acid synthesis and myelin sheath formation [30].
Interestingly, a study of recently abstinent methamphetamine-dependent subjects yielded almost opposite results to those reported here, i.e., low NAA and high choline in the anterior cingulum [23]. Importantly, the same group has also been able to show that attentional control in abstinent methamphetamine abusers measured in the Stroop interference task correlates with reduced NAA/Cr metabolite ratios in the ACC [31]. However, it still needs to be established whether neurometabolite alterations in abstinent psychostimulant users are primarily due to drug effects or may also reflect brain pathology preceding stimulant use.
In summary, our results build upon the rapidly expanding literature on functional disturbances in the ACC in ADHD. It is now becoming increasingly apparent that subtle structural abnormalities of the ACC underlie neuropsychological deficits associated with ADHD. Conceivably, stimulant treatment may alleviate ADHD symptomatology at least in part by rectifying neurometabolite disturbances and/or by inducing compensatory structural changes in the ACC.
References
Alexander MP, Stuss DT, Picton T, Shallice T, Gillingham S (2007) Regional frontal injuries cause distinct impairments in cognitive control. Neurology 68(18):1515–1523
Bartzokis G (2007) Acetylcholinesterase inhibitors may improve myelin integrity. Biol Psychiatry 62(4):294–301
Boulanger Y, Labelle M, Khiat A (2000) Role of phospholipase A(2) on the variations of the choline signal intensity observed by 1H magnetic resonance spectroscopy in brain diseases. Brain Res Brain Res Rev 33(2–3):380–389
Braus DF, Ende G, Weber-Fahr W, Demirakca T, Tost H, Henn FA (2002) Functioning and neuronal viability of the anterior cingulate neurons following antipsychotic treatment: MR-spectroscopic imaging in chronic schizophrenia. Eur Neuropsychopharmacol 12(2):145–152
Brown JW, Braver TS (2005) Learned predictions of error likelihood in the anterior cingulated cortex. Science 307:1118–1121
Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, Rosen BR, Biederman J (1999) Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the counting stroop. Biol Psychiatry 45(12):1542–1552
Bustillo J, Barrow R, Paz R, Tang J, Seraji-Bozorgzad N, Moore GJ, Bolognani F, Lauriello J, Perrone-Bizzozero N, Galloway MP (2006) Long-term treatment of rats with haloperidol: lack of an effect on brain N-acetyl aspartate levels. Neuropsychopharmacology 31(4):751–756
Clark JB (1998) N-acetyl aspartate: a marker for neuronal loss or mitochondrial dysfunction. Dev Neurosci 20:271–276
Colla M, Ende G, Alm B, Deuschle M, Heuser I, Kronenberg G (2007) Cognitive MR spectroscopy of anterior cingulate cortex in ADHD: elevated choline signal correlates with slowed hit reaction times. J Psychiatr Res [Epub ahead of print]
Colla M, Ende G, Bohrer M, Deuschle M, Kronenberg G, Henn FA, Heuser I (2003) MR spectroscopy in Alzheimer’s disease: gender differences in probabilistic learning capacity. Neurobiol Aging 24(4):545–552
Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L (1988) The continous performance test, identical pairs version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatry Res 26:223–238
Deicken RF, Zhou L, Schuff N, Weiner MW (1997) Proton magnetic resonance spectroscopy of the anterior cingulate region in schizophrenia. Schizophr Res 27(1):65–71
DuPaul G, Power T, Anastopoulos A, Reid R (1998) ADHD rating scale IV: checklists, norms and clinical interpretation. Guilford Press, New York
Ende G, Braus DF, Walter S, Weber-FAhr W, Soher B, Maudsley AA, Henn FA (2000) Effects of age, medication, and illness duration on N-acetyl-aspartate signal of the anterior cingulate region in schizophrenia. Schizophr Res 41:389–395
Ende G, Laxer KD, Knowlton RC, Matson GB, Schuff N, Fein G, Weiner MW (1997) Temporal lobe epilepsy: bilateral hippocampal metabolite changes revealed at proton MR spectroscopic imaging. Radiology 202:809–817
Fagundes AO, Rezin GT, Zanette F, Grandi E, Assis LC, Dal-Pizzol F, Quevedo J, Streck EL (2007) Chronic administration of methylphenidate activates mitochondrial respiratory chain in brain of young rats. Int J Dev Neurosci 25(1):47–51
Fallgatter AJ, Ehlis AC, Seifert J, Strik WK, Scheuerpflug P, Zillessen KE, Herrmann MJ, Warnke A (2004) Altered response control and anterior cingulate function in attention-deficit/hyperactivity disorder boys. Clin Neurophysiol 115(4):973–981
Horn W (1983) Leistungspruefsystem. HansHuber Verlag, Bern
Krauel K, Duzel E, Hinrichs H, Santel S, Rellum T, Baving L (2007) Impact of emotional salience on episodic memory in attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study. Biol Psychiatry 61(12):1370–1379
Martins MR, Reinke A, Petronilho FC, Gomes KM, Dal-Pizzol F, Quevedo J (2006) Methylphenidate treatment induces oxidative stress in young rat brain. Brain Res 1078:189–197
Maudsley AA, Mattson GB, Hugg JW, Weiner MW (1994) Reduced phase encoding in spectroscopic imaging. Magn Reson Med 31(6):645–651
Merz J, Lehrl S, Galster JV, Erzigkeit H (1975) MWT-B—ein Intelligenzkurztest. Psychiatr Neurol Med Psychol (Leipzig) 27:423–428
Nordahl TE, Salo R, Possin K, Gibson DR, Flynn N, Leamon M, Galloway GP, Pfefferbaum A, Spielman DM, Adalsteinsson E, Sullivan EV (2002) Low N-acetyl-aspartate and high choline in the anterior cingulum of recently abstinent methamphetamine-dependent subjects: a preliminary proton MRS study. Magnetic resonance spectroscopy. Psychiatry Res 116(1–2):43–52
Ochsner KN, Kosslyn SM, Cosgrove GR, Cassem EH, Price BH, Nierenberg AA, Rauch SL (2001) Deficits in visual cognition and attention following bilateral anterior cingulotomy. Neuropsychologia 39(3):219–230
Perlov E, Philipsen A, Hesslinger B, Buechert M, Ahrendts J, Feige B, Bubl E, Hennig J, Ebert D, Tebartz van Elst L (2007) Reduced cingulate glutamate/glutamine-to-creatine ratios in adult patients with attention deficit/hyperactivity disorder—a magnet resonance spectroscopy study. J Psychiatr Res 41(11): 934–941
Posner MI, DiGirolamo GJ, Fernandez-Duque D (1997) Brain mechanisms of cognitive skills. Conscious Cogn 6(2/3):267–290
Retz W, Retz-Junginger P, Schneider M, Scherk H, Hengesch G, Rosler M (2007) Drug addiction in young prison inmates with and without attention deficit hyperactivity disorder (ADHD). Fortschr Neurol Psychiatr 75(5):285–292
Roessner V, Becker A, Rothenberger A, Rohde LA, Banaschewski T (2007) A cross-cultural comparison between samples of Brazilian and German children with ADHD/HD using the child behavior checklist. Eur Arch Psychiatry Clin Neurosci 257(6):352–359 Epub 2007 Jul 14
Rosler M, Retz W, Retz-Junginger P, Hengesch G, Schneider M, Supprian T, Schwitzgebel P, Pinhard K, Dovi-Akue N, Wender P, Thome J (2004) Prevalence of attention deficit-/hyperactivity disorder (ADHD) and comorbid disorders in young male prison inmates. Eur Arch Psychiatry Clin Neurosci 254(6):365–371
Russell VA, Oades RD, Tannock R, Killeen PR, Auerbach JG, Johansen EB, Sagvolden T (2006) Response variability in attention-deficit/hyperactivity disorder: a neuronal and glial energetics hypothesis. Behav Brain Funct 2:30
Salo R, Nordahl TE, Natsuaki Y, Leamon MH, Galloway GP, Waters C, Moore CD, Buonocore MH (2007) Attentional control and brain metabolite levels in methamphetamine abusers. Biol Psychiatry 61(11):1272–1280
Sartorius A, Vollmayr B, Neumann-Haefelin C, Ende G, Hoehn M, Henn FA (2003) Specific creatine rise in learned helplessness induced by electroconvulsive shock treatment. Neuroreport 14(17):2199–2201
Sayal K (2007) Epidemiology of attention-deficit/hyperactivity disorder in the community. Br J Hosp Med (Lond) 68(7):352–355
Scheffler RM, Hinshaw SP, Modrek S, Levine P (2007) The global market for ADHD medications. Health Aff (Millwood) 26(2):450–457
Sobanski E, Brüggemann D, Alm B, Kern S, Deschner M, Schubert T, Philipsen A, Rietschel M (2007) Psychiatric comorbidity and functional impairment in a clinically referred sample of adults with attention-deficit/hyperactivity disorder (ADHD). Eur Arch Psychiatry Clin Neurosci [Epub ahead of print]
Soher BJ, Young K, Govindaraju V, Maudsley AA (1998) Automated spectral analysis III: application to in vivo proton MR spectroscopy and spectroscopic imaging. Magn Reson Med 40(6):822–831
Théberge J, Al-Semaan Y, Drost DJ, Malla AK, Neufeld RW, Bartha R, Manchanda R, Menon R, Densmore M, Schaefer B, Williamson PC (2004) Duration of untreated psychosis vs. N-acetylaspartate and choline in first episode schizophrenia: a 1H magnetic resonance spectroscopy study at 4.0 Tesla. Psychiatry Res 131(2):107–114
van Meel CS, Heslenfeld DJ, Oosterlaan J, Sergeant JA (2007) Adaptive control deficits in attention-deficit/hyperactivity disorder (ADHD): the role of error processing. J Psychiatr Res 151:211–220
Weiss G, Hechtman L, Milroy T, Perlman T (1985) Psychiatric status of hyperactives as adults: a controlled prospective 15-year follow-up of 63 hyperactive children. J Am Acad Child Psychiatry 24(2):211–220
Wood SJ, Yücel M, Wellard RM, Harrison BJ, Clarke K, Fornito A, Velakoulis D, Pantelis C (2007) Evidence for neuronal dysfunction in the anterior cingulate of patients with schizophrenia: a proton magnetic resonance spectroscopy study at 3 T. Schizophr Res 94(1–3):328–331
Yano M, Steiner H (2005) Methylphenidate (Ritalin) induces Homer 1a and zif 268 expression in specific corticostriatal circuits. Neuroscience 132(3):855–865
Young K, Govindaraju V, Soher BJ, Maudsley AA (1998a) Automated spectral analysis I: formation of a priori information by spectral simulation. Magn Reson Med 40(6):812–815
Young K, Soher BJ, Maudsley AA (1998b) Automated spectral analysis II: application of wavelet shrinkage for characterization of non-parameterized signals. Magn Reson Med 40(6):816–821
Acknowledgements
The authors wish to thank Ulrike Kersting for assistance with neuropsychological testing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kronenberg, G., Ende, G., Alm, B. et al. Increased NAA and reduced choline levels in the anterior cingulum following chronic methylphenidate. Eur Arch Psychiatry Clin Neurosc 258, 446–450 (2008). https://doi.org/10.1007/s00406-008-0810-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-008-0810-2